Abstract
Adaptive (stationary-phase) mutagenesis occurs in the gram-positive bacterium Bacillus subtilis. Furthermore, taking advantage of B. subtilis as a paradigm for the study of prokaryotic differentiation and development, we have shown that this type of mutagenesis is subject to regulation involving at least two of the genes that are involved in the regulation of post-exponential phase prokaryotic differentiation, i.e., comA and comK. On the other hand, a functional RecA protein was not required for this type of mutagenesis. The results seem to suggest that a small subpopulation(s) of the culture is involved in adaptive mutagenesis and that this subpopulation(s) is hypermutable. The existence of such a hypermutable subpopulation(s) raises important considerations with respect to evolution, the development of specific mutations, the nature of bacterial populations, and the level of communication among bacteria in an ecological niche.
For over a decade, there has been considerable interest in a phenomenon that has been called adaptive, or stationary-phase, mutagenesis. The result of the mechanism(s) responsible for this phenomenon is the production of mutations that arise in nondividing or stationary-phase bacteria when the cells are subjected to nonlethal selective pressure, such as nutrient-limited environments (6, 11, 15, 32, 61). While most of the research has involved Escherichia coli model systems, similar observations have been made in other prokaryotes (43) as well as in eukaryotic organisms (69).
In the F′ lac frameshift reversion assay system in E. coli, stationary-phase mutations that lead to the generation of Lac+ cells can be distinguished from normal growth-dependent spontaneous Lac+ mutations (21, 59, 63). Specifically, Lac+ mutations are generated in stationary-phase cells via a molecular mechanism that requires a functional homologous recombination system (11, 21, 36, 37), F′ transfer functions (20, 23), and a component(s) of the SOS system (50). Genetic evidence suggests that DNA polymerase III (18, 35) and DNA polymerase IV (51, 52) are responsible for the synthesis of errors that lead to these mutations. Furthermore, for the Lac+ mutations, different sequence spectra are generated for the stationary-phase mutations than for the types of mutations generated during growth.
For instance, a majority of the Lac+ mutations that arise during stationary phase have a −1 deletion at mononucleotide repeats within the target gene. On the other hand, for the spontaneous mutations that arise during growth, various types of mutations occur in seemingly random locations (19, 62). These characteristics suggested that stationary-phase Lac+ reversions occur via a different molecular mechanism(s) than for those reversions of the same lac allele that are generated during growth. However, there is also evidence that demonstrates that the mutations generated by this lac system during stationary phase are the result of gene amplification followed by SOS-induced mutagenesis and selection (39).
Although the very observations of adaptive or stationary-phase mutagenesis could have suggested the existence of a Lamarckian type of genetics, subsequent research has demonstrated that this type of mutagenesis is not necessarily directed only to the selected genes (17, 46, 73). In fact, some studies have suggested that in a starving or stressed culture, a small subpopulation of the cells seem to have an overall increased mutation frequency (5, 15, 33, 46, 73). Basically, the results mentioned above were used to generate a proposal suggesting that a physiologically stressed bacterial community may differentiate a hypermutable subpopulation and that these hypermutable cells generate mutations randomly. If one or more of the mutations help the cell survive longer or grow under the stressful conditions, then the organism will appear to have “adapted” to its environment.
The majority of research efforts on stationary-phase mutagenesis have focused on the mechanisms that generate these mutations. The equally exciting elucidation of the mechanism(s) that regulates the activation of this mutation system(s) or even the development of the proposed transitory hypermutable state has not, as yet, generated the same level of interest. There has been increasing evidence for and discussion about the existence of transitory subpopulations in stressed or stationary-phase prokaryotic cultures (52, 66). Such subpopulations could play an important role in the generation of mutants under stressful conditions like those that exist in stationary-phase cells. While such mutations could be the result of stochastic physiological events, it is intriguing to consider that basic regulatory mechanisms involved in gene expression during stationary phase might control the mutagenic potential of these cells. In fact, using a different model system for the study of stationary-phase mutagenesis (the araB lacZ MCS2 strain of E. coli [65]), Gómez-Gómez and colleagues demonstrated that two growth phase-regulatory proteins (H-NS and σS) of E. coli significantly altered the production of adaptive or stationary-phase mutations (24).
Accordingly, we began the investigations described in this paper by proposing that bacterial differentiation and development play a role in the regulation of a hypermutable subpopulation(s) that enhances diversity in nutritionally stressed cells. To study this possibility, we used the genetic and molecular biological strengths of the bacterium Bacillus subtilis. We chose this organism because it is a paradigm for prokaryotic differentiation and development (26, 67). In addition, there has been evidence to suggest that mutation rates in this organism may be influenced by the stringent response (64), a response associated with physiological stress.
In order to test our hypothesis, we first had to demonstrate that stationary-phase mutagenesis occurs in B. subtilis. Furthermore, for this hypothesis to be correct, it is essential to implicate prokaryotic differentiation processes in the control of stationary-phase mutagenesis. Accordingly, in this report we demonstrate that B. subtilis exhibits the stationary-phase mutation phenomenon when cells are placed under amino acid starvation pressure. In addition, we found that at least two genes involved in the regulation of differentiation in postexponential growth of B. subtilis, comA and comK, also appear to regulate aspects of stationary-phase mutagenesis in this bacterium.
MATERIALS AND METHODS
Strains.
The bacterial strains used in these experiments are listed in Table 1. B. subtilis YB955 is a prophage-“cured” strain that contains the hisC952, metB5, and leuC427 alleles (this report) (80, 81). The metB5 and hisC952 alleles are the result of nonsense mutations (GAA→TAA at position 346 and CAG→TAG at position 952, respectively; Tables 1, 2, and 3) and have been shown to be suppressed by the sup-3 allele in addition to less well characterized nonsense suppressor mutations (72, 81). leuC427 is a missense mutation in which the 143rd amino acid residue was changed from Gly to Arg (GGA→AGA at position 427; Tables 1 to 3).
TABLE 1.
B. subtilis strains
| Strain | Relevant genotype | Reference |
|---|---|---|
| YB955 | hisC952 metB5 leuC427 xin-1 SpβSENS | 81 |
| YB9000 | YB955 carrying ΔcomA::tet (comA gene distrupted with a tetracycline cassette) | 27, 28; also see Materials and Methods |
| YB9100 | YB955 carrying ΔcomK::kan | See Materials and Methods |
| YB9102 | YB955 carrying ΔsigB::cat (transformed with PB105 genomic DNA in order to disrupt the sigB gene) | 4; also see Materials and Methods |
| YB9200 | YB955 carrying both ΔcomA::tet and ΔcomK::kan gene disruptions | See Materials and Methods |
| YB9300 | YB955 carrying ΔrecA::cat (recA gene disrupted by a chloramphenicol cassette) | 13; also see Materials and Methods |
TABLE 2.
Base changes in mutant alleles found in YB955a
| Allele | Position of mutation (bp) | Type of mutation | DNA change | Result of mutation |
|---|---|---|---|---|
| hisC952 | 450 | Transition | C→T | Asp→Aspb |
| 799 | Transversion | T→G | Tyr→Aspb | |
| 952 | Transition | C→T | Gln→stop (amber mutation) | |
| metB5 | 346 | Transversion | G→T | Glu→stop (ochre mutation) |
| leuC427 | 427 | Transition | G→A | Gly→Arg (missense mutation) |
Mutant alleles found in strain YB955 and the changes in the DNA sequences of the mutant alleles compared to the published genome of B. subtilis (http://genolist.pasteur.fr/SubtiList).
These point mutations in the hisC952 allele do not play any significant role in the inactivity of the gene product. These two changes may actually represent errors in the published genome.
TABLE 3.
Base changes in revertants of mutant alleles
| Revertant allelea | Position of mutation (bp) | No. of revertants sequenced | Type of mutation | DNA change | Result of mutation |
|---|---|---|---|---|---|
| hisC (day 2 His+) | 953 | 2 | Transition | A→G | Stop→Trp |
| hisC (days 3-7 His+) | 952 | 5 | Transition | T→C | Stop→Gln |
| 952 | 1 | Transversion | T→A | Stop→Lys | |
| 953 | 4 | Transition | A→G | Stop→Trp | |
| 954 | 1 | Transversion | G→T | Stop→Tyr | |
| metB (day 2 Met+) | 346 | 2 | Transition | T→C | Stop→Gln |
| 346 | 1 | Transversion | T→G | Stop→Glu | |
| 348 | 1 | Transversion | A→T | Stop→Tyr | |
| metB (days 3-7 Met+) | 346/348 | 2 | Transition/transversion | T→C/A→C | Stop→His |
| 346 | 2 | Transition | T→C | Stop→Gln | |
| leuC (day 2 Leu+) | 427 | 3 | Transition | A→G | Arg→Gly |
| leuC (days 3-7 Leu+) | 427 | 12 | Transition | A→G | Arg→Gly |
| 429 | 1 | Transversion | A→T | Arg→Ser | |
| ? | 6 | ? | ? | ? |
The day after plating on which revertants were isolated is shown in parentheses.
Procedures for transformation and isolation of chromosomal and plasmid DNA were as described previously (13, 70, 73). B. subtilis strains were maintained on tryptose blood agar base medium (TBAB; Difco Laboratories, Detroit, Mich.), and liquid cultures were routinely grown in PB medium (antibiotic medium 3; Difco) supplemented with appropriate antibiotics (10 μg of kanamycin/ml, as well as 5 μg of chloramphenicol and of tetracycline/ml). E. coli cultures were grown in Luria-Bertani (LB) supplemented with 100 μg of ampicillin/ml as needed.
Bacillus strains YB9000, YB9100, and YB9200 are isogenic derivatives of YB955 (Table 1) that are impaired or deficient in their ability to become competent. Strain YB9000 was constructed by transforming YB955 with a linearized derivative of plasmid pMK3 (70), which carries a defective comA (28) allele whose reading frame has been disrupted with the insertion of a tetracycline resistance cassette (27). Essentially, by using PCR (1) and the DNA sequences of the comA+ gene (http://genolist.pasteur.fr/SubtiList/), we cloned out this gene onto the pMK3 plasmid with the primers 5′-AACTGCAGAAGAGTGAGTAAAAGGGAGGAA-3′ for the 5′ end of the gene and 5′-CGGGATCCATCTCTACACCCCCCAAC-3′ for the 3′ end. The PCR fragment was then ligated into pMK3 opened at the SmaI site following the addition of thymine tails to the vector (1). The cloned comA+ gene on the pMK3 vector was opened with the restriction endonuclease XmnI (obtained from Promega), and the tetracycline cassette (cut with SmaI and HindIII from plasmid pDG1515) (28) was then inserted following usage of the Klenow fragment enzyme to fill in the single-stranded ends (1). For cloning purposes, E. coli strain DH5α, as described before (1), was used.
Strain YB9200 was constructed by transforming YB9000 with genomic DNA isolated from BD2121 (30). This strain carries a comK allele that has been interrupted by the insertion of an antibiotic cassette (provided by David Dubnau). This transformation resulted in a strain deficient in both ComA and ComK activity. Strain YB9100 was constructed by transforming YB955 with genomic DNA isolated from strain YB9200 and selecting for interruption of the comK gene. Strain YB9300 is recombination deficient due to the lack of a functional recA gene product and was constructed by transforming YB955 with genomic DNA isolated from YB3000 (recA240; Table 1) (13). Strain YB9300 was checked to demonstrate the lack of RecA activity as previously described (13). Strain YB9102 was constructed by transforming YB955 with genomic DNA isolated from strain PB105 (4). This strain carries a chloramphenicol resistance cassette that interrupts the structural gene for σB, an alternative σ factor that is involved in a general response to environmental stress (22, 75).
Procedures for stationary-phase mutagenesis assay.
Procedures for the stationary-phase mutagenesis assay are described below. Essentially, 10 ml of cells was grown in PB medium supplemented with appropriate antibiotics in a Nephloflask at 37°C with aeration (250 rpm) to 90 min after the cessation of exponential growth (designated T90). Growth was monitored with a Klett-Summerson colorimeter (no. 66 filter; Klett Mfg. Co., Inc.; 1 kU ≈106 CFU/ml). The cells were harvested by centrifugation at 10,000 × g for 10 min at room temperature and then resuspended in 10 ml of 1× Spizizen salt solution (68) in order to reduce the amount of trace nutrients. The cells were then plated in quintuplicate and incubated at 37°C on Spizizen minimal medium (SMM; 1× Spizizen salts supplemented with 0.5% glucose and either 50 or 95 g or 200 ng of the required amino acid/ml and 50 μg each of isoleucine and glutamic acid/ml). The experiments were repeated at least three times.
In addition, each time an isogenic derivative strain of YB955 was examined for its ability to perform stationary-phase mutagenesis, a YB955 control was tested simultaneously. The concentration of the amino acid used depended on the reversion that was being selected. For instance, when selecting His+ revertants, 50 μg of methionine and leucine/ml was added to the medium and 200 ng of histidine/ml was added. Isoleucine and glutamic acid were added as described previously (71) in order to protect the viability of the cells. Appropriate antibiotic concentrations were maintained throughout the experiments. The number of revertants was scored daily. The initial number of bacteria plated for each experiment was determined by serial dilution of the bacterial cultures and then by plating the cells on a minimal medium containing all three essential amino acids.
The survival rates of the bacteria plated on the minimal selective medium were also determined via two mechanisms. First, the cells (as described above) were plated on multiple petri dishes containing minimal medium (supplemented with 200 ng of the essential amino acids/ml), and the plates were incubated at 37°C for various periods of time. Each day, 10 ml of 1× Spizizen salts was added to two plates, the cells were scraped off the medium, and the resuspended cells were then diluted and plated on SMM containing all the essential amino acids (50 μg/ml). The number of colonies was then determined following 48 h of growth at 37°C.
Since this first method involved a medium that differed slightly from the medium used for scoring for reversions, a second approach was used. In these experiments, three agar plugs were removed from each selection plate daily. The plugs were removed with sterile Pasteur pipettes and taken from areas of the plates where no growth of revertants was observed. The plugs were suspended in 400 μl of 1× Spizizen salts mixed, diluted, and plated on SMM containing all the essential amino acids (50 μg/ml). Again, the number of colonies was determined following 48 h of growth at 37°C.
Procedures for gene amplification and for sequencing DNA.
The genes of interest were amplified by polymerase chain reactions (PCRs) with the primers listed in Table 4. Three individual 100-μl reactions were set up with the conditions described before (42). Basically, 0.5 μg of genomic DNA was used in a 100-μl PCR mixture that contained 10 mM Tris-HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 0.25 mM dNTPs, 0.25 μM concentrations of each primer, and 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.). The PCRs were carried out in a thermocycler (Eppendorf; Mastercycler gradient) for 30 cycles. The PCR products were purified by gel extraction in order to separate the amplified sequences from the unused primers and from the primer dimers. Basically, a 1% agarose (Gibco-BRL, Rockville, Md.) gel was used for the electrophoresis separation, and then the amplified DNA fragments were extracted from the gel with the Qiaex II gel extraction system (Qiagen, Valencia, Calif.).
TABLE 4.
DNA primers used for PCR and DNA sequencing reactions
| Usea | Gene | 5′ primer | 3′ primer | DNA sequenceb | Used for PCRc | Used for sequencingd |
|---|---|---|---|---|---|---|
| Entire gene | hisC | + | 5′ ATCAGGCGCTGCAGGAGTTTGAGG 3′ | + | + | |
| + | 5′ GACCGGCGAGCAATATTGTATCTTTCA 3′ | + | + | |||
| Internal | hisC | + | 5′ CAAACACGGTTACTGCTGC 3′ | + | ||
| + | 5′ AAGGCATACGGTCTGGCAG 3′ | + | ||||
| Entire gene | metB | + | 5′ AACTGCAGAAACGGGGAAATAATGGAGGTG 3′ | + | + | |
| + | 5′ CGGGATCCGGTGCCCTGTCAAAAAGACTTG 3′ | + | + | |||
| Internal | metB | + | 5′ TGCGCCGATCGAGCATTTGG 3′ | + | ||
| + | 5′ CATGATTTCCTTGAGCTCTTCCCA 3′ | + | ||||
| Entire gene | leuC | + | 5′ ACGCGTCGACAATACAATTTCTAATGTGTGACAG 3′ | + | + | |
| + | 5′ ACGCGTCGACATCCGATTTAATACGGGTGCTTTCC 3′ | + | + | |||
| Internal | leuC | + | 5′ CAGTGTGGATCAAGGGATTGTC 3′ | + | ||
| + | 5′ ACGATGGATGAACGAATGACTG 3′ | + | ||||
| + | 5′ TTGAAGACATTAAAGTGGAGCAC 3′ | + |
Entire gene, primers used for sequencing of the entire genes used for stationary-phase mutagenesis assay; Internal, primers used for internal sequencing of the gene.
Bold sequences are not part of the coding region of the gene but were used for cloning purposes.
Primers used in PCRs to amplify the genes, as described in Materials and Methods.
Primers used for sequencing as described in Materials and Methods.
The purified PCR products were used as templates for sequencing reactions. The sequences of the DNA fragments were determined with a Thermo Sequenase radiolabeled terminator cycle sequencing kit (USB, Cleveland, Ohio). Essentially, 100 ng of template DNA was used in each reaction, and the reactions were carried out in a thermocycler for 25 cycles. The radiolabeled DNA fragments were separated with a denaturing (8 M urea) 6% polyacrylamide gel (J. T. Baker) with a glycerol-tolerant gel buffer, as recommended by USB. Exposed Kodak Bio-Max film was used to obtain the sequence from the finished gel.
Analysis of mutation rates.
The growth-dependent reversion frequencies for the His+, Met+, and Leu+ alleles were measured by fluctuation tests. In order to determine the mutation rates of various strains, the bacteria were grown to saturation at 37°C with aeration in PB medium. The saturated cultures were then used to make a 10−4-fold dilution into fresh PB medium and dispensed into 1 ml of PB. Thirty-eight 18-mm test tubes containing bacteria, each containing almost the same number of cells, were incubated overnight to saturation (about 14 to 16 h with aeration at 37°C). The saturated cultures were then pelleted and resuspended in 100 μl of 1× Spizizen salts medium. The cells were then spread onto selection medium as described previously for detecting His+, Met+, and Leu+ stationary-phase-generated mutations. The revertants were scored and recorded within 48 h after plating. The median (r) is the mean of the 19th and 20th values of r (observed number of mutants per culture) when the r's are ranked. The number of mutations per culture (m) is calculated with the Lea-Coulson formula, r/m − ln(m) = 1.24. Three parallel cultures were used to determine the total number of CFU plated on each plate (Nt) by titration. The mutation rates were calculated with the formula m/2Nt (45, 58, 76).
The mutation rates for the generation of rifampin-resistant cells were also determined by the method mentioned above but with fluctuation tests that each involved 40 growth tubes. The rifampin-resistant bacteria were selected on TBAB containing 5 μg of rifampin (Fluka Biochemicals, Switzerland)/ml. After 24 h of incubation at 37°C, the number of rifampin-resistant colonies was determined.
RESULTS
Stationary-phase mutagenesis.
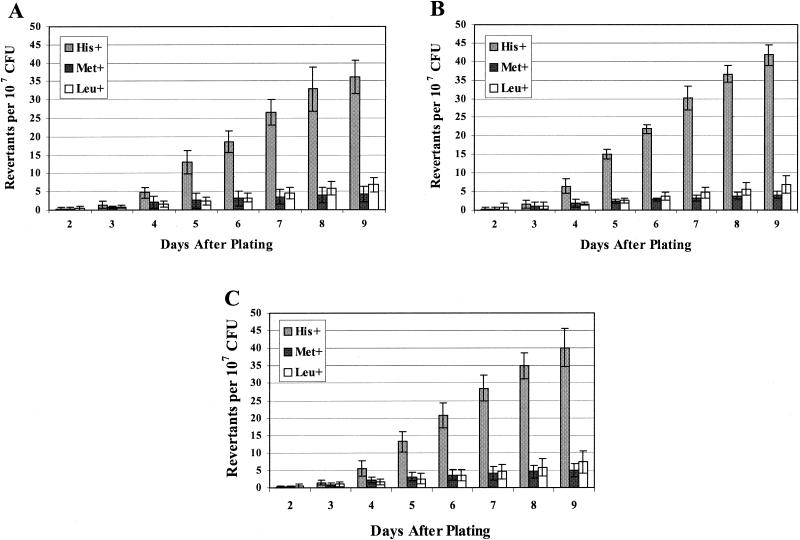
The number of mutant colonies that appeared on each day of incubation was scored as described in Materials and Methods. From the results shown in Fig. 1, it seemed that stationary-phase mutations were accumulating in the B. subtilis cultures that had been placed under conditions of nutrient deprivation or stress. Essentially, revertant colonies appeared on the selective plates and new colonies arose each day of incubation. To try to eliminate the possibility that these results were due to artifacts, the stationary-phase mutagenesis experiment on strain YB955 was performed at least five times. In addition, each time a strain isogenic to YB955 was investigated for its mutagenic potential, YB955 was run simultaneously as a control. The results in Fig. 1 demonstrate the consistency of the data that were obtained. Figures 1A and 1B are the results of single experiments that were performed on different days. Figure 1C shows the data when the results from seven different experiments were averaged together.
FIG. 1.
Reversion frequencies for the his, met, and leu alleles of YB955. (A and B) Results from two independent stationary-phase mutagenesis experiments, representing the average number of revertants (from five individual plates) on each day. Error bars represent 1 standard error for the five different plates. (C) Results presented are the average number of revertants from seven individual experiments performed on different days. Error bars represent 1 standard error for the seven experiments.
Of course, the possibility existed that the revertants that accumulated on the plates were the result of slow-growing bacteria whose mutations had occurred during growth in the liquid medium prior to plating. To rule out this possibility, revertant colonies were picked, purified, and tested for their ability to form colonies on the selection medium. In all cases, the revertants yielded colonies within 48 h of having been plated on the selection medium. These results strongly suggested that these were not slow-growing variants.
Following the initial report of adaptive mutagenesis in E. coli (12), there was a subsequent paper (57) that indicated that the original lac+ revertants might actually have been generated during the growth phase. It was suggested that these mutants, while able to grow at normal rates in liquid medium, appeared to be slow growing when placed on a solid medium in the presence of ≈107 to 108 Lac− cells. Such a phenotype could cause revertants to be incorrectly classified as having been generated during the stationary phase.
In order to determine if this might be a phenomenon associated with our stationary-phase mutagenesis, revertants were isolated and tested for this type of slow growth behavior. Accordingly, DNAs from four of the Leu+ revertants (two isolated on day 5 and one each isolated on days 6 and 7) were obtained and used to transform strain YB955 to a Leu+ genotype. Essentially, 108 Leu− cells of YB955/ml were made competent (see Materials and Methods) and exposed to the appropriate transforming DNA, and the cells were then plated on a medium lacking leucine. If the Leu+ revertants originally isolated were the result of slow-growing growth phase mutations, then the transformants should not appear before day 5 after plating (Fig. 1). Our results demonstrated that in all cases, the transformants appeared within 48 h after plating, and the numbers ranged from 4,000 to 12,000 transformants per μg of DNA (depending on the DNA preparation that was used).
While these results appear to demonstrate that we had isolated revertants that grew normally, an argument could still be made that the genetic background of the original revertants might have prevented these cells from forming colonies until after several days of incubation. Therefore, the revertants might not actually be examples of stationary-phase mutagenesis. Specifically, we needed to know whether there were secondary site mutations in the original revertants that slowed the growth of these revertants when they were incubated with much larger numbers of auxotrophic cells. In order to rule out this possibility, approximately 500 Leu+, Met+, and His+ CFU per ml (revertants described above) were mixed with 108 CFU of strain YB955/ml or the strain from which they had been isolated (that had been grown as described for the adaptive mutagenesis experiments), and 0.1 ml of this mixed population of cells was plated on petri dishes containing SMM and used to select Leu+, Met+, and His+ revertants (Materials and Methods). After 48 h of growth at 37°C, approximately 50 Leu+, Met+, or His+ colonies were detected on each plate (Table 5). Thus, these revertants did not demonstrate any slow growth phenotype.
TABLE 5.
Growth of revertants in mixed culturesa
| Strain and revertantsb | Avg no. of colonies from 3 plates
|
||
|---|---|---|---|
| His+ | Met+ | Leu+ | |
| YB955 | |||
| A | 36.7 | 41.0 | 41.3 |
| B | 40.3 | 34.7 | 46.7 |
| C | 29.0 | 42.0 | 45.0 |
| BG | 1.0 | 1.3 | 0.3 |
| YB9000 (comA) | |||
| A | 25.3 | 55.7 | 28.0 |
| B | 24.0 | 23.7 | 28.3 |
| C | 38.3 | 25.7 | 27.0 |
| BG | 1.7 | 0.7 | 0.3 |
| YB9100 (comK) | |||
| A | 44.7 | 41.3 | 25.3 |
| B | 32.3 | 23.3 | 24.3 |
| C | 38.7 | 26.3 | 24.3 |
| BG | 0.7 | 1.0 | 0.7 |
| YB9200 (comA comK) | |||
| A | 31.7 | 27.0 | 29.3 |
| B | 25.0 | 27.0 | 23.0 |
| C | 29.7 | 30.3 | 32.3 |
| BG | 1.0 | 0.7 | 0.7 |
His+, Met+, and Leu+ revertants that arose on day 6 from each strain were mixed with about 5 × 108 CFU of bacteria representing the strain from which they had originally been isolated, plated on minimal medium, and incubated at 37°C. All colonies were scored and recorded within 48 h.
Nine stationary-phase mutagenesis-generated revertants were isolated from each strain. A, B, and C each represent one set of three isolates; each set includes one His+, one Met+, and one Leu+ strain. BG is the number of background revertants that appeared on the medium after 48 h of incubation at 37°C (number of revertants generated during growth-dependent mutagenesis).
Published results for the E. coli system indicated that the mechanism(s) for generating adaptive or stationary-phase mutations may have differed from those involved in growth-associated mutagenesis (16, 19, 39, 62). Since both the hisC952 and metB5 alleles are nonsense mutations (Table 2), we decided to investigate whether the types of revertants obtained 2 days after the cells had been placed on the selective medium (nonsense suppressors versus true revertants) differed from those obtained after longer incubations. Specifically, revertants generated during bacterial growth should yield colonies on the appropriate selective minimal medium approximately 24 to 48 h after the plating. On the other hand, stationary-phase-generated mutations should not appear before 48 h after plating of the cells on the selective minimal medium.
Accordingly, His+ colonies were picked 48 h after the initial plating and checked for their ability to grow on a medium that lacked histidine as well as on a medium that lacked methionine. The data (Table 6) show that 20% of these His+ revertants were also phenotypically Met+. These His+ Met+ colonies were most probably due to the generation of a nonsense suppressor mutation, since DNA sequencing did not reveal any base changes in the hisC gene itself. Similarly, His+ colonies that arose 3, 4, 5, and 6 days after plating (presumed stationary-phase mutations) were checked for their ability to grow in the absence of exogenous methionine. In these cases, the frequency of His+ cells that were phenotypically Met+ was 3.3%, 2.7%, 4%, and 8.3%, respectively (Table 6). The average number of His+ cells that were also Met+ was approximately 4% for those revertants picked from day 3 to day 6 after the initial plating. Thus, there could be a difference in the types of His+ revertants that were generated depending on when the mutations arose. Importantly, the data in Table 6 also substantiate that all of the revertants continued to grow when transferred to fresh medium lacking the formerly required amino acid; i.e., we had revertants and not phenocopies.
TABLE 6.
Growth of isolated revertants on various selective mediaa
| Dayb | Selection | No. of revertants that grew/no. tested (% that grew)
|
||
|---|---|---|---|---|
| His− medium | Met− medium | Leu− medium | ||
| 2 | His+ | 34/34 (100) | 7/34 (20.6) | 0/34 (0) |
| Met+ | 24/33 (72.7) | 33/33 (100) | 0/33 (0) | |
| Leu+ | 0/50 (0) | 0/50 (0) | 50/50 (100) | |
| 3 | His+ | 150/150 (100) | 5/150 (3.3) | 0/150 (0) |
| Met+ | 49/50 (98) | 50/50 (100) | 0/50 (0) | |
| Leu+ | 0/30 (0) | 0/30 (0) | 30/30 (100) | |
| 4 | His+ | 150/150 (100) | 4/150 (2.7) | 0/150 (0) |
| Met+ | 52/53 (98) | 53/53 (100) | 0/53 (0) | |
| Leu+ | 1/20 (5) | 1/20 (5) | 20/20 (100) | |
| 5 | His+ | 150/150 (100) | 6/150 (4) | 0/150 (0) |
| Met+ | 32/32 (100) | 32/32 (100) | 2/32 (6.2) | |
| Leu+ | 0/41 (0) | 0/41 (0) | 41/41 (100) | |
| 6 | His+ | 84/84 (100) | 7/84 (8.3) | 0/84 (0) |
| Met+ | 22/22 (100) | 22/22 (100) | 0/22 (0) | |
| Leu+ | 0/32 (0) | 0/32 (0) | 32/32 (100) | |
Revertants were selected initially for their growth on one type of medium (i.e., medium that lacked one essential amino acid). The cells were regrown on the original selection medium to eliminate background and then checked for their ability to grow on medium lacking the other essential amino acids. The three types of selective medium used in these experiments lacked histidine, methionine, or leucine.
Day after the initial plating on which the revertants were isolated.
Based on the results described so far, we believe that adaptive or stationary-phase mutagenesis occurs in B. subtilis and that the Leu+, His+, and Met+ revertants (described in Fig. 1) isolated after the second day following plating on the selective medium represent mutations that arose via this mutagenesis process.
Dynamics of stationary-phase mutagenesis.
The results in Fig. 1 represent the reversion frequencies for the three auxotrophic characteristics displayed by parental strain YB955. These data strongly indicate that stationary-phase mutagenesis has sequence specificity. The three different alleles tested showed diverse stationary-phase mutation frequencies. For instance, compared with Met+ and Leu+ reversion kinetics, the His+ revertants accumulated at a higher rate and earlier after plating (being placed in a stressful condition).
Greater than 90% of the limited number of revertants obtained that were initially selected for being Met+ were the result of the generation of a nonsense suppressor, since these cells were also His+ (Table 6). Thus, true Met+ reversions were rarely obtained in our initial screening, indicating that the site of the nonsense mutation in the metB5 allele may be a cold spot for stationary-phase-directed mutagenesis. As shown in Table 2, eight Met+ His− revertants had their DNA sequenced to determine the type of mutation that occurred. The data demonstrate that only one of the eight (a revertant obtained on day 2 after plating) had the appropriate transversion mutation necessary for restoring the tyrosine to the peptide (base change of TAA→GAA at position 346) that is seen in the wild-type sequence.
Although one transition mutation and two different transversion mutations would produce a viable revertant (Table 4), these mutations still occurred very infrequently (Tables 2 and 6) compared with the generation of the nonsense suppression mutation. On the other hand, the vast majority of the His+ cells represented reversions within the hisC gene (both transitions and transversions; Table 2), and these revertants accumulated at a significantly higher frequency than did the nonsense suppressor mutation that led to the Met+ His+ phenotype (Table 6). Leu+ reversions were also generated (Fig. 1) at a frequency significantly less than that obtained for the His+ mutations. In the case of the Leu+ reversions, 15 isolates demonstrated a transition mutation at site 427 that resulted in restoration of the original wild-type sequence (AGA→GGA; Table 2). For six of the Leu+ colonies tested, we cannot yet identify the mutation responsible for the apparent reversion (despite having sequenced all of the genes known to be directly involved in leucine metabolism; data not shown). In these cases, we are dealing with either some type of suppressor mutation or the activation of a cryptic gene or a type of adaptive amplification (38). These possibilities are presently being investigated.
A functional RecA protein is not required for stationary-phase mutagenesis in B. subtilis.
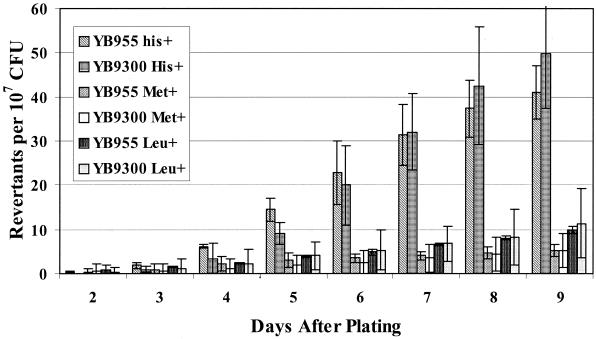
In the lacZ +1 frameshift E. coli model, a functional recombination system and a functional SOS system are required for the generation of stationary-phase mutants (8, 36, 39, 50, 60). Consequently, in this model, stationary-phase mutagenesis requires a functional RecA protein. In order to determine if the recA gene and its product are required for stationary-phase mutagenesis in B. subtilis, an isogenic derivative of strain YB955 (YB9300) that lacked a functional recA gene (Table 1) was examined (Fig. 2). The results clearly demonstrate that a functional recA gene is not necessary for this type of stationary-phase mutagenesis in B. subtilis.
FIG. 2.
Reversion frequencies in YB955 and YB9300 (recA mutant) strains. Results presented are the average number of revertants on each day from five different selection plates. Error bars represent 1 standard error. These results are representative of experiments repeated at least three times.
Genetic regulation of stationary-phase mutagenesis.
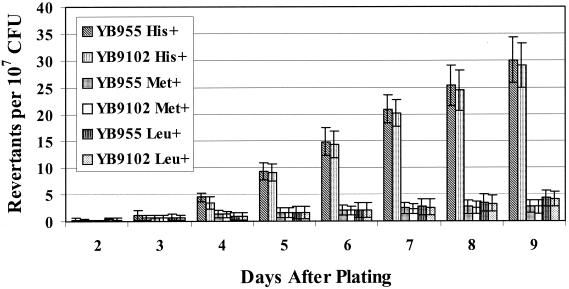
An original premise for beginning this project was our belief that previous research performed to examine stationary-phase mutagenesis indicated that this process might involve a form of prokaryotic differentiation. Essentially, we wanted to test the model that advocated that this type of mutagenesis was the result of a small subset of cells that were hypermutable. In order to begin to test this model, we examined the effects that selected mutations in specific regulatory genes would have on stationary-phase mutagenesis. Gómez-Gómez and colleagues had demonstrated that RpoS (an alternative sigma subunit of the E. coli RNA polymerase) was required for the generation of one type of stationary-phase mutagenesis in E. coli (24). The σS subunit is required for the expression of a variety of genes in this bacterium under starvation conditions (40). An analogous but not completely identical σ factor in B. subtilis is the product of the sigB gene (22, 75). Therefore, using strain YB9102 (an isogenic derivative of YB955 that lacks a functional sigB gene), we examined stationary-phase mutagenesis and found that the lack of σB did not affect this process, as demonstrated by the data presented in Fig. 3.
FIG. 3.
Reversion frequencies in YB955 and YB9102 (sigB mutant) strains. Results presented here are the average number of revertants on each day from five different selection plates. Error bars represent 1 standard error. These results are representative of experiments repeated at least three times.
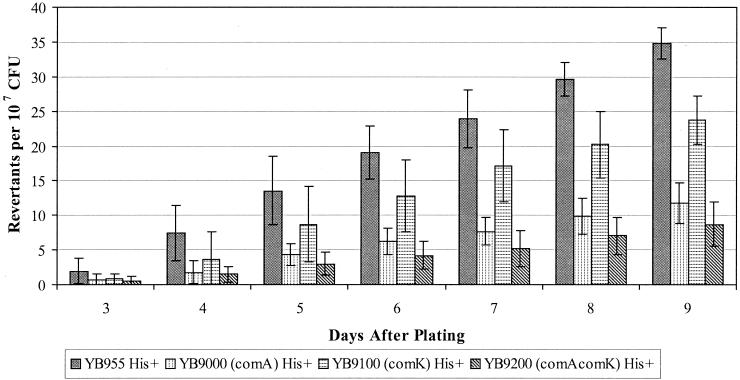
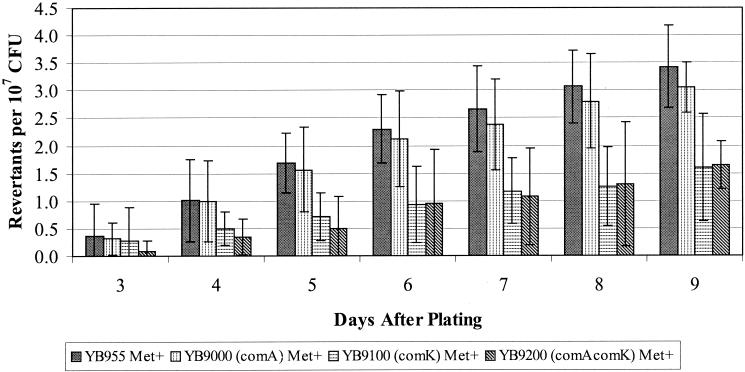
The next regulatory genes we chose to investigate were two (comA and comK) that control pathways in the prokaryotic differentiation processes (14, 30, 54, 74). The results presented in Fig. 4, 5, and 6 demonstrate the effects on stationary-phase mutagenesis that occur when a functional ComA and/or ComK protein is eliminated. Essentially, strains carrying a knockout of the comA gene or knockouts of both the comA and comK genes have up to a two-thirds decreased reversion frequency for the His+ mutations (Fig. 4). However, the strain carrying the comK mutation alone had a reversion frequency for the hisC allele that was only slightly reduced compared with what was found in the wild-type or parent strain. Whether or not the comK and comA alleles are affecting the production of the Met+ revertants is difficult to determine due to the small number of these colonies that arose, although elimination of the ComK protein did appear to decrease the Met+ reversion frequency (Fig. 5).
FIG. 4.
His+ reversion frequencies in YB955, YB9000 (comA), YB9100 (comK), and YB9200 (comA comK). Results presented here are the average number of revertants on each day from five different selection plates. Error bars represent 1 standard error. These results are representative of experiments repeated at least three times
FIG. 5.
Met+ reversion frequencies in YB955, YB9000 (comA), YB9100 (comK), and YB9200 (comA comK). Results presented here are the average number of revertants on each day from five different selection plates. Error bars represent 1 standard error. These results are representative of experiments repeated at least three times.
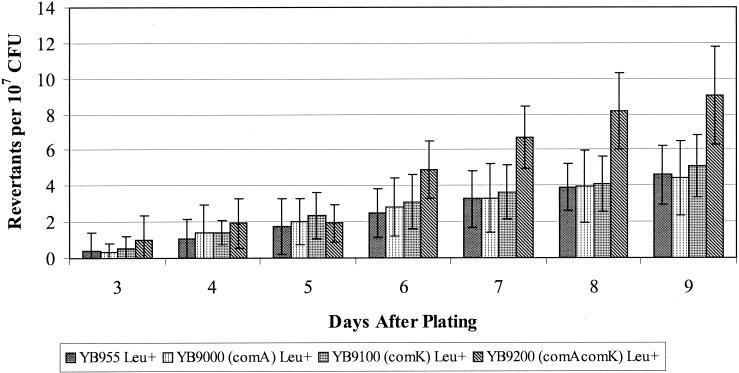
While strains lacking a functional ComK and/or ComA protein appeared to have the same ability to generate Leu+ reversions via the stationary-phase system(s) as did the wild-type strain (Fig. 6), there does appear to be a trend for more Leu+ revertants generated in the comA comK background than in any of the other backgrounds examined. As mentioned in the figure legends, these results were consistent each time the experiment was performed. Thus, in this last case, it seems that absence of the ComA and ComK proteins actually enhanced the mechanism(s) that generated the Leu+ revertants.
FIG. 6.
Leu+ reversion frequencies in YB955, YB9000 (comA), YB9100 (comK), and YB9200 (comA comK). Results presented here are the average number of revertants on each day from five different selection plates. Error bars represent 1 standard error. These results are representative of experiments repeated at least three times.
Speculation as to why the comA and comK mutations should have different effects on the reversion and mutation frequencies for these different genes is presented in the Discussion section. However, we do not believe that these differential effects can be attributed to changes in growth parameters or survivability, since all of the strains used have similar growth and survival rates. Specifically, as described in Materials and Methods, the bacteria that had been plated on the medium lacking essential amino acids were checked daily for survival. In all of the strains, between 10 and 50% of the bacteria survived 9 days on the minimal medium that lacked all of the appropriate essential amino acids.
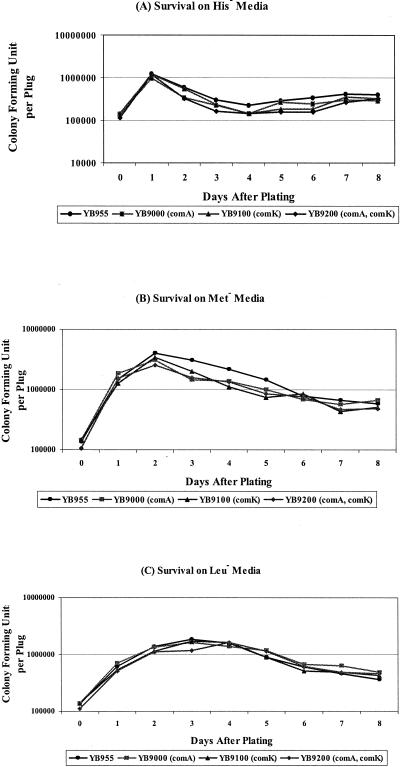
Since the minimal medium lacking all of the required amino acids differs slightly from the selective medium that was used to screen for the appearance of revertants, we also used a second approach for looking at the survival of the cells (see Materials and Methods). In this second approach, agar plugs were removed from the selective medium each day and the number of CFU was determined. As shown in Fig. 7, despite some fluctuations in the number of surviving CFU from each medium, there was no significant difference between YB955 and its isogenic derivatives with respect to bacterial viability. Therefore, the variations in the production of adaptive mutations in these strains cannot be attributed to different survival rates.
FIG. 7.
Ability of B. subtilis strains to survive under amino acid starvation. Three plugs of agar containing bacteria were taken out each day to test the viability of bacteria on the selection plate; see Materials and Methods for details. (A) Viability of bacteria under histidine starvation. (B) Viability of bacteria under methionine starvation. (C) Viability of bacteria under leucine starvation. The results are representative, and the experiments were performed at least twice.
Mutation rates for strain YB955, its isogenic derivatives, and revertants generated during “stationary-phase” mutagenesis.
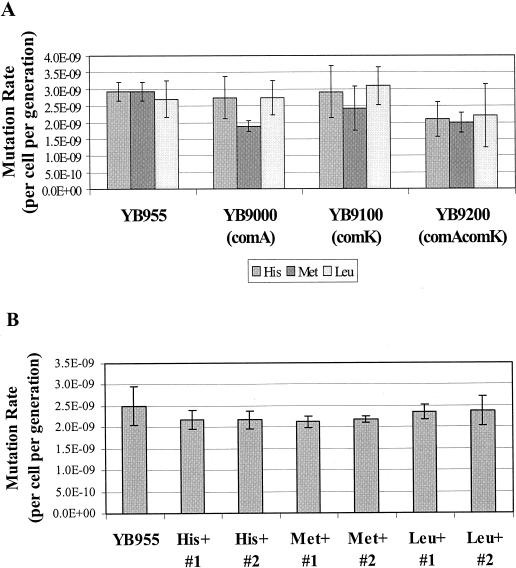
Strains YB955, YB9000, YB9100, and YB9200 were tested for their ability to generate His+, Met+, and Leu+ revertants during exponential growth. The data in Fig. 8A demonstrate that growth-dependent mutation rates were similar for all of these strains. Thus, the presence or absence of ComA and/or ComK does not affect the spontaneous mutation rate of these bacteria.
FIG. 8.
Analysis of mutation frequencies. (A) YB955 and isogenic strains of YB955 carrying the comA and/or comK allele were tested for their ability to produce His+, Met+, and Leu+ revertants during exponential growth as described in Materials and Methods. The revertants were scored and recorded 48 h after plating. The median (r) is the mean of the 19th and 20th values of r (observed number of mutants per culture in a 38-test-tube fluctuation test) when the r's are ranked. The number of mutations per culture (m) was calculated with the Lea-Coulson formula, r/m − ln(m) = 1.24. Three parallel cultures were used to determine the Nt by titration. The mutation rates were calculated with the formula m/2Nt (45). Results presented are the average mutation rates from three individual fluctuation tests. Error bars represent 1 standard error. (B) YB955 and His+, Met+, and Leu+ revertants (that arose on the sixth day following plating) were tested for their abilities to generate growth-dependent spontaneous rifampin resistance mutations (see Materials and Methods for details). The mutation rates for rifampin resistance were also determined by the method described above for panel A but with a 40-test-tube fluctuation test. The rifampin-resistant bacteria were selected on TBAB containing 5 μg of rifampin/ml (Fluka Biochemicals). After 24 h of incubation at 37°C, the number of rifampin-resistant colonies was determined. Results presented are the average mutation rates from three individual fluctuation tests. Error bars represent 1 standard error.
In order to determine whether stationary-phase mutagenesis might be the result of a permanent mutator phenotype in the surviving revertants, day 6 His+, Met+, and Leu+ colonies were picked, reisolated, and tested for their ability to generate growth phase-dependent rifampin resistance mutations (Fig. 8B) as described in Materials and Methods. The results presented in Fig. 8B clearly demonstrate that the growth-dependent mutation rate for the selected revertants was similar to that seen in the parental strain YB955. Therefore, these late-arising stationary-phase revertants are not the result of a permanent mutator phenotype.
DISCUSSION
The last 10 years have seen a dramatic increase in the interest shown in “directed” and stress-induced mutagenesis. As recently reviewed by Wright, there are data that strongly support the existence of stress-related “directed” mutagenesis mechanisms (77). Significantly, for the B. subtilis model system, our laboratory has established a relationship between prokaryotic differentiation and the induction of stress responses (3, 47, 48), including the diverse phenomena associated with the SOS regulon of this bacterium (79).
Because of the early observations on the adaptive or stationary-phase mutagenesis phenomenon in E. coli (16), we began this investigation into whether or not this type of mutagenesis occurred in B. subtilis, whether or not this phenomenon was part of programmed prokaryotic differentiation or development, and whether or not this type of mutagenesis was in anyway directed. B. subtilis would appear to be an ideal organism for studying this possibility because it is a paradigm for investigations into prokaryotic differentiation and development (26, 41, 54, 67).
As mentioned previously, the F′ lac system for studying adaptive mutagenesis in E. coli is dependent upon a functional RecA. However, other detection systems have been shown to be independent of recombination (7, 16, 31). Therefore, it was important to determine the involvement of recombination in any stationary-phase mutagenesis that existed in B. subtilis. The data presented in Fig. 1 and 2 demonstrate that stationary-phase mutagenesis does occur in B. subtilis and that this type of mutagenesis does not require a functional RecA protein. In B. subtilis the RecA protein is required for homologous recombination and for the induction of many of the SOS phenomena (78). However, unlike the E. coli SOS regulon, in B. subtilis, filamentation (a type II SOS phenomenon) and type III competence-related SOS phenomena are induced via RecA-independent mechanisms (78). Although our results cannot eliminate the possibility that stationary-phase mutagenesis in B. subtilis is part of the SOS regulon, we can eliminate the need for a functional RecA protein in the generation of these types of mutations (Fig. 2).
Do stationary-phase mutations occur in a hypermutable subpopulation? An argument that was generated by some for the initial description of adaptive or stationary-phase mutagenesis concerned whether or not the mutations were limited to those genes or metabolic processes for which selective pressure was applied (12). For instance, do we obtain Leu+ reversions only on medium that lacks leucine? In the E. coli model, substantial evidence has been provided to indicate that under the experimental conditions, mutations occur in genes that control metabolic processes unrelated to the initial selective pressure. Although there is still considerable debate, it is argued that most if not all (9) random adaptive mutations are occurring in a hypermutable subpopulation.
During the course of our experiments, three colonies were isolated (out of 500 colonies that were checked) that showed prototrophy for all three amino acid requirements. In addition, two more of these 500 colonies (chosen randomly) were demonstrated, upon DNA sequencing, to have two mutations each in the same gene. Thus, this initial screen yielded 5 revertants out of 500 (1%) that had more than one mutation. While these double mutations could have been the result of normal sequential mutagenesis probabilities, the frequency of the double mutations would argue for, but certainly not prove, the existence of hypermutable cells. Furthermore, as was the case for E. coli, such a proposed hypermutable subpopulation must be transient, since upon subsequent growth, the isolated revertants did not continue to have an increased mutation frequency (Fig. 8B).
While our results support the existence of a hypermutable subpopulation(s), we still cannot definitively prove the existence of such a subpopulation or even eliminate the possibility that some of the adaptive mutations are directed. However, these possibilities are presently being examined.
Stationary-phase mutagenesis mechanism(s) demonstrates hot spots and cold spots for mutagenic activity.
The vast majority of Met+ revertants (>90%) generated by either the growth-dependent spontaneous mutagenesis or stationary-phase mutagenesis pathway were also His+ (Table 6). Further testing of these His+ and Met+ cells demonstrated that these bacteria carried no base changes in the auxotrophic structural genes (data not shown). These results strongly suggest that the Met+ His+ revertants were caused by the generation of a suppressor mutation. A previous study demonstrated that only 20% of the UV- and ethyl methane sulfonate-induced Met+ revertants in strain YB955 were true revertants, while 80% of the Met+ revertants were the result of the generation of a nonsense suppressor (81). Taken collectively, it would appear that the DNA sequence within the metB gene is a cold spot for growth-dependent spontaneous mutagenesis and stationary-phase mutagenesis and for the generation of SOS-induced mutations (at least with respect to reversion of the nonsense mutation in the metB5 allele). On the other hand, the data presented in Fig. 1 and Table 6 indicate that the DNA sequence within the hisC gene appears to be a hot spot for reversion of the hisC952 nonsense allele.
Genetic control of eubacterial differentiation and stationary-phase mutagenesis.
Since the results presented indicate the existence of stationary-phase mutagenesis in B. subtilis, one of the next steps would be to begin to elucidate the mechanism(s) that controls or regulates this type of mutagenesis. Unlike the system described by Gómez-Gómez et al. (24) for E. coli, the alternative stress-associated σ factor SigB is not essential for the functioning of stationary-phase mutagenesis in B. subtilis, at least with the systems that we are using to observe this phenomenon (Fig. 3). However, ComA and ComK do appear to be involved in this process.
ComA and ComK are both transcription factors, and among other things, they regulate the expression of genes involved in the development of competence. ComA is part of a two-component regulatory system (56) and is involved in the initiation of early competence and maximizing sporulation processes, while ComK activates the expression of late competence genes (26, 34, 74). In addition, recent gene array analysis has demonstrated that ComK directly or indirectly influences the transcription of at least 100 genes (2, 55). Neither our results (Fig. 8A) nor previous reports suggest that either ComA or ComK has any effect on the generation of mutations during growth. In fact, the results presented in this report clearly show that isogenic derivatives of YB955 that are deficient in the production of ComA and/or ComK have growth-dependent mutation frequencies similar to those found in the parent strain (Fig. 8A). On the other hand, our data demonstrate that the presence of functional ComA and probably ComK proteins plays an important role in some aspect of stationary-phase mutagenesis in B. subtilis (Fig. 4, 5, and 6).
We believe that ComA and ComK are not directly involved in the mutagenesis process but rather regulate the gene expression necessary for stationary-phase mutagenesis and for the differentiation of a hypermutable subpopulation(s). It is important to note that the influence that ComA and ComK have on stationary-phase mutagenesis but not on growth-phase mutagenesis strongly argues against the possibility that revertants arising during stationary phase are merely the result of limited growth of some cells on the exhausted medium.
It is conceivable that there is more than one type of hypermutable subpopulation in stressed B. subtilis cultures. These different subpopulations could utilize various molecular mechanisms for obtaining increased mutation frequencies. For instance, in one subpopulation, mismatch repair could be inactivated, while in another subpopulation, aspects of base excision or nucleotide excision repair could be inactivated or depressed. If this is the case, an argument could be made for stochastic maintenance of this phenomenon. However, we suggest that these subpopulations would have to communicate with each other through regulation and sensing mechanisms to control the number of cells actually engaged in generating mutations via these mechanisms. Without this type of control, the culture could quickly accumulate too many mutations and reach an unsupportable genetic load.
Known models for the presence of coexisting subpopulations that communicate with each other, limit population size, and enhance diversity as well as the ability to survive can actually be found in studies of the regulation of sporulation, competence development, and production of secondary metabolites in B. subtilis (26, 41, 44). These studies have revealed the existence of multiple interacting and complex regulatory networks that permit the cells within the culture to sense and adapt to their environments. Specifically, it has been found that with respect to the development of competence, only 10 to 20% of the cells in a culture obtain this state (14); quorum sensing is an essential component (26), and this developmental process actually does occur in the soil, the natural environment of this bacterium (25). Furthermore, it has been clearly demonstrated that while some SOS phenomena are spontaneously induced in the component subpopulation of B. subtilis, other SOS functions are prevented from such induction in the same subpopulation (53, 78).
The existence of multiple types of stationary-phase mutagenesis mechanisms is also suggested by the collective analysis of the data that have been obtained from studies with E. coli (16, 66) and other model systems (as reviewed in reference 54). Recently, Makinoshima and his colleagues were able to fractionate subpopulations of an E. coli culture (49) just as the competent B. subtilis subpopulation can be separated from the noncompetent bacteria in a single culture (29). These results again suggest the existence of multiple subpopulations within stationary-phase cultures.
With the library of regulatory genes that have been elucidated in B. subtilis, we are now delineating the mechanisms that control the production of the proposed subpopulation or populations in this bacterium that are involved in stationary-phase mutagenesis. More explicitly, we are examining the ability of the cells deficient in various developmental regulatory gene products to undergo stationary-phase mutagenesis.
In summary, our present data strongly suggest that a previously defined developmental genetic network in B. subtilis also controls the diversity within this bacterium that can be generated by stationary-phase mutagenesis. The continued elucidation of these networks should provide an opportunity for better understanding the role played by stress-induced genetic diversity mechanisms in species survival and pathogenesis as well as in evolution.
Acknowledgments
We thank Juan González and Mario Pedraza-Reyes for helpful suggestions. Strains and research results were generously provided by D. Dubnau, C. Price, and L. Hamoen.
This research was supported by MCB-9975140 from the National Science Foundation. These results represent partial fulfillment of the doctoral degree requirements for H.-M. Sung.
REFERENCES
- 1.Ausubel, F., R. Brent, R. Kingston, J. Seidman, J. A. Smith, and K. Struhl. 2001. Current protocols in molecular biology, CD version. Wiley, New York, N.Y.
- 2.Berka, R. M., J. Hahn, M. Albano, I. Draskovic, M. Persuh, X. Cui, A. Sloma, W. Widner, and D. Dubnau. 2002. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol. Microbiol. 43:1331-1345. [DOI] [PubMed] [Google Scholar]
- 3.Bol, D., and R. E. Yasbin. 1990. Characterization of an inducible oxidative stress system in Bacillus subtilis. J. Bacteriol. 172:3503-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boylan, S. A., M. D. Thomas, and C. W. Price. 1991. Genetic method to identify regulons controlled by nonessential elements: isolation of a gene dependent on alternate transcription factor sigma B of Bacillus subtilis. J. Bacteriol. 173:7856-7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridges, B. A. 1997. Hypermutation under stress. Nature 387:557-558. [DOI] [PubMed] [Google Scholar]
- 6.Bridges, B. A. 1998. The role of DNA damage in stationary phase (‘adaptive’) mutation. Mutat. Res. 408:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Bridges, B. A. 1993. Spontaneous mutation in stationary-phase Escherichia coli WP2 carrying various DNA repair alleles. Mutat. Res. 302:173-176. [DOI] [PubMed] [Google Scholar]
- 8.Bull, H. J., G. J. McKenzie, P. J. Hastings, and S. M. Rosenberg. 2000. Evidence that stationary-phase hypermutation in the Escherichia coli chromosome is promoted by recombination. Genetics 154:1427-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull, H. J., G. J. McKenzie, P. J. Hastings, and S. M. Rosenberg. 2000. Response to John Cairns: the contribution of transiently hypermutable cells to mutation in stationary phase. Genetics 156:925-926. [Google Scholar]
- 10.Cairns, J. 2000. The contribution of bacterial hypermutators to mutation in stationary phase. Genetics 156:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145. [DOI] [PubMed] [Google Scholar]
- 13.Cheo, D. L., K. W. Bayles, and R. E. Yasbin. 1991. Cloning and characterization of DNA damage-inducible promoter regions from Bacillus subtilis. J. Bacteriol. 173:1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubnau, D. 1991. Genetic competence in Bacillus subtilis. Microbiol. Rev. 55:395-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster, P. L. 1998. Adaptive mutation: has the unicorn landed? Genetics 148:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster, P. L. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster, P. L. 1997. Nonadaptive mutations occur on the F′ episome during adaptive mutation conditions in Escherichia coli. J. Bacteriol. 179:1550-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, P. L., G. Gudmundsson, J. M. Trimarchi, H. Cai, and M. F. Goodman. 1995. Proofreading-defective DNA polymerase II increases adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:7951-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster, P. L., and J. M. Trimarchi. 1994. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science 265:407-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster, P. L., and J. M. Trimarchi. 1995. Adaptive reversion of an episomal frameshift mutation in Escherichia coli requires conjugal functions but not actual conjugation. Proc. Natl. Acad. Sci. USA 92:5487-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster, P. L., J. M. Trimarchi, and R. A. Maurer. 1996. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics 142:25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaidenko, T. A., and C. W. Price. 1998. General stress transcription factor 32 σB and sporulation transcription factor σH each contribute to survival of Bacillus subtilis under extreme growth conditions. J. Bacteriol. 180:3730-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galitski, T., and J. R. Roth. 1995. Evidence that F plasmid transfer replication underlies apparent adaptive mutation. Science 268:421-423. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Gómez, J., J. Blázquez, F. Baquero, and J. Martinez. 1997. H-NS and RpoS regulate emergence of Lac Ara+ mutants of Escherichia coli MCS2. J. Bacteriol. 179:4620-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham, J. B., and C. A. Istock. 1978. Genetic exchange in Bacillus subtilis in soil. Mol. Gen. Genet. 166:287-290. [DOI] [PubMed] [Google Scholar]
- 26.Grossman, A. D. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu. Rev. Genet. 29:477-508. [DOI] [PubMed] [Google Scholar]
- 27.Guérout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 28.Guillen, N., Y. Weinrauch, and D. A. Dubnau. 1989. Cloning and characterization of the regulatory Bacillus subtilis competence genes comA and comB. J. Bacteriol. 171:5354-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadden, C., and E. W. Nester. 1968. Purification of competent cells in the Bacillus subtilis transformation system. J. Bacteriol. 95:876-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn, J., A. Luttinger, and D. Dubnau. 1996. Regulatory inputs for the synthesis of ComK, the competence transcription factor of Bacillus subtilis. Mol. Microbiol. 21:763-775. [DOI] [PubMed] [Google Scholar]
- 31.Hall, B. G. 1995. Genetics of selection-induced mutations: I. uvrA, uvrB, uvrC, and uvrD are selection-induced specific mutator loci. J. Mol. Evol. 40:86-93. [DOI] [PubMed] [Google Scholar]
- 32.Hall, B. G. 1997. On the specificity of adaptive mutations. Genetics 145:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall, B. G. 1990. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics 126:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamoen, L. W., A. F. Van Werkhoven, G. Venema, and D. Dubnau. 2000. The pleiotropic response regulator DegU functions as a priming protein in competence development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 97:9246-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris, R. S., H. J. Bull, and S. M. Rosenberg. 1997. A direct role for DNA polymerase III in adaptive reversion of a frameshift mutation in Escherichia coli. Mutat. Res. 375:19-24. [DOI] [PubMed] [Google Scholar]
- 36.Harris, R. S., S. Longerich, and S. M. Rosenberg. 1994. Recombination in adaptive mutation. Science 264:258-260. [DOI] [PubMed] [Google Scholar]
- 37.Harris, R. S., K. J. Ross, and S. M. Rosenberg. 1996. Opposing roles of the Holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics 142:681-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hastings, P. J., H. J. Bull, J. R. Klump, and S. M. Rosenberg. 2000. Adaptive amplification: an inducible chromosomal instability mechanism. Cell 103:723-731. [DOI] [PubMed] [Google Scholar]
- 39.Hendrickson, H., E. S. Slechta, U. Bergthorsson, D. I. Andersson, and J. R. Roth. 2002. Amplification-mutagenesis: evidence that “directed” adaptive mutation and general hypermutability result from growth with a selected gene amplification. Proc. Natl. Acad. Sci. USA 99:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hengge-Aronis, R. 1993. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell 72:165-168. [DOI] [PubMed] [Google Scholar]
- 41.Hoch, J. A. 2000. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 3:165-170. [DOI] [PubMed] [Google Scholar]
- 42.Innis, M. A., D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.). 1990. PCR protocols: a guide to methods and applications. Academic Press, New York, N.Y.
- 43.Kasak, L., R. Horak, and M. Kivisaar. 1997. Promoter-creating mutations in Pseudomonas putida: a model system for the study of mutation in starving bacteria. Proc. Natl. Acad. Sci. USA 94:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroos, L., B. Zhang, H. Ichikawa, and Y. T. Yu. 1999. Control of sigma factor activity during Bacillus subtilis sporulation. Mol. Microbiol. 31:1285-1294. [DOI] [PubMed] [Google Scholar]
- 45.Lea, D. E., and C. A. Coulson. 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:264-285. [DOI] [PubMed] [Google Scholar]
- 46.Lombardo, M. J., J. Torkelson, H. J. Bull, G. J. McKenzie, and S. M. Rosenberg. 1999. Mechanisms of genome-wide hypermutation in stationary phase. Ann. N. Y. Acad. Sci. 870:275-289. [DOI] [PubMed] [Google Scholar]
- 47.Love, P. E., M. J. Lyle, and R. E. Yasbin. 1985. DNA damage inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc. Natl. Acad. Sci. USA 82:6201-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love, P. E., and R. E. Yasbin. 1984. Genetic characterization of the inducible “SOS-like” system of Bacillus subtilis. J. Bacteriol. 160:910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makinoshima, H., A. Nishimura, and A. Ishihama. 2002. Fractionation of Escherichia coli cell populations at different stages during growth transition to stationary phase. Mol. Microbiol. 43:269-279. [DOI] [PubMed] [Google Scholar]
- 50.McKenzie, G. J., R. S. Harris, P. L. Lee, and S. M. Rosenberg. 2000. The SOS response regulates adaptive mutation. Proc. Natl. Acad. Sci. USA 97:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKenzie, G. J., P. L. Lee, M. J. Lombardo, P. J. Hastings, and S. M. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. [DOI] [PubMed] [Google Scholar]
- 52.McKenzie, G. J., and S. M. Rosenberg. 2001. Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr. Opin. Microbiol. 4:586-594. [DOI] [PubMed] [Google Scholar]
- 53.McVeigh, R., and R. E. Yasbin. 1996. The smart phages of B. subtilis: type 4 SOS response. J. Bacteriol. 178:3399-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Msadek, T. 1999. When the going gets tough: survival strategies and environmental signaling networks in Bacillus subtilis. Trends Microbiol. 7:201-207. [DOI] [PubMed] [Google Scholar]
- 55.Ogura, M., H. Yamaguchi, K. Kobayashi, N. Ogasawara, Y. Fujita, and T. Tanaka. 2002. Whole-genome analysis of genes regulated by the Bacillus subtilis competence transcription factor ComK. J. Bacteriol. 184:2344-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogura, M., H. Yamaguchi, K. Yoshida, Y. Fujita, and T. Tanaka. 2001. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 29:3804-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prival, M., and T. Cebula. 1996. Adaptive mutation and slow-growing revertants of an Escherichia coli lacZ amber mutant. Genetics 144:1337-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosche, W. A., and P. L. Foster. 2000. Determining mutation rates in bacterial populations. Methods 20:4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg, S. M. 1997. Mutation for survival. Curr. Opin. Genet. Dev. 7:829-834. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg, S. M., R. S. Harris, S. Longerich, and A. M. Galloway. 1996. Recombination-dependent mutation in non-dividing cells. Mutat. Res. 350:69-76. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg, S. M., R. S. Harris, and J. Torkelson. 1995. Molecular handles on adaptive mutation. Mol. Microbiol. 18:185-189. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg, S. M., S. Longerich, P. Gee, and R. S. Harris. 1994. Adaptive mutation by deletions in small mononucleotide repeats. Science 265:405-407. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg, S. M., C. Thulin, and R. S. Harris. 1998. Transient and heritable mutators in adaptive evolution in the lab and in nature. Genetics 148:1559-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudner, R., A. Murray, and N. Huda. 1999. Is there a link between mutation rates and the stringent response in Bacillus subtilis? Ann. N. Y. Acad. Sci. 870:418-422. [DOI] [PubMed] [Google Scholar]
- 65.Shapiro, J. A. 1984. Observations on the formation of clones containing araB-lacZ cistron fusions. Mol. Gen. Genet. 194:79-90. [DOI] [PubMed] [Google Scholar]
- 66.Shapiro, J. A. 1998. Thinking about bacterial populations as multicellular organisms. Annu. Rev. Microbiol. 52:81-104. [DOI] [PubMed] [Google Scholar]
- 67.Sonenshein, A. L., J. A. Hoch, and R. Losick (ed.). 1993. Bacillus subtilis and other gram-positive bacteria: physiology, biochemistry, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 68.Spizizen, J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 44:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steele, D. F., and S. Jinks-Robertson. 1992. An examination of adaptive reversion in Saccharomyces cerevisiae. Genetics 132:9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan, M. A., R. E. Yasbin, and F. E. Young. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 29:21-26. [DOI] [PubMed] [Google Scholar]
- 71.Sung, H. M., and R. E. Yasbin. 2000. Transient growth requirement in Bacillus subtilis following the cessation of exponential growth. Appl. Environ. Microbiol. 66:1220-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tevethia, M. J., J. N. Baptist, and M. Mandel. 1974. Pleiotropic effects of suppressor mutations in Bacillus subtilis. J. Bacteriol. 119:961-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torkelson, J., R. S. Harris, M. J. Lombardo, J. Nagendran, C. Thulin, and S. M. Rosenberg. 1997. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J 16:3303-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Sinderen, D., A. Luttinger, L. Kong, D. Dubnau, G. Venema, and L. Hamoen. 1995. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol. Microbiol. 15:455-462. [DOI] [PubMed] [Google Scholar]
- 75.Volker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Borstel, R. C. 1978. Measuring spontaneous mutation rates in yeast. Methods Cell Biol. 20:1-24. [DOI] [PubMed] [Google Scholar]
- 77.Wright, B. E. 2000. A biochemical mechanism for nonrandom mutations and evolution. J. Bacteriol. 182:2993-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yasbin, R. E., D. Cheo, and D. Bol. 1993. DNA repair systems, p. 529-537. In A. L. Sonenshein, R. Losick, and J. Hoch (ed.), Bacillus subtilis and other gram-positive organisms. American Society for Microbiology, Washington, D.C.
- 79.Yasbin, R. E., D. L. Cheo, and K. W. Bayles. 1992. Inducible DNA repair and differentiation in Bacillus subtilis: interactions between global regulons. Mol. Microbiol. 6:1263-1270. [DOI] [PubMed] [Google Scholar]
- 80.Yasbin, R. E., P. I. Fields, and B. J. Andersen. 1980. Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene 12:155-159. [DOI] [PubMed] [Google Scholar]
- 81.Yasbin, R. E., R. Miehl-Lester, and P. E. Love. 1987. Mutagenesis in Bacillus subtilis, p. 73-84. In M. Alacevic, D. Hranueli, and Z. Tomen (ed.), Genetics of industrial microorganisms. GIM-86, Split, Yugoslavia.
- 82.Yasbin, R. E., M. Stranathan, and K. W. Bayles. 1991. The recE(A)+ gene of B. subtilis and its gene product: further characterization of this universal protein. Biochimie 73:245-250. [DOI] [PubMed] [Google Scholar]
- 83.Yasbin, R. E., G. A. Wilson, and F. E. Young. 1973. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J. Bacteriol. 113:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]