Abstract
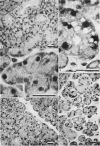
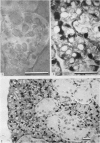
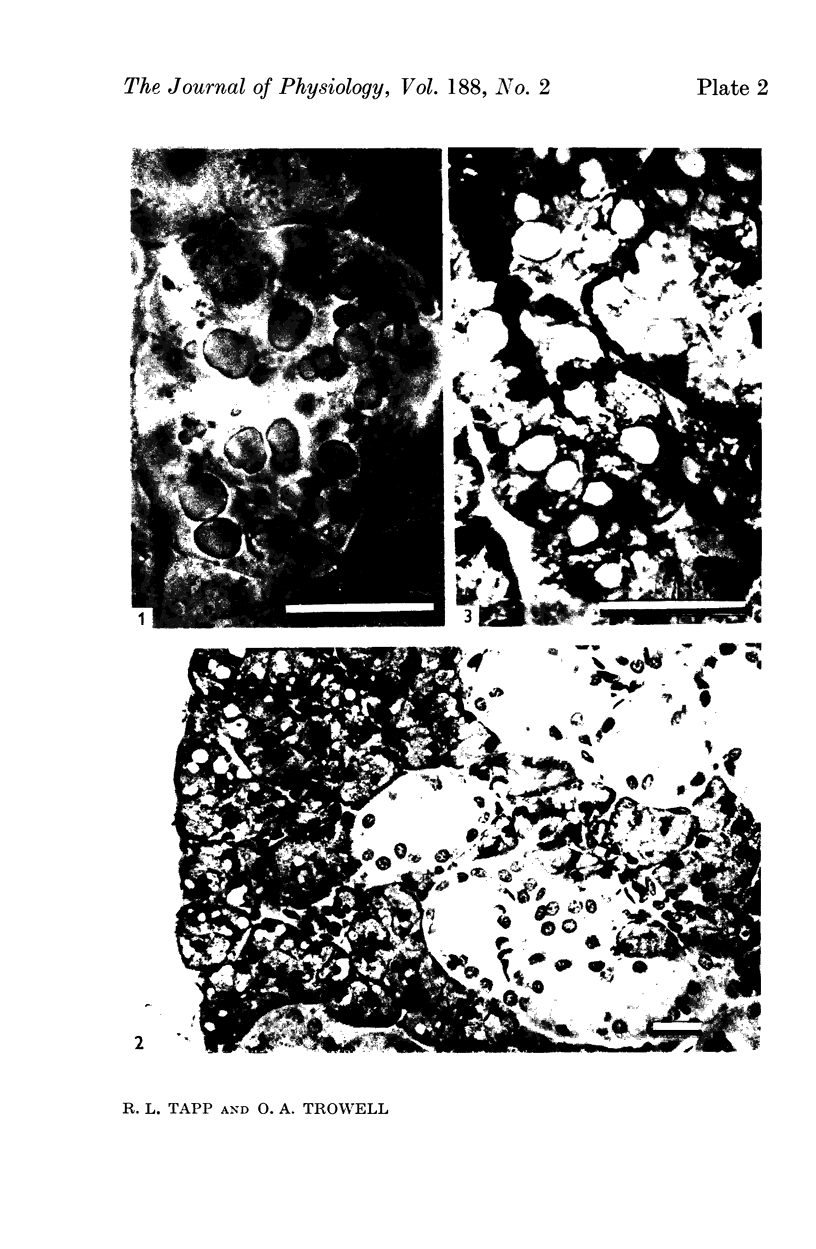
1. Watery vacuolation in the acinar cells of the rat submandibular gland is described. The vacuoles are cytoplasmic, membrane-walled, and 2-20 μ in diameter. They are visible in living cells and appear to contain a watery fluid.
2. Vacuolation occurred regularly in the following experimental situations: (1) in vitro—under anoxic conditions (2) post mortem—in animals killed by anoxia, and (3) in vivo—during secretion.
3. By in vitro experiments it was shown that vacuolation occurs only when the cells are both anoxic and exposed to an excess of extracellular fluid containing calcium and bicarbonate. It was further shown that vacuolation is reversible in oxygen and that both its development and recovery are temperature dependent.
4. Evidence is presented that the vacuolation is not a degenerative or necrotic change, that it is accompanied by the entry of fluid into the cells, and that it is not caused by simple osmosis.
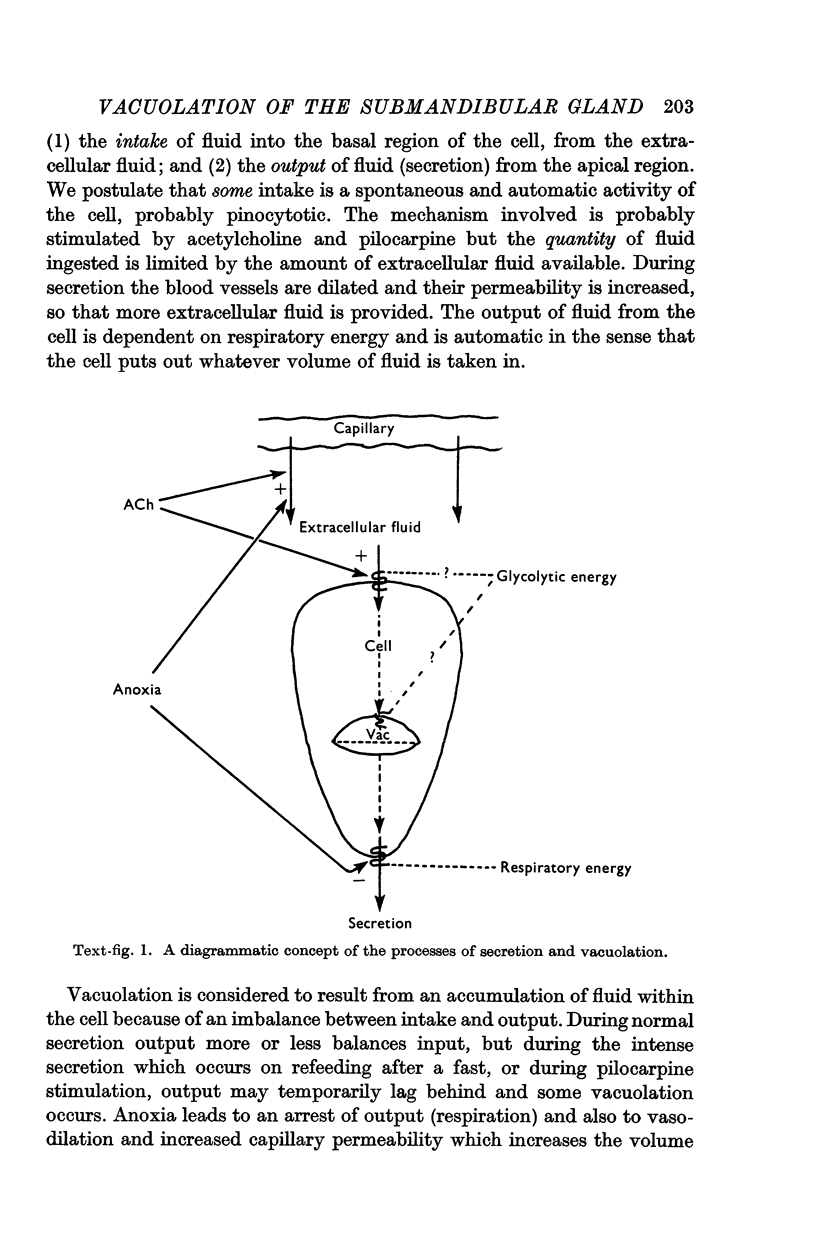
5. The mechanism of vacuolation and its possible relation to secretion are discussed. It is suggested that vacuolation represents an imbalance between the ingestion and secretion of water and salts.
6. Similar vacuoles, apparently produced by the same mechanism, were observed in the acinar cells of the parotid gland and the pancreas of the rat.
7. The close similarity of this vacuolation to that previously described in rat liver cells was noted.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRNBAUM D., HOLLANDER F. Inhibition of pancreatic secretion by the carbonic anhydrase inhibitor 2-acetylamino-1,3,4-thiadiazole-5-sulfonamide, diamox (#6063). Am J Physiol. 1953 Aug;174(2):191–195. doi: 10.1152/ajplegacy.1953.174.2.191. [DOI] [PubMed] [Google Scholar]
- Brewer D. B., Heath D. Electron microscopy of anoxic vacuolation in the liver cell and its comparison with sucrose vacuolation. J Pathol Bacteriol. 1965 Oct;90(2):437–441. doi: 10.1002/path.1700900211. [DOI] [PubMed] [Google Scholar]
- CHAUNCEY H. H., WEISS P. A. Composition of human saliva; parotid gland secretion: flow rate, pH and inorganic composition after oral administration of a carbonic anhydrase inhibitor. Arch Int Pharmacodyn Ther. 1958 Jan 1;113(3-4):377–383. [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. The influence of calcium on the secretory response of the submaxillary gland to acetylcholine or to noradrenaline. J Physiol. 1963 Mar;165(3):528–541. doi: 10.1113/jphysiol.1963.sp007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTER H. Pinocytosis. Int Rev Cytol. 1959;8:481–504. doi: 10.1016/s0074-7696(08)62738-2. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Essential absence of glucose in the foetal fluids of the sheep. Nature. 1962 Oct 27;196:379–379. doi: 10.1038/196379a0. [DOI] [PubMed] [Google Scholar]
- JACOBY F., LEESON C. R. The postnatal development of the rat submaxillary gland. J Anat. 1959 Apr;93(2):201–216. [PMC free article] [PubMed] [Google Scholar]
- OUDEA P. R. Anoxic changes of liver cells. Electron microscopic study after injection of colloidal mercury. Lab Invest. 1963 Mar;12:386–394. [PubMed] [Google Scholar]
- TROWELL O. A. A modified technique for organ culture in vitro. Exp Cell Res. 1954 Feb;6(1):246–248. doi: 10.1016/0014-4827(54)90169-x. [DOI] [PubMed] [Google Scholar]
- Trowell O. A. The experimental production of watery vacuolation of the liver. J Physiol. 1946 Dec 6;105(3):268–297. [PMC free article] [PubMed] [Google Scholar]