Abstract
1. The responses of primary and secondary afferent fibres from muscle spindles in cat soleus were studied during constant velocity stretching.
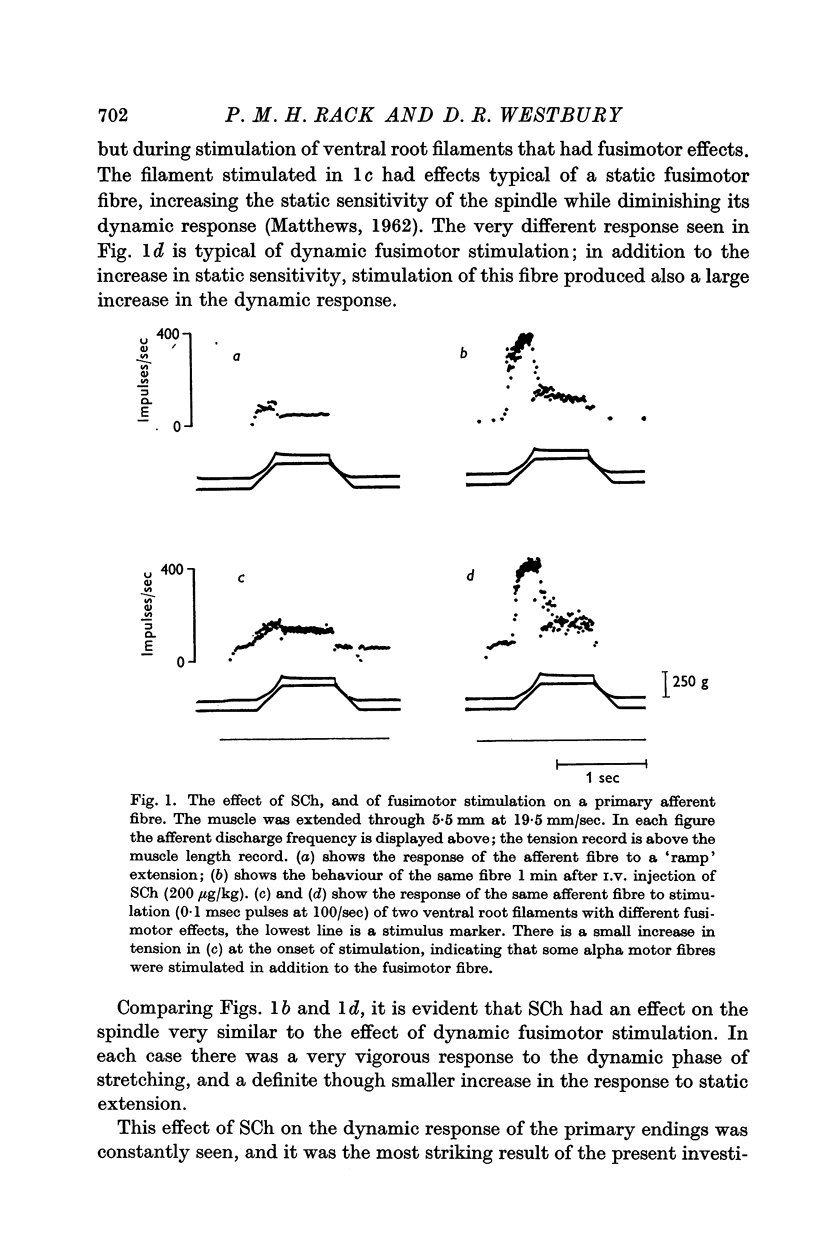
2. Intravenous Suxamethonium (SCh) caused a large increase in the response of the primary afferents to the dynamic phase of stretching, and a smaller increase in their response to static extension. The effects of SCh were similar to the effects of dynamic fusimotor stimulation.
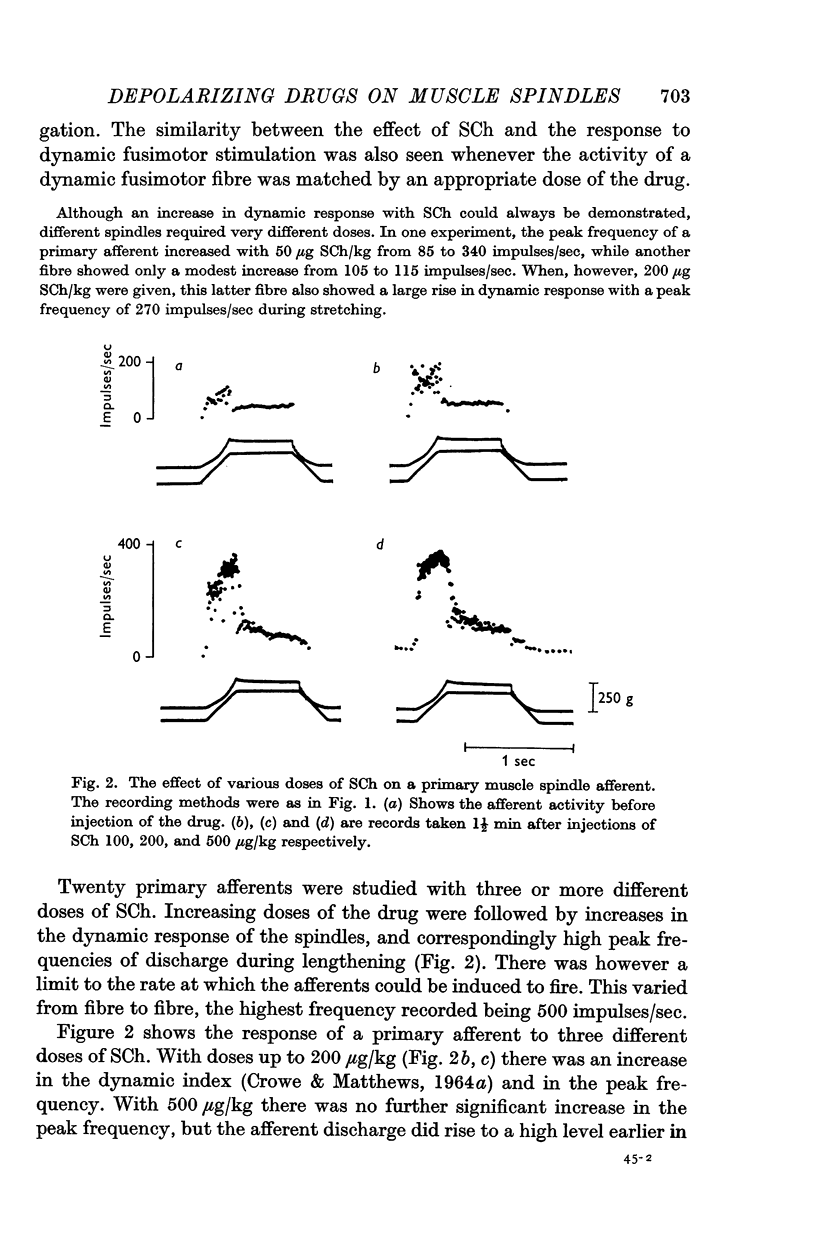
3. Increasing doses of SCh increased the response of primary afferents to dynamic stretching up to a point, but a peak discharge frequency was encountered beyond which the afferent fibre could not be induced to discharge.
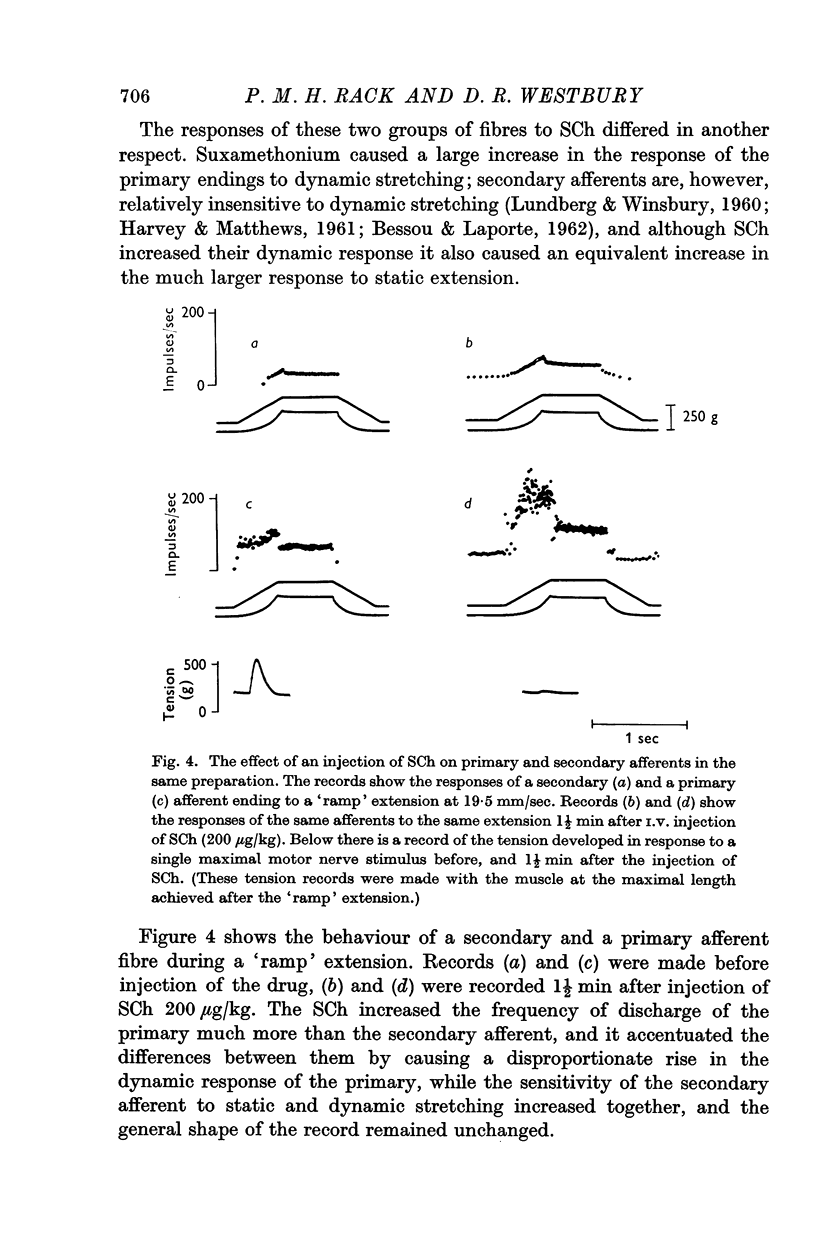
4. Suxamethonium increased the response of the secondary afferent fibres by a smaller amount than the primaries, and in particular caused a smaller increase in the response to dynamic extension.
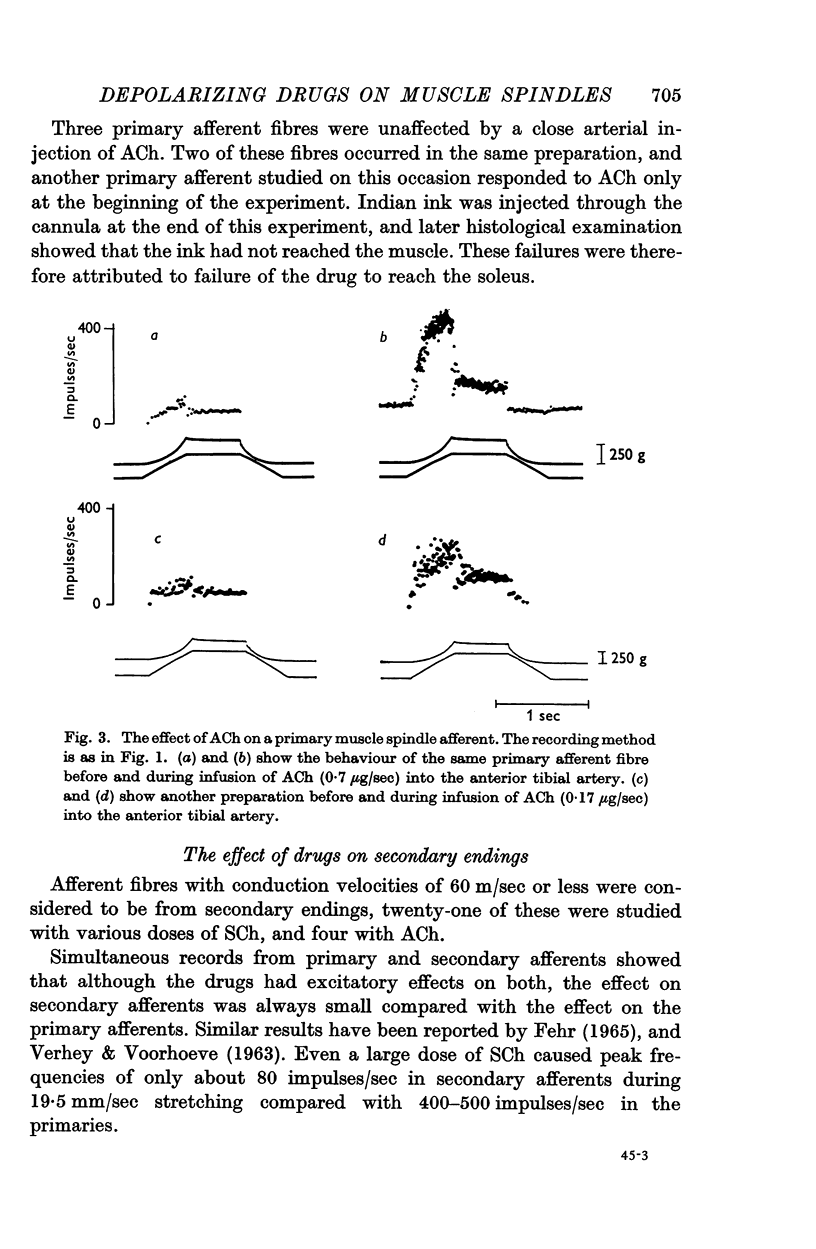
5. Acetylcholine given by close arterial injection had an effect similar to the effect of SCh.
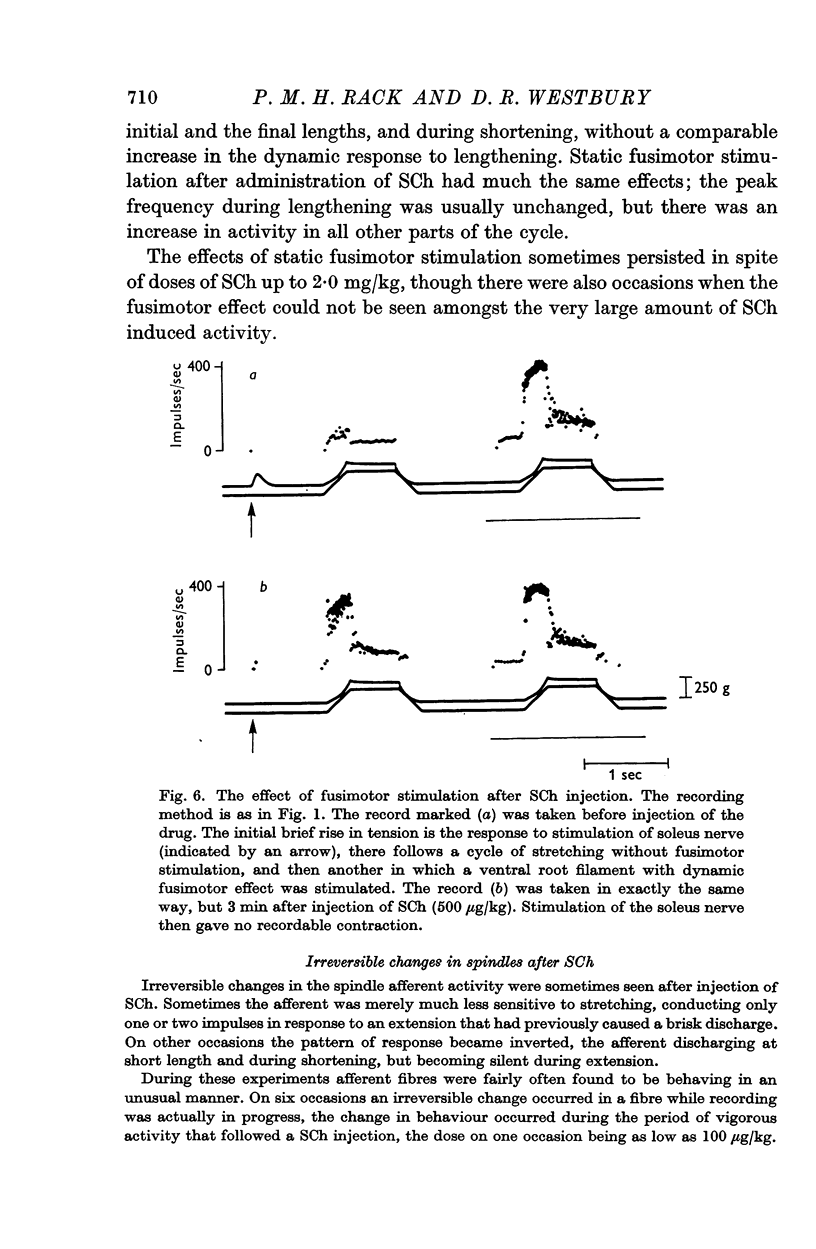
6. The effects of static and dynamic fusimotor stimulation on the response of primary afferents summed with the effects of small doses of SCh. When large amounts of SCh were used fusimotor stimulation sometimes had no further effect on the afferent discharge. It was not possible to say whether the fusimotor activity was then inhibited, or submerged in the SCh activity.
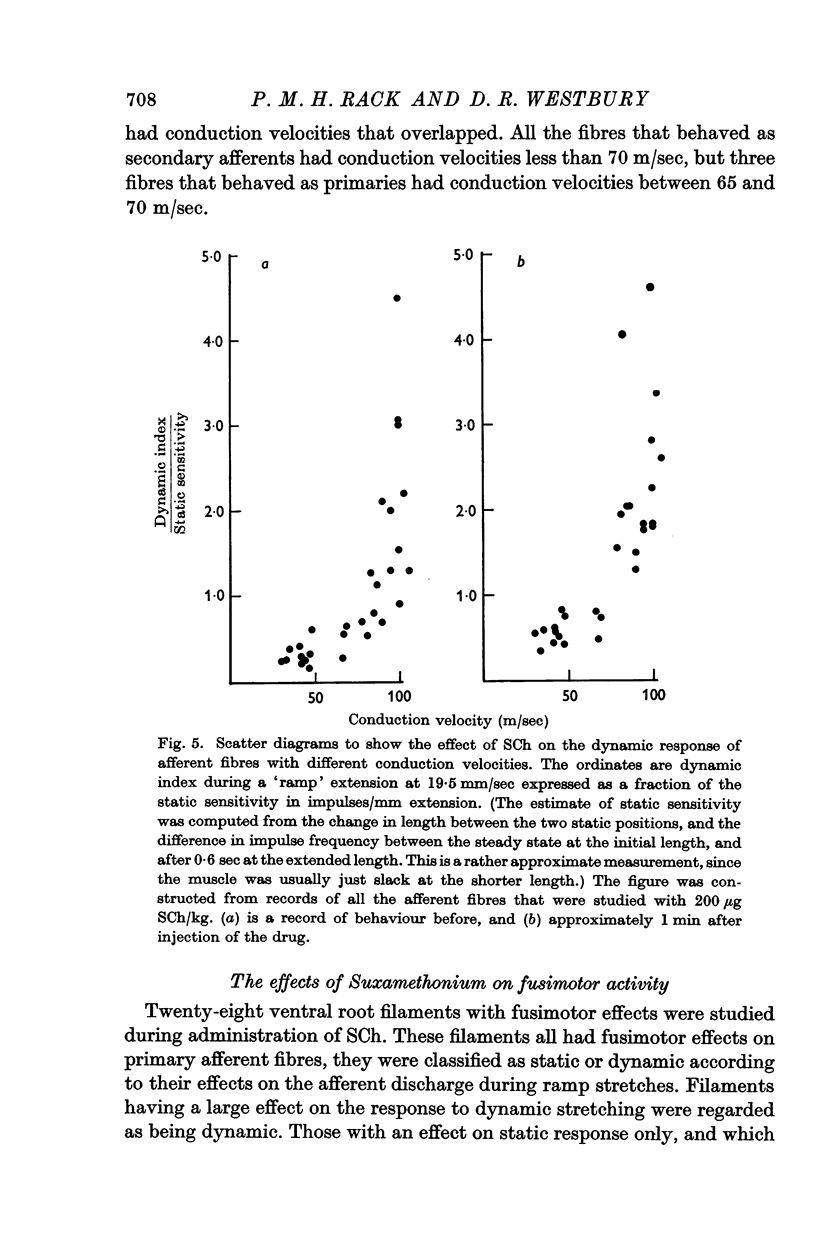
7. The actions of SCh and of acetylcholine emphasized the differences in response of primary and secondary afferent endings to dynamic stretching. The use of these drugs enabled us to classify fibres of intermediate conduction velocity.
8. Suxamethonium is known to activate slow muscle fibre systems with distributed nerve endings. The similarity between dynamic fusimotor activity and the effect of SCh suggests that the dyanimic fusimotor fibres act on slow intrafusal muscle fibres through multiple distributed endings.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS B. D., PATON W. D. M. Depolarization of the motor end-plate by decamethonium and acetylcholine. J Physiol. 1951 Sep;115(1):41–73. doi: 10.1113/jphysiol.1951.sp004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCHILL-DAVIDSON H. C. Suxamethonium (succinylcholine) chloride and muscle pains. Br Med J. 1954 Jan 9;1(4853):74–75. doi: 10.1136/bmj.1.4853.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEHR H. U. ACTIVATION BY SUXAMETHONIUM OF PRIMARY AND SECONDARY ENDINGS OF THE SAME DE-EFFERENTED MUSCLE SPINDLE DURING STATIC STRETCH. J Physiol. 1965 May;178:98–110. doi: 10.1113/jphysiol.1965.sp007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., SKOGLUND S., THESLEFF S. Activation of muscle spindles by succinylcholine and decamethonium, the effects of curare. Acta Physiol Scand. 1953;28(2-3):134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- GRAY E. G. The spindle and extrafusal innervation of a frog muscle. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):416–430. doi: 10.1098/rspb.1957.0021. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., MATTHEWS P. B. The response of de-efferented muscle spindle endings in the cat's soleus to slow extension of the muscle. J Physiol. 1961 Jul;157:370–392. doi: 10.1113/jphysiol.1961.sp006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS A., PILAR G. SLOW FIBRES IN THE EXTRAOCULAR MUSCLES OF THE CAT. J Physiol. 1963 Dec;169:780–798. doi: 10.1113/jphysiol.1963.sp007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS A. Two kinds of motor nerve endings on mammalian intrafusal muscle fibers revealed by the cholinesterase technique. Anat Rec. 1961 Feb;139:173–183. doi: 10.1002/ar.1091390209. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C. The mammalian small-nerve fibers: a system for efferent nervous regulation of muscle spindle discharge. Res Publ Assoc Res Nerv Ment Dis. 1952;30:24–47. [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Properties of the 'slow' skeletal muscles fibres of the frog. J Physiol. 1953 Aug;121(2):318–340. doi: 10.1113/jphysiol.1953.sp004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- OTTOSON D. The effect of acetylcholine and related substances on the isolated muscle spindle. Acta Physiol Scand. 1961 Nov-Dec;53:276–287. doi: 10.1111/j.1748-1716.1961.tb02286.x. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Mattews P. B. Differences in variability of discharge frequency between primary and secondary muscle spindle afferent endings of the cat. Nature. 1965 Dec 18;208(5016):1217–1218. doi: 10.1038/2081217a0. [DOI] [PubMed] [Google Scholar]
- THESLEFT S. The mode of neuromuscular block caused by acetylcholine, nicotine, decamethonium and succinylcholine. Acta Physiol Scand. 1955 Oct 27;34(2-3):218–231. doi: 10.1111/j.1748-1716.1955.tb01242.x. [DOI] [PubMed] [Google Scholar]
- VERHEY B. A., VOORHOEVE P. E. ACTIVATION OF GROUP IA AND GROUP II MUSCLE SPINDLE AFFERENTS BY SUCCINYLCHOLINE AND OTHER CHOLINERGIC DRUGS. Acta Physiol Pharmacol Neerl. 1963 Apr;12:23–29. [PubMed] [Google Scholar]


