Abstract
1. Acetylcholine (ACh) has been collected from the visual cortex of anaesthetized rabbits during stimulation of the lateral geniculate body and after cutting central nervous pathways. ACh has also been collected from the visual cortex of conscious, free-moving rabbits.
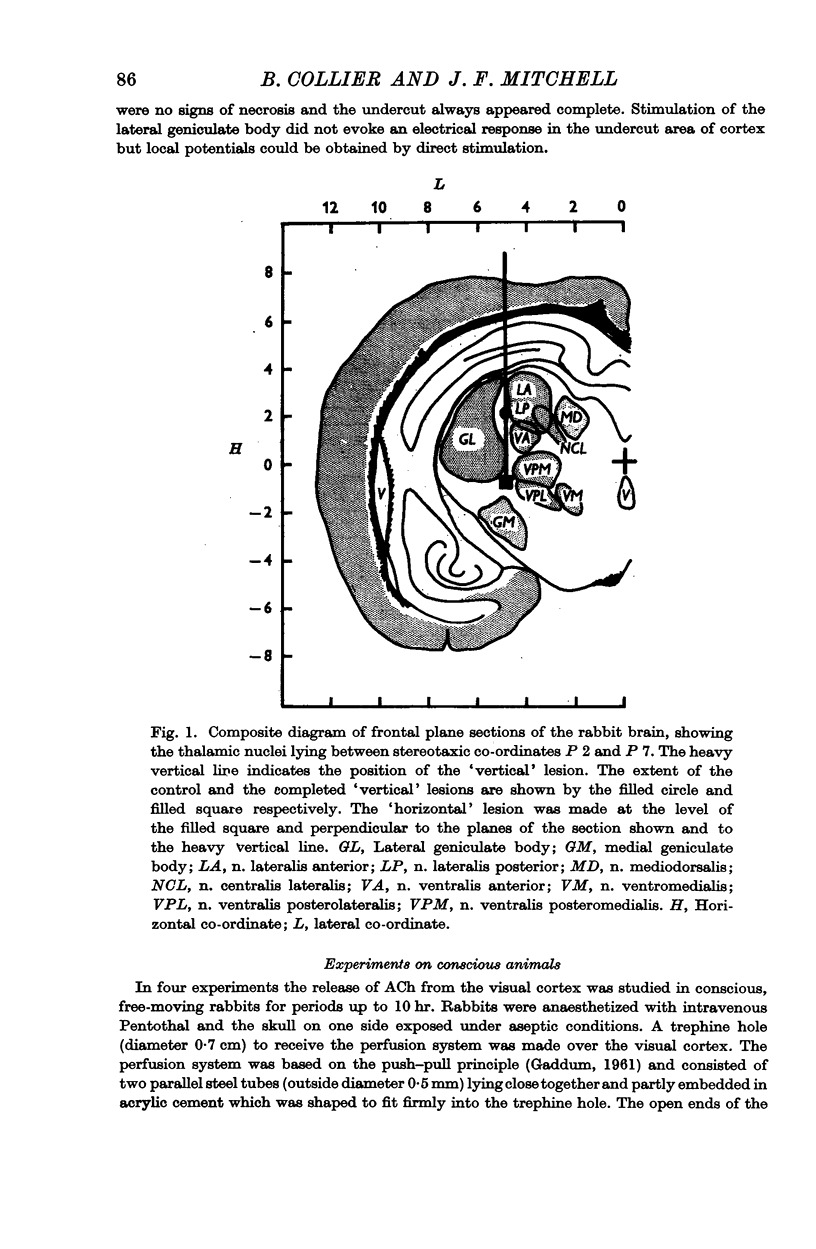
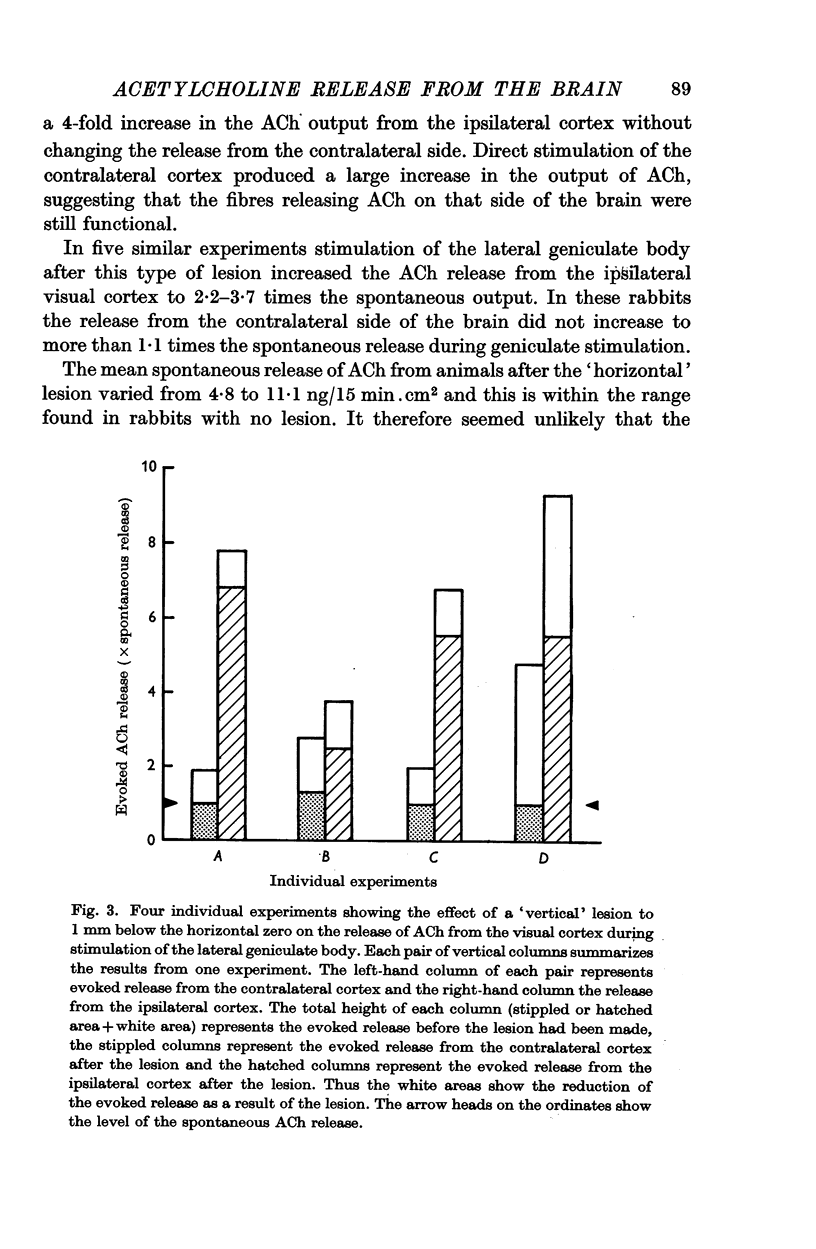
2. After a unilateral `vertical' lesion separating the geniculate body from more centrally situated nuclei, ACh release evoked from the contralateral cortex by geniculate body stimulation was abolished but evoked release from the ipsilateral cortex was only reduced.
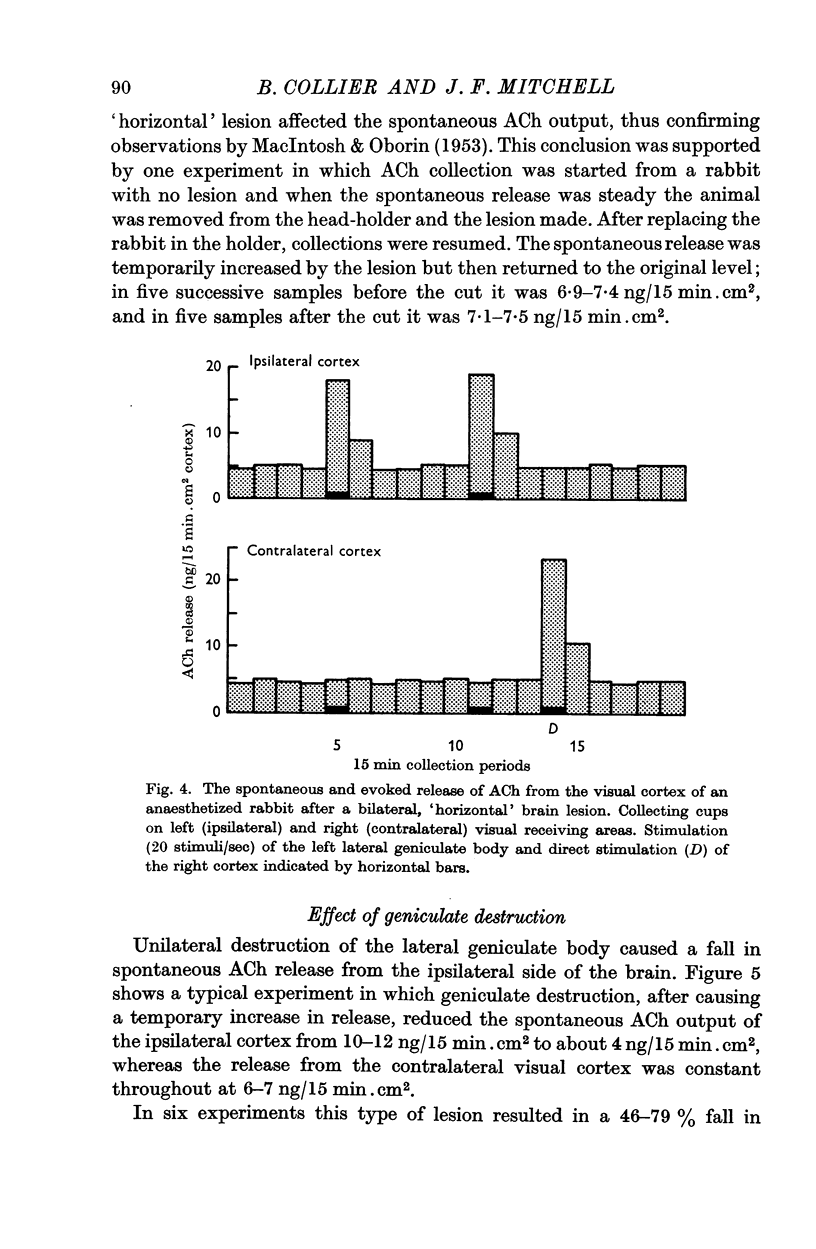
3. After a bilateral, `horizontal' lesion separating the thalamic nuclei from the reticular formation, unilateral geniculate stimulation gave an increased ACh release from the ipsilateral but not from the contralateral visual cortex.
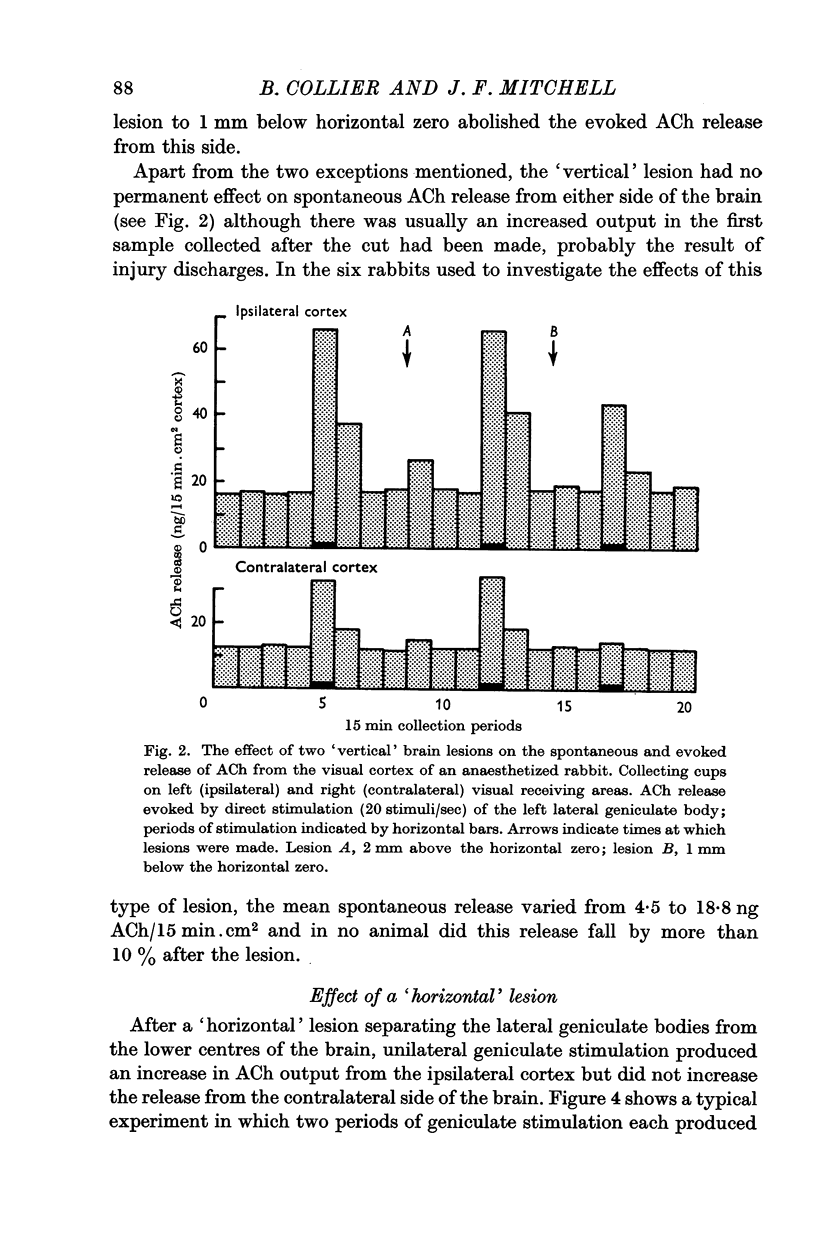
4. The `vertical' and `horizontal' lesions had no permanent effect on the spontaneous release of ACh from the visual cortex.
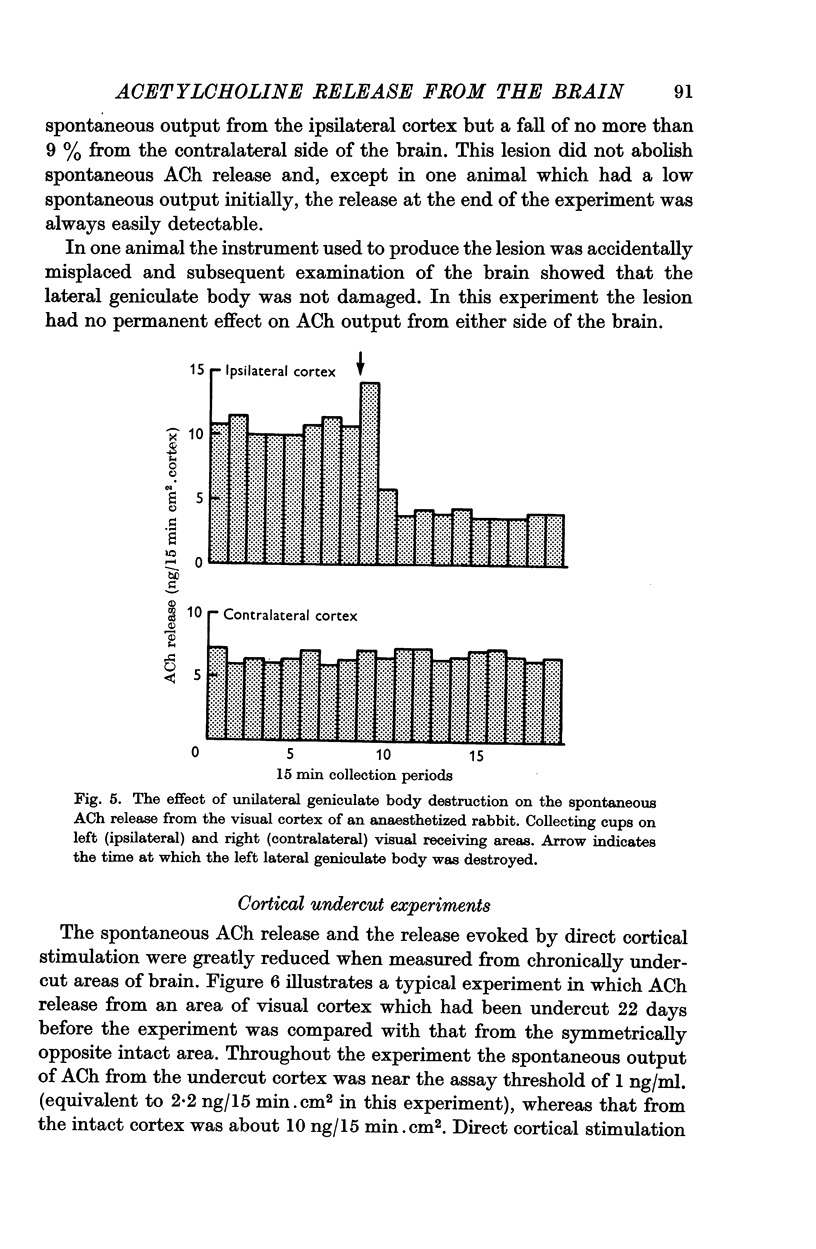
5. Unilateral destruction of the geniculate body reduced the spontaneous release of ACh from the ipsilateral cortex but did not affect the contralateral release.
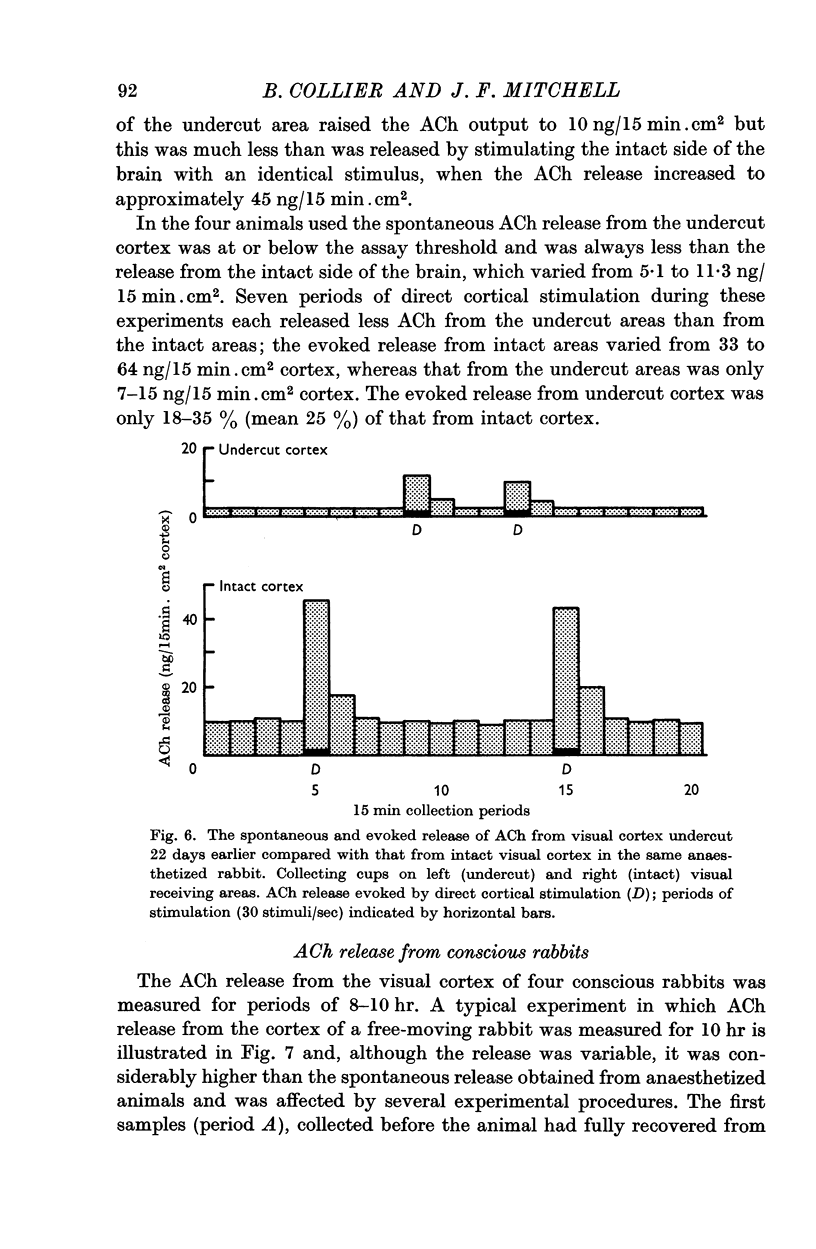
6. The spontaneous and directly evoked ACh release from chronically undercut areas of cortex was found to be considerably lower than from intact areas of cortex.
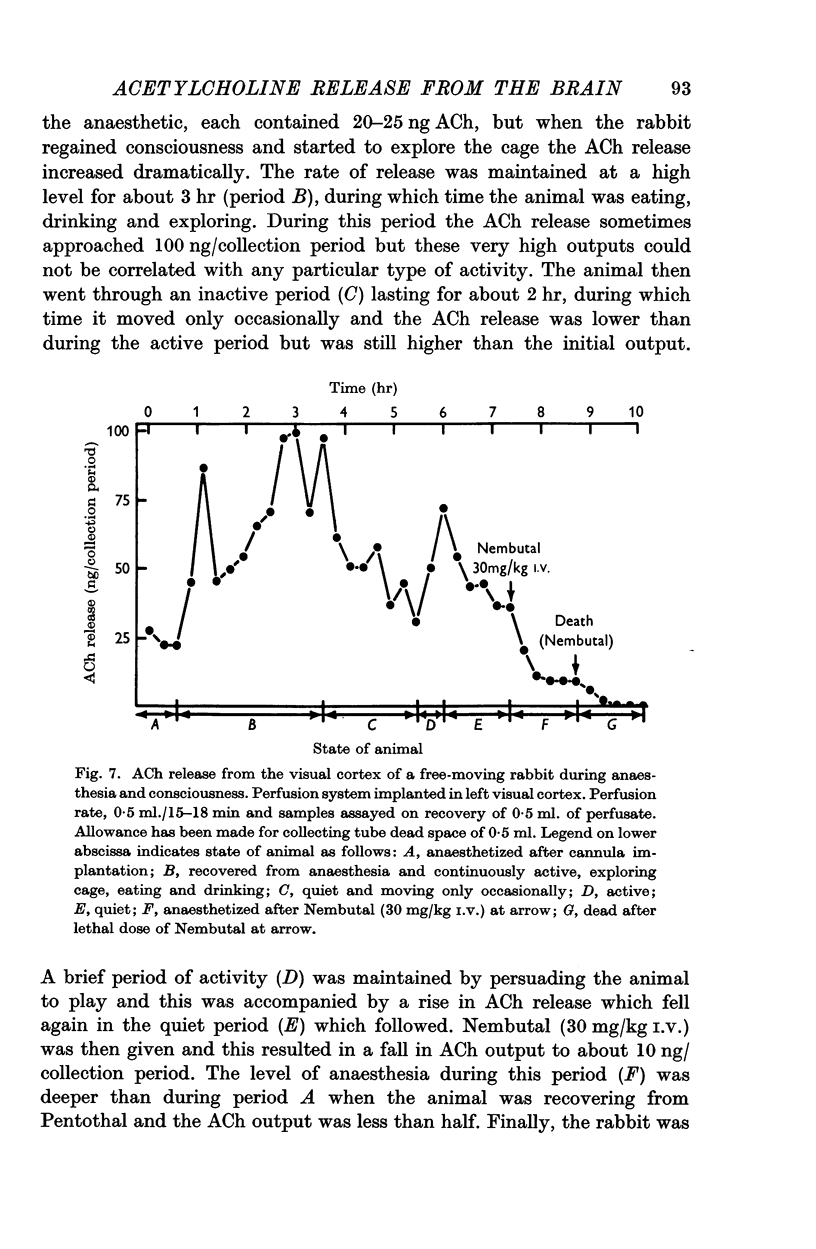
7. A high output of ACh was obtained from the visual cortex of conscious, free-moving rabbits. The rate of ACh release was closely related to the activity and state of arousal of the animals.
8. These results support an earlier suggestion that two major ascending cholinergic systems exist in the rabbit brain. One pathway is the non-specific reticulo-cortical tract responsible for cortical arousal and the other is the more specific thalamo-cortical pathway associated with augmenting and repetitive after-discharge responses. The functional significance of these two cholinergic pathways and their role in the conscious animal are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier B., Mitchell J. F. The central release of acetylcholine during stimulation of the visual pathway. J Physiol. 1966 May;184(1):239–254. doi: 10.1113/jphysiol.1966.sp007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Silver A. A histochemical study of cholinergic fibres in the cerebral cortex. J Anat. 1965 Oct;99(Pt 4):711–759. [PMC free article] [PubMed] [Google Scholar]
- MARLEY E., PATON W. D. The output of sympathetic amines from the cat's adrenal gland in response to splanchnic nerve activity. J Physiol. 1961 Jan;155:1–27. doi: 10.1113/jphysiol.1961.sp006610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. F. The spontaneous and evoked release of acetylcholine from the cerebral cortex. J Physiol. 1963 Jan;165(1):98–116. doi: 10.1113/jphysiol.1963.sp007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER C. H., EVERETT J. W., GREEN J. D. The rabbit diencephalon in stereotaxic coordinates. J Comp Neurol. 1954 Dec;101(3):801–824. doi: 10.1002/cne.901010307. [DOI] [PubMed] [Google Scholar]


