Abstract
1. Glucose transport, uptake, utilization, and lactate production by intestinal slices from embryos and young chicks have been determined by means of the in vitro tissue accumulation method. Changes in these parameters with age, after feeding, and in the presence of phlorrhizin have been measured, in most cases, under both aerobic and anaerobic conditions.
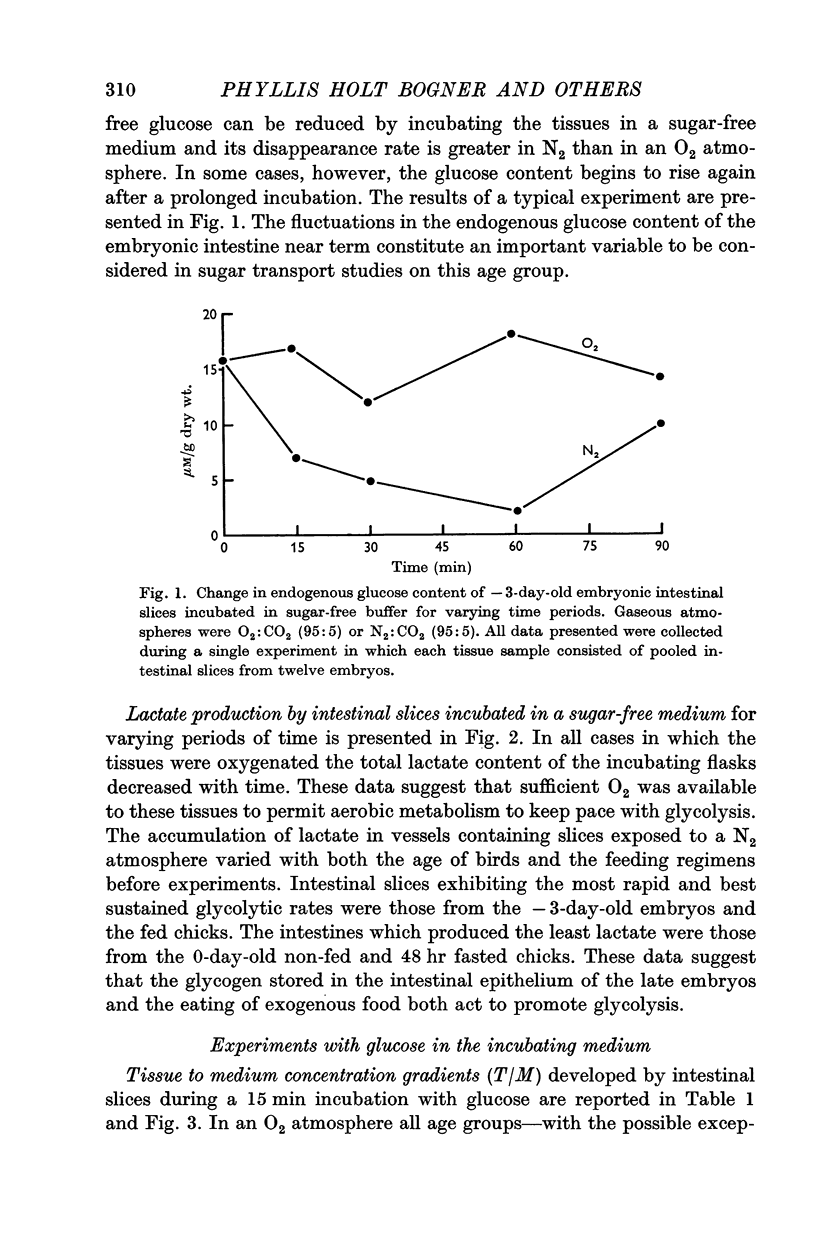
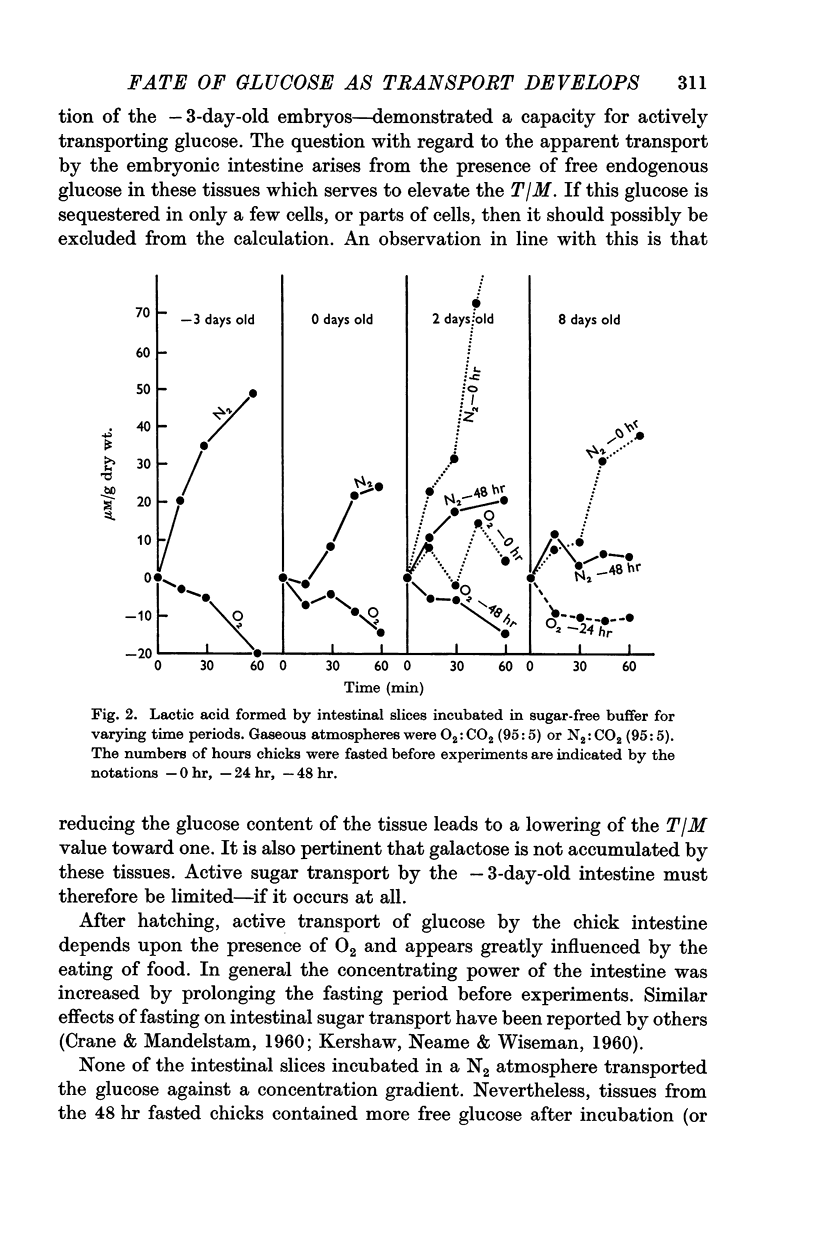
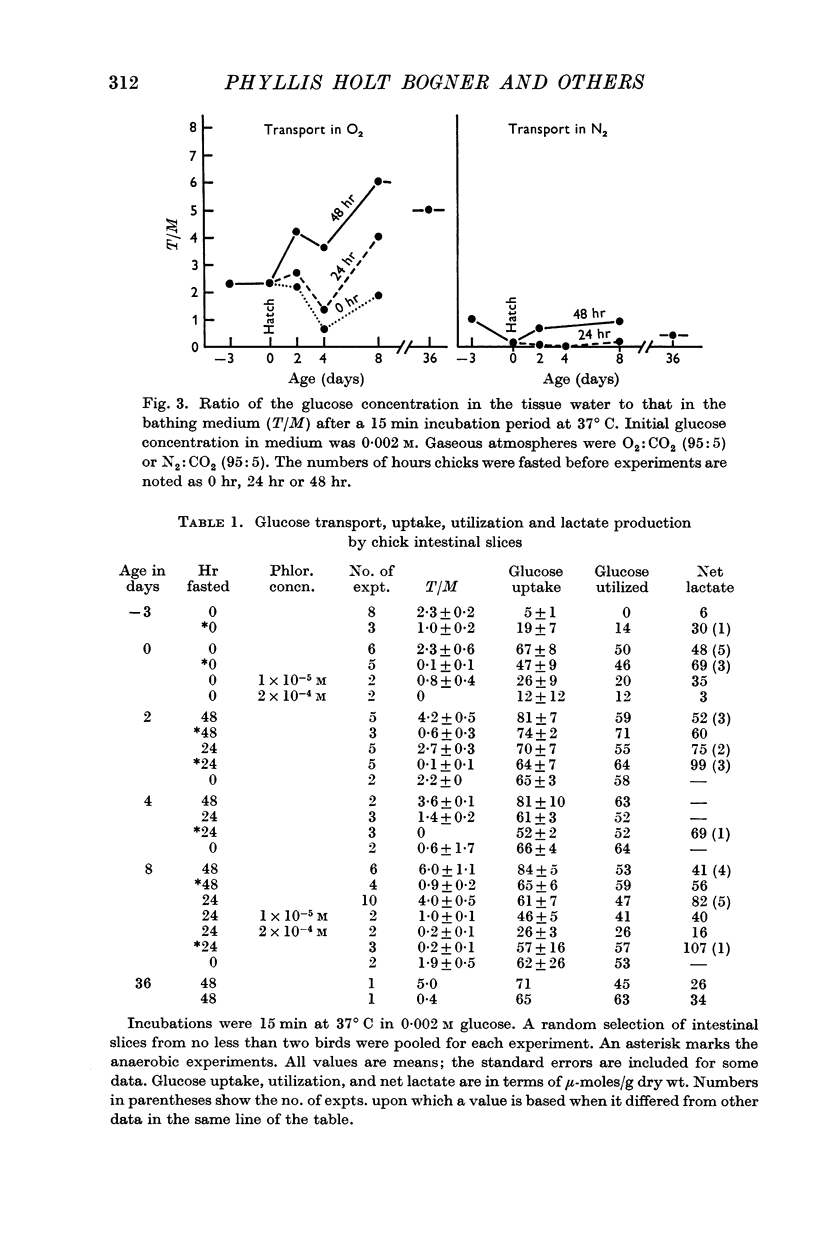
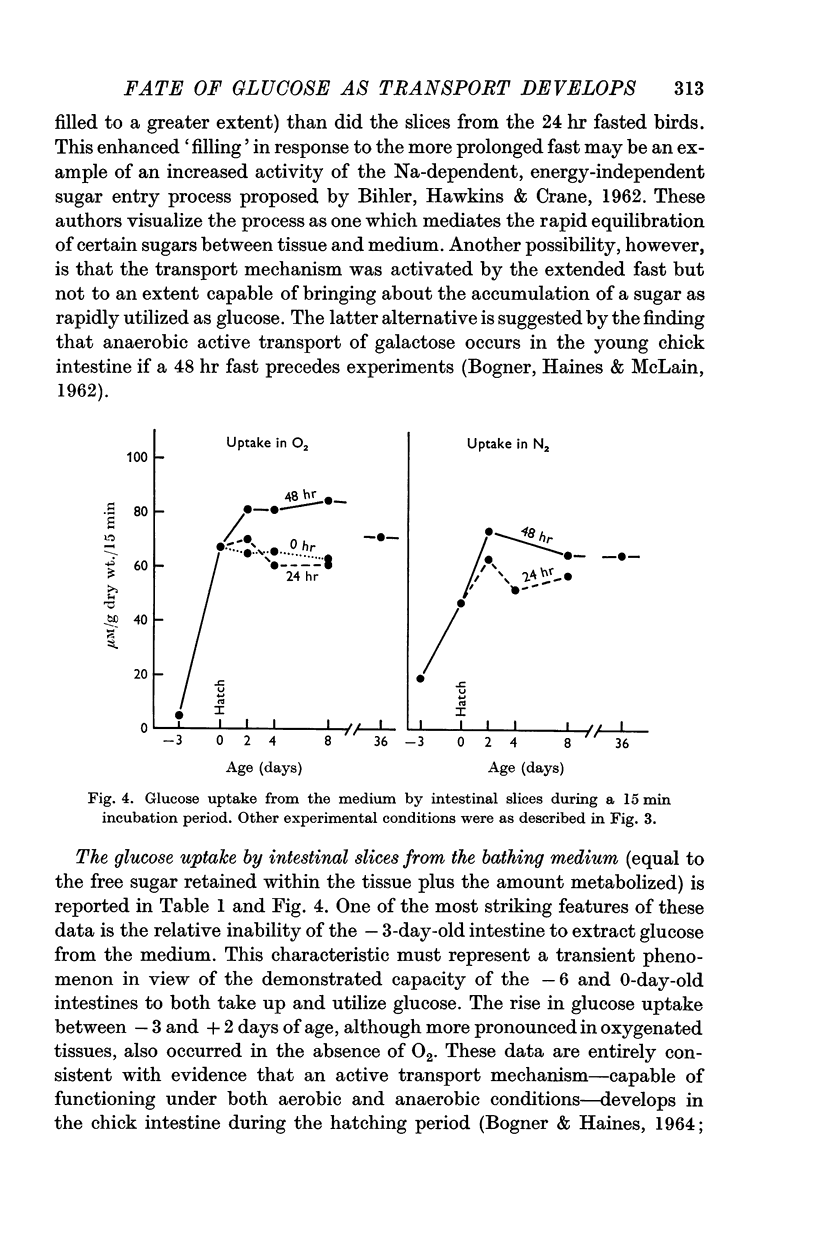
2. The embryonic intestine, 3 days before hatching, took up and utilized only a negligible quantity of glucose from the incubating medium and net lactate production was limited. The transport and utilization of exogenous sugar thus seem to be minimal at this age.
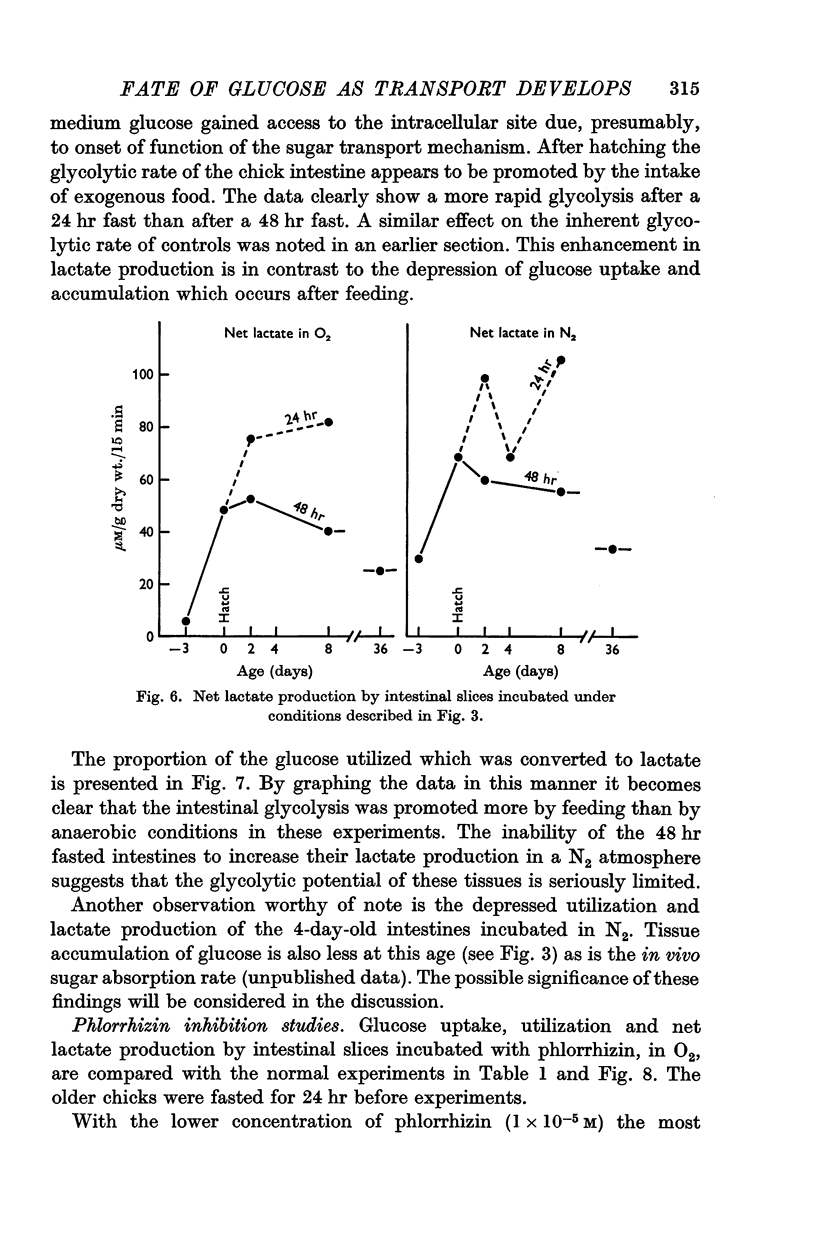
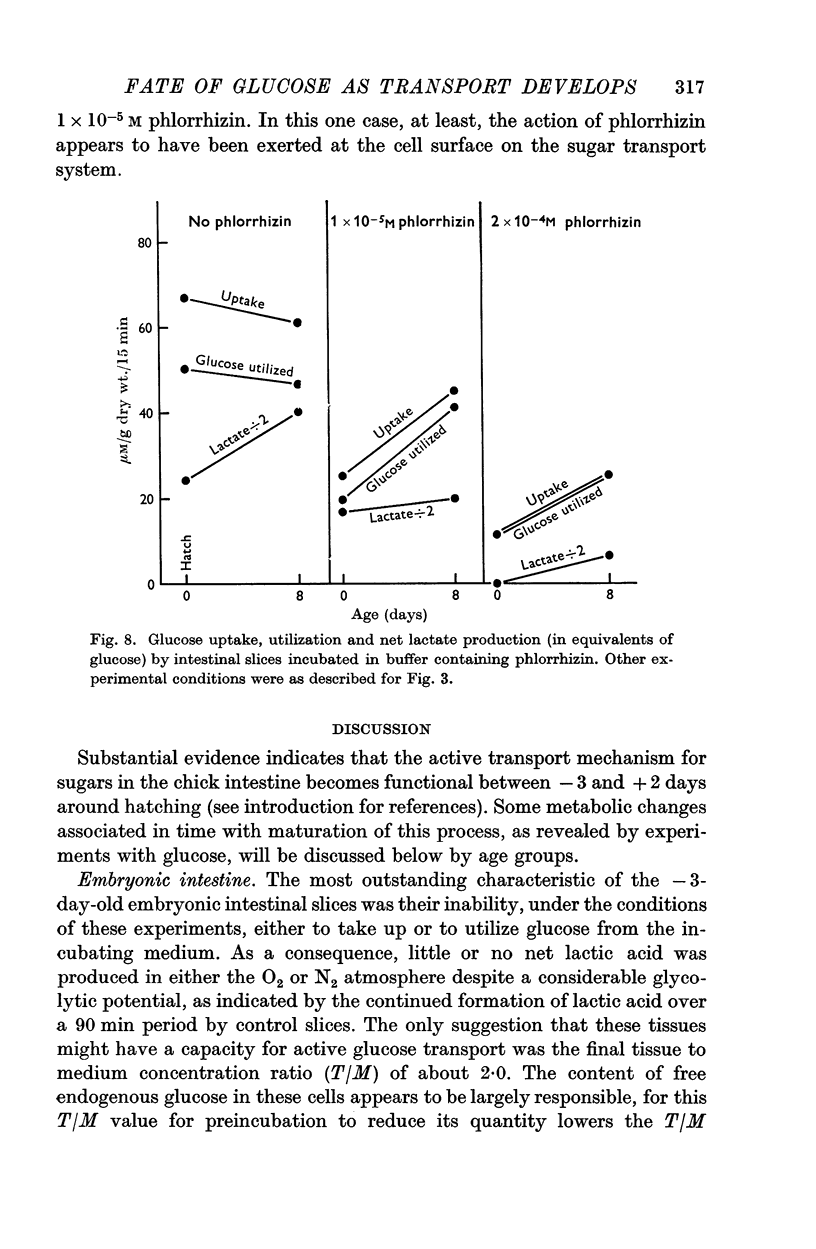
3. On the day of hatching, the intestine concentrated and utilized glucose, and lactate production was significant as was the inhibition of glucose uptake by phlorrhizin. This capacity to metabolize exogenous sugar appears to be consequent to onset of function of the sugar transport mechanism just before hatching.
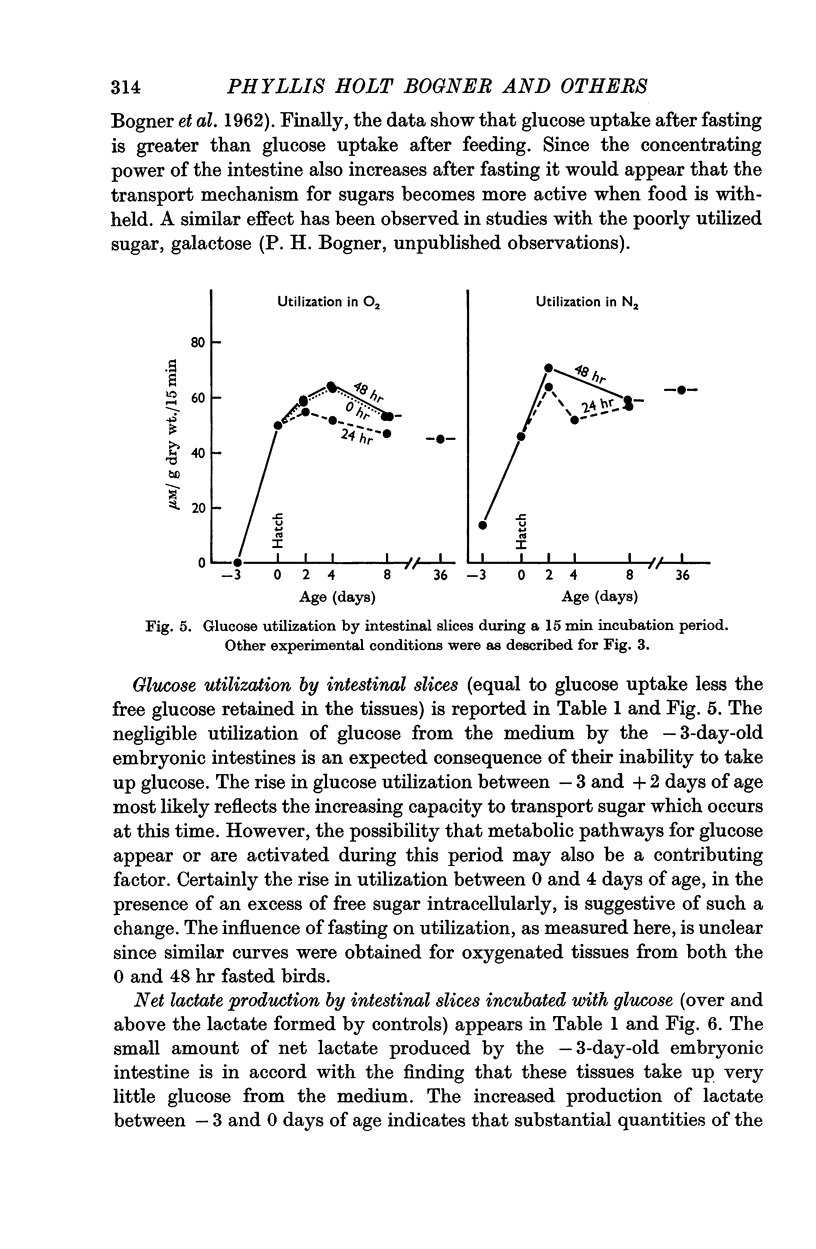
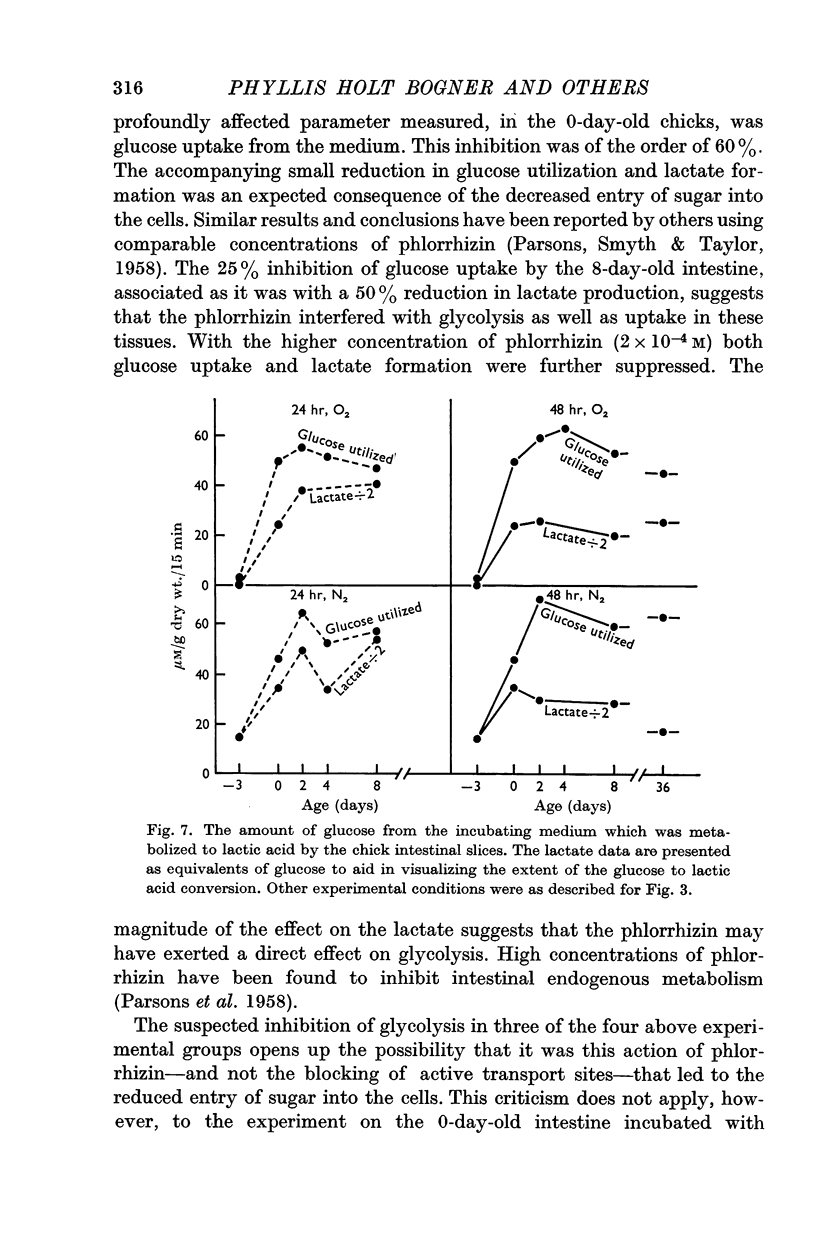
4. At 2 days of age intestinal slices concentrated and utilized more glucose than at 0 days of age, if the chicks were not fed. After eating, glucose transport was decreased while lactate production was enhanced. Feeding schedules thus influence sugar transport and metabolism by the young chick intestine.
5. The metabolic parameters measured showed essentially the same relationships in intestinal slices from 8- and 36-day-old chicks as in 2-day-old birds. Although there were indications that intestinal maturation continues well into post-natal life, the most striking changes in functional capacity, observed in these studies, occurred during the several days around hatching.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGAR W. T., HIRD F. J., SIDHU G. S. The uptake of amino acids by the intestine. Biochim Biophys Acta. 1954 May;14(1):80–84. doi: 10.1016/0006-3002(54)90134-1. [DOI] [PubMed] [Google Scholar]
- BIHLER I., HAWKINS K. A., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. VI. The specificity and other properties of Na ion-dependent entrance of sugars into intestinal tissue under anaerobic conditions, in vitro. Biochim Biophys Acta. 1962 May 7;59:94–102. doi: 10.1016/0006-3002(62)90700-x. [DOI] [PubMed] [Google Scholar]
- BOGNER P. H., HAINES I. A. FUNCTIONAL DEVELOPMENT OF ACTIVE SUGAR TRANSPORT IN THE CHICK INTESTINE. Am J Physiol. 1964 Jul;207:37–41. doi: 10.1152/ajplegacy.1964.207.1.37. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., MANDELSTAM P. The active transport of sugars by various preparations of hamster intestine. Biochim Biophys Acta. 1960 Dec 18;45:460–476. doi: 10.1016/0006-3002(60)91482-7. [DOI] [PubMed] [Google Scholar]
- KERSHAW T. G., NEAME K. D., WISEMAN G. The effect of semistarvation on absorption by the rat small intestine in vitro and in vivo. J Physiol. 1960 Jun;152:182–190. doi: 10.1113/jphysiol.1960.sp006480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOG F., WENGER E. L. The occurrence of a neutral mucopolysaccharide at sites of high alkaline phosphatase activity. Am J Anat. 1952 May;90(3):339–377. doi: 10.1002/aja.1000900303. [DOI] [PubMed] [Google Scholar]
- NUNNALLY D. A. The functional differentiation of the small intestine. X. Duodenal succinic dehydrogenase in chick embryos and hatched chicks. J Exp Zool. 1962 Mar;149:103–115. doi: 10.1002/jez.1401490203. [DOI] [PubMed] [Google Scholar]
- PARSONS B. J., SMYTH D. H., TAYLOR C. B. The action of phlorrhizin on the intestinal transfer of glucose and water in vitro. J Physiol. 1958 Dec 30;144(3):387–402. doi: 10.1113/jphysiol.1958.sp006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- WASHKO M. E., RICE E. W. Determination of glucose by an improved enzymatic procedure. Clin Chem. 1961 Oct;7:542–545. [PubMed] [Google Scholar]
- WILSON T. H., LIN E. C. Active transport by intestines of fetal and newborn rabbits. Am J Physiol. 1960 Dec;199:1030–1032. doi: 10.1152/ajplegacy.1960.199.6.1030. [DOI] [PubMed] [Google Scholar]


