Abstract
1. The oxygen consumption of isolated anterior byssus retractor muscle of Mytilus edulis (ABRM) has been measured at rest and after phasic contractions induced by a.c. stimulation.
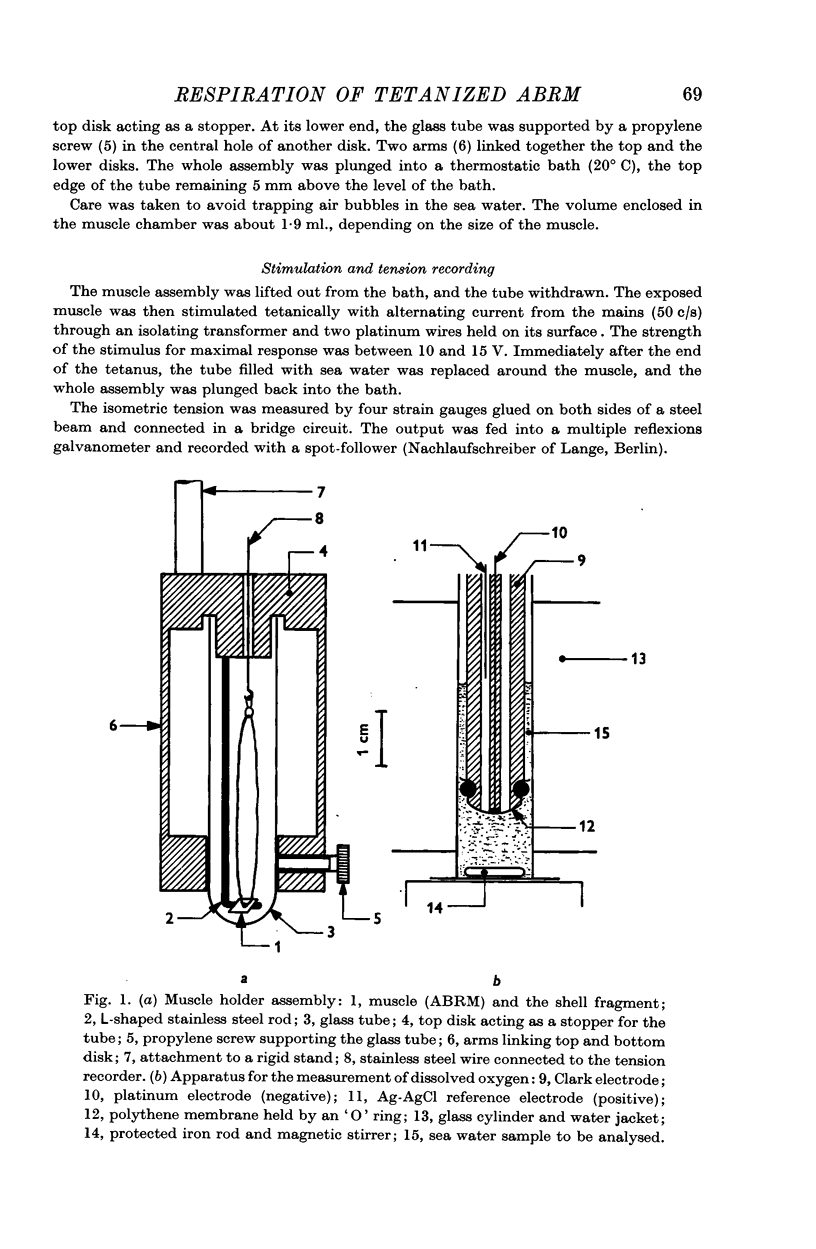
2. The respiration was measured with a Clark oxygen electrode in successive periods of 5 or 15 min, at 20° C.
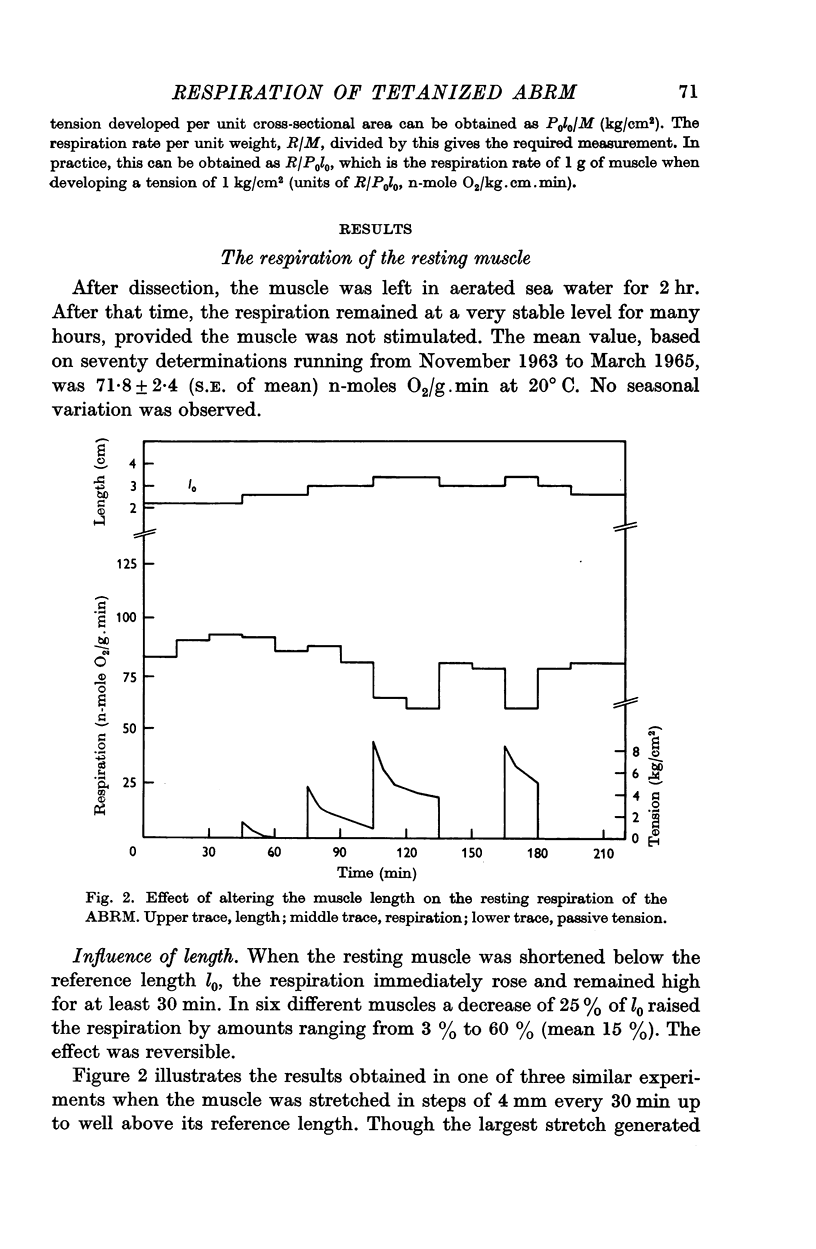
3. The resting respiration is 71·8 ± 2·4 n-moles O2/g wet weight.min (mean ± S.E., n = 70). It is increased by a release and decreased by a passive stretch.
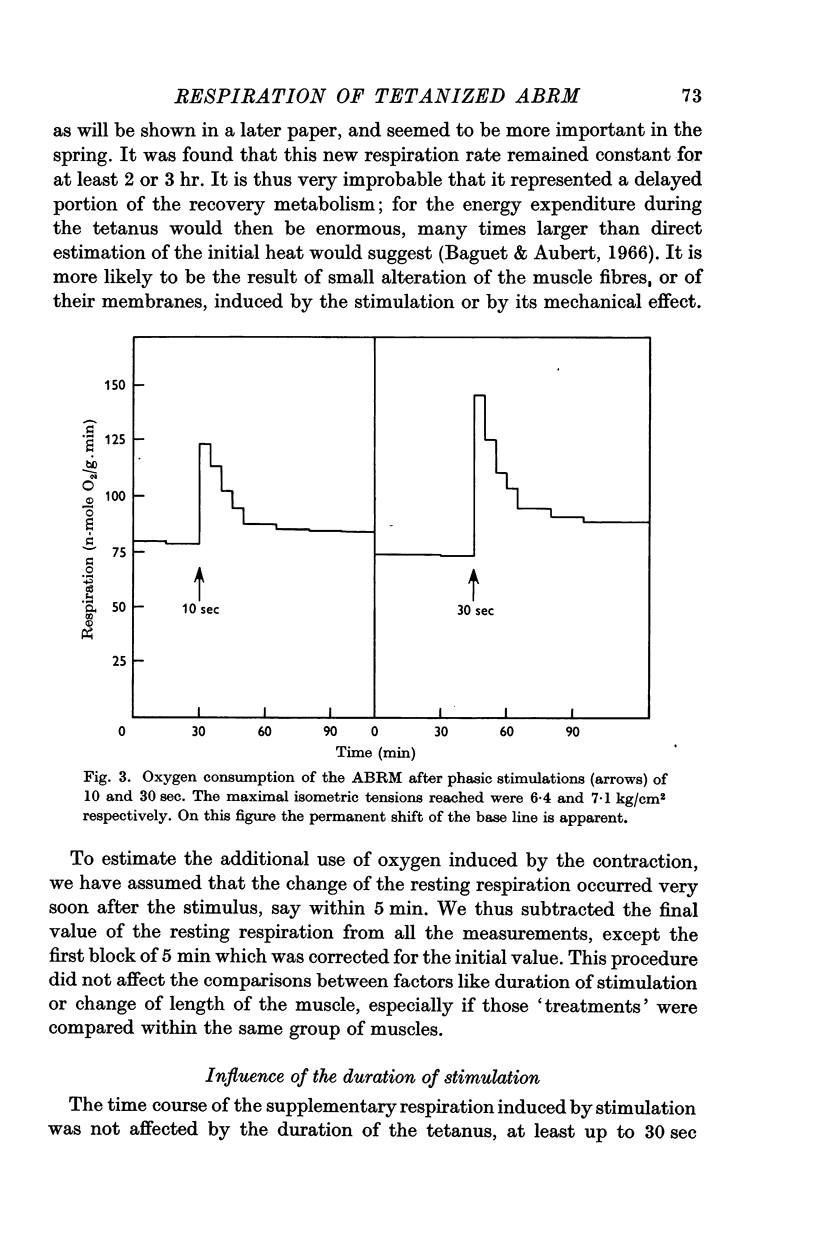
4. After phasic stimulation of up to 30 sec the respiration is increased and returns to a slightly higher level than the resting level in an exponential fashion with a time constant of about 10 min.
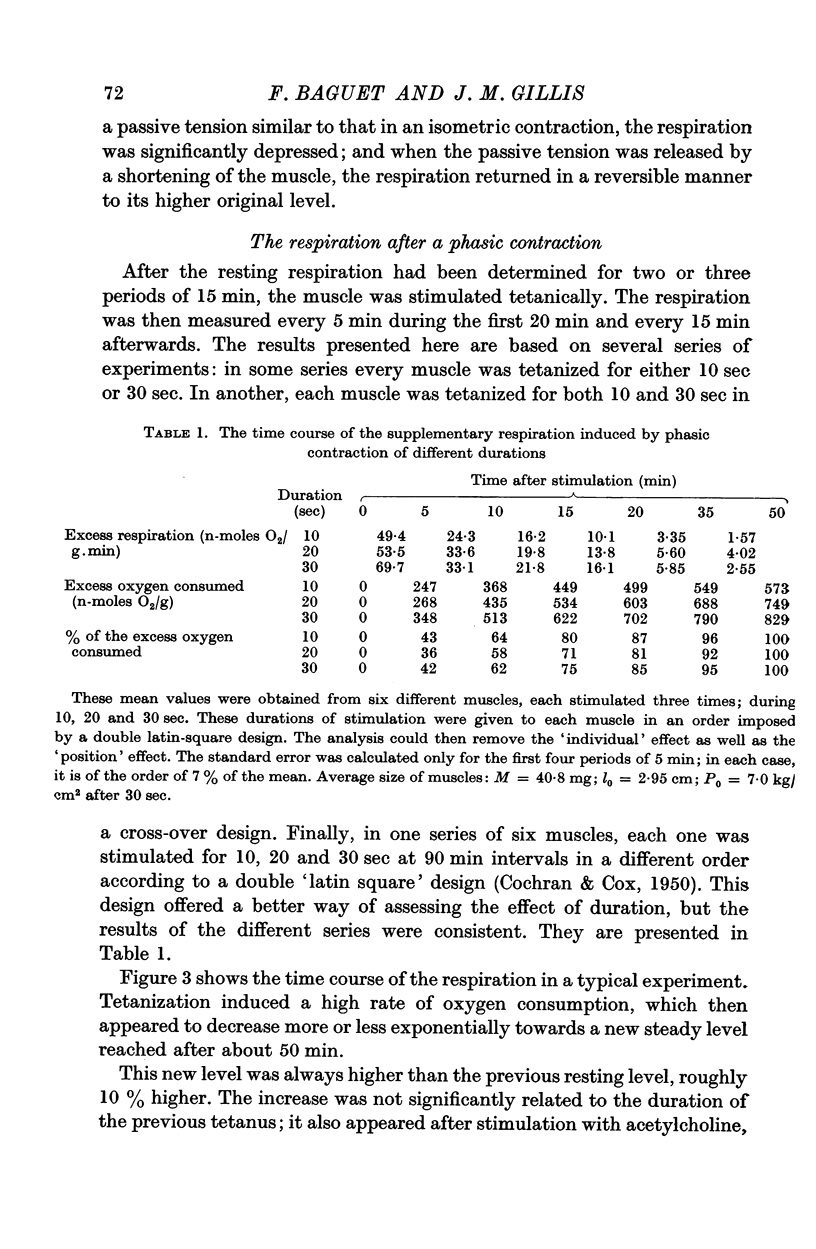
5. The duration of stimulation does not change the time course of the excess respiration but it affects its magnitude. The amount of extra oxygen consumed, in n-moles O2/g, is made up of a constant amount, 449 ± 102, and an amount that depends on the duration of stimulation (t, sec), which is given by t × 13·2 ± 4·3. When due account is taken for the tension developed, these parameters become 83·1 ± 20·7 and t × 1·24 ± 0·66 n-moles O2/g muscle and kg/cm2 of tension. This regression analysis is based on forty-eight data, with a residual error based on 5 degrees of freedom.
6. Release of the tension after the last stimulus of a 30 sec tetanus reduces by half the extra oxygen consumed during the recovery whereas the same release applied 5 min later has a much smaller effect. This suggests that relaxation is an active process.
7. From these measurements of the recovery metabolism the energy cost of the contraction was estimated and compared with this cost in vertebrate striated muscle. The constant item has about the same magnitude, but the item related to the duration of stimulation is about 250 times smaller.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., LOWY J. Contraction in mulluscan smooth muscle. J Physiol. 1958 May 28;141(3):385–397. doi: 10.1113/jphysiol.1958.sp005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAGUET F., MARECHAL G., AUBERT X. [Thermogenesis of smooth muscle of lamellibranchs during phasic contraction]. Arch Int Physiol Biochim. 1962 Jun;70:416–417. [PubMed] [Google Scholar]
- BARANY M., BARANY K., RECKARD T., VOLPE A. MYOSIN OF FAST AND SLOW MUSCLES OF THE RABBIT. Arch Biochem Biophys. 1965 Jan;109:185–191. doi: 10.1016/0003-9861(65)90304-8. [DOI] [PubMed] [Google Scholar]
- BRECHT K., UTZ G., LUTZ E. Uber die Atmung quergestreifter und glatter Muskeln von Kaltblütern in Ruhe, Dehnung, Kontraktion und Kontraktur. Pflugers Arch. 1955;260(6):524–537. doi: 10.1007/BF00363669. [DOI] [PubMed] [Google Scholar]
- Baguet F. Consommation d'oxygène et contraction phasique chez un muscle de lamellibranche. Arch Int Physiol Biochim. 1965 Mar;73(2):389–391. [PubMed] [Google Scholar]
- Bozler E. The heat production of smooth muscle. J Physiol. 1930 Jun 27;69(4):442–462. doi: 10.1113/jphysiol.1930.sp002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T. P. The effect of length on the resting metabolism of muscle. J Physiol. 1932 Apr 26;74(4):441–454. doi: 10.1113/jphysiol.1932.sp002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF TENSION IN PROLONGING THE ACTIVE STATE IN A TWITCH. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:589–595. doi: 10.1098/rspb.1964.0021. [DOI] [PubMed] [Google Scholar]
- HILL A. V., WOLEDGE R. C. An examination of absolute values in myothermic measurements. J Physiol. 1962 Jul;162:311–333. doi: 10.1113/jphysiol.1962.sp006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree W., Hill A. V. The regulation of the supply of energy in muscular contraction. J Physiol. 1921 May 24;55(1-2):133–158. doi: 10.1113/jphysiol.1921.sp001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R. The nature of the phasic and the tonic responses of the anterior byssal retractor muscle of Mytilus. J Physiol. 1959 Dec;149:154–177. doi: 10.1113/jphysiol.1959.sp006332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWY J., MILLMAN B. M., HANSON J. STRUCTURE AND FUNCTION IN SMOOTH TONIC MUSCLES OF LAMELLIBRANCH MOLLUSCS. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:525–536. doi: 10.1098/rspb.1964.0068. [DOI] [PubMed] [Google Scholar]
- MARECHAL G., MOMMAERTS W. F. The metabolism of phosphocreatine during an isometric tetanus in the frog sartorius muscle. Biochim Biophys Acta. 1963 Feb 19;70:53–67. doi: 10.1016/0006-3002(63)90718-2. [DOI] [PubMed] [Google Scholar]
- Minihan K., Davies R. E. Energy requirements for relaxation from tonic contractions ('catch') in an invertebrate muscle. Nature. 1965 Dec 25;208(5017):1327–1329. doi: 10.1038/2081327a0. [DOI] [PubMed] [Google Scholar]
- PRINGLE J. W. Models of muscle. Symp Soc Exp Biol. 1960;14:41–68. [PubMed] [Google Scholar]
- Sréter F. A., Gergely J. Comparative studies of the MG activated ATPase activity and Ca uptake of fractions of white and red muscle homogenates. Biochem Biophys Res Commun. 1964 Jul 27;16(5):438–443. doi: 10.1016/0006-291x(64)90372-9. [DOI] [PubMed] [Google Scholar]
- Winton F. R. The changes in viscosity of an unstriated muscle (Mytilus edulis) during and after stimulation with alternating, interrupted and uninterrupted direct currents. J Physiol. 1937 Jan 18;88(4):492–511. doi: 10.1113/jphysiol.1937.sp003455. [DOI] [PMC free article] [PubMed] [Google Scholar]


