Abstract
1. The influence of adenosine cyclic 3′,5′-monophosphate (3′,5′-AMP) and of drugs believed to increase or decrease its concentration in the tissues has been determined on the response of vascular and uterine smooth muscles to catecholamines. Generally, drugs believed to increase tissue content of 3′,5′-AMP potentiated the responses and those believed to decrease it depressed them.
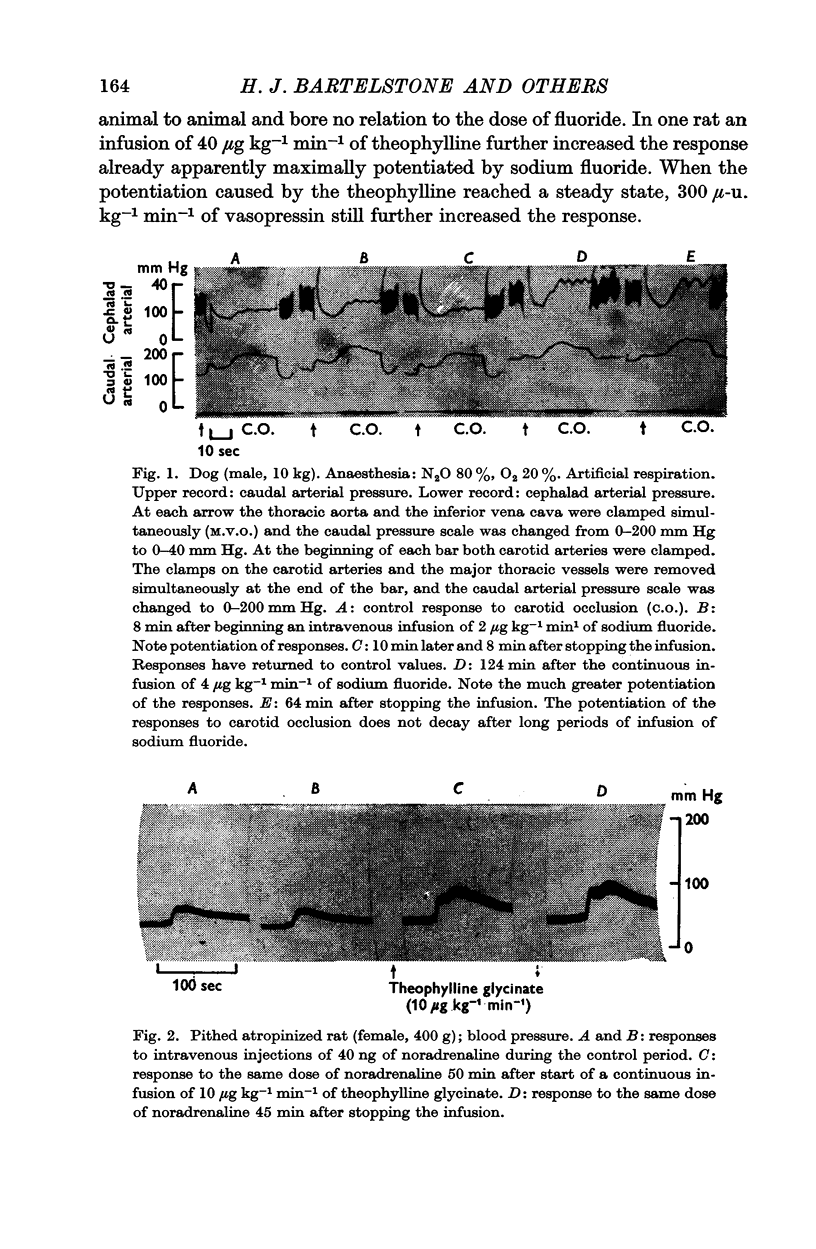
2. The cardiovascular responses of dogs (with major vessels occluded in the chest) to carotid occlusion were potentiated by infusions of theophylline and sodium fluoride. Infusion of theophylline also potentiated the response of the occluded abdominal vessels to noradrenaline.
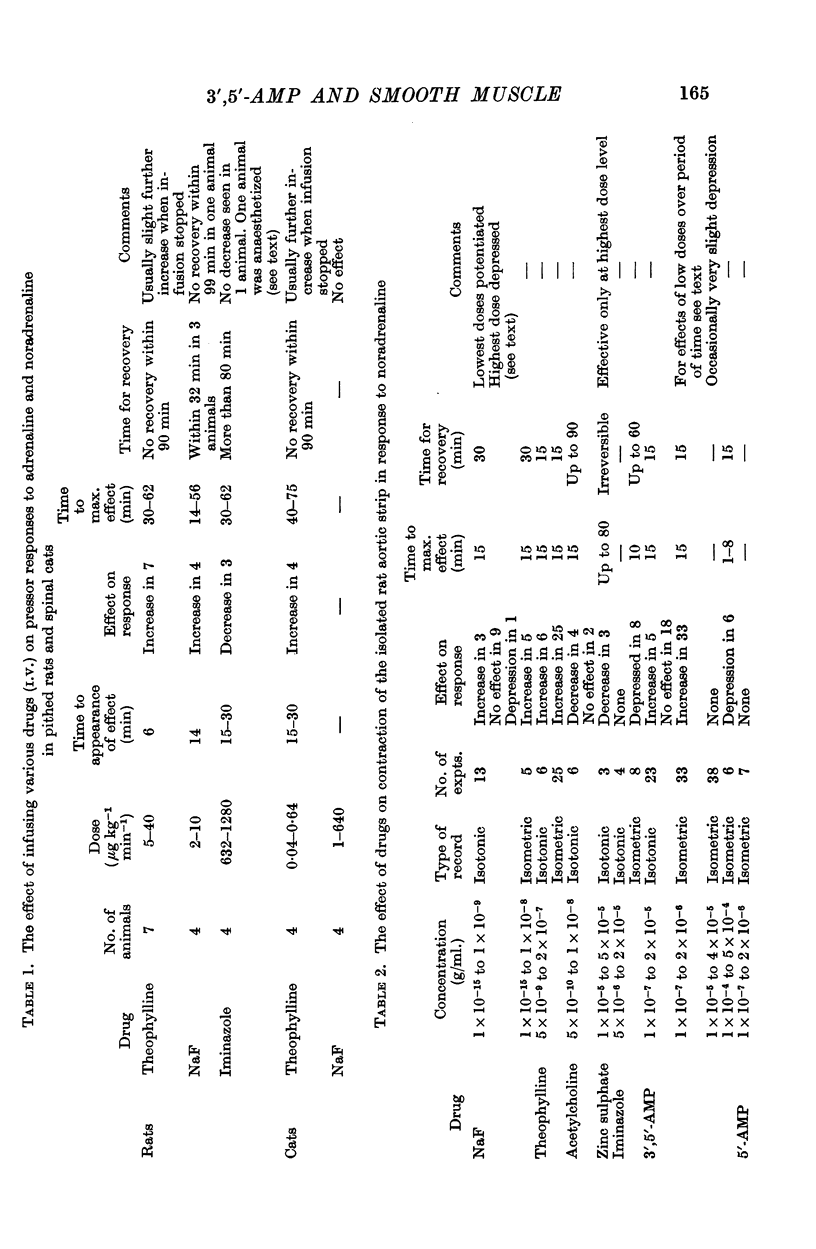
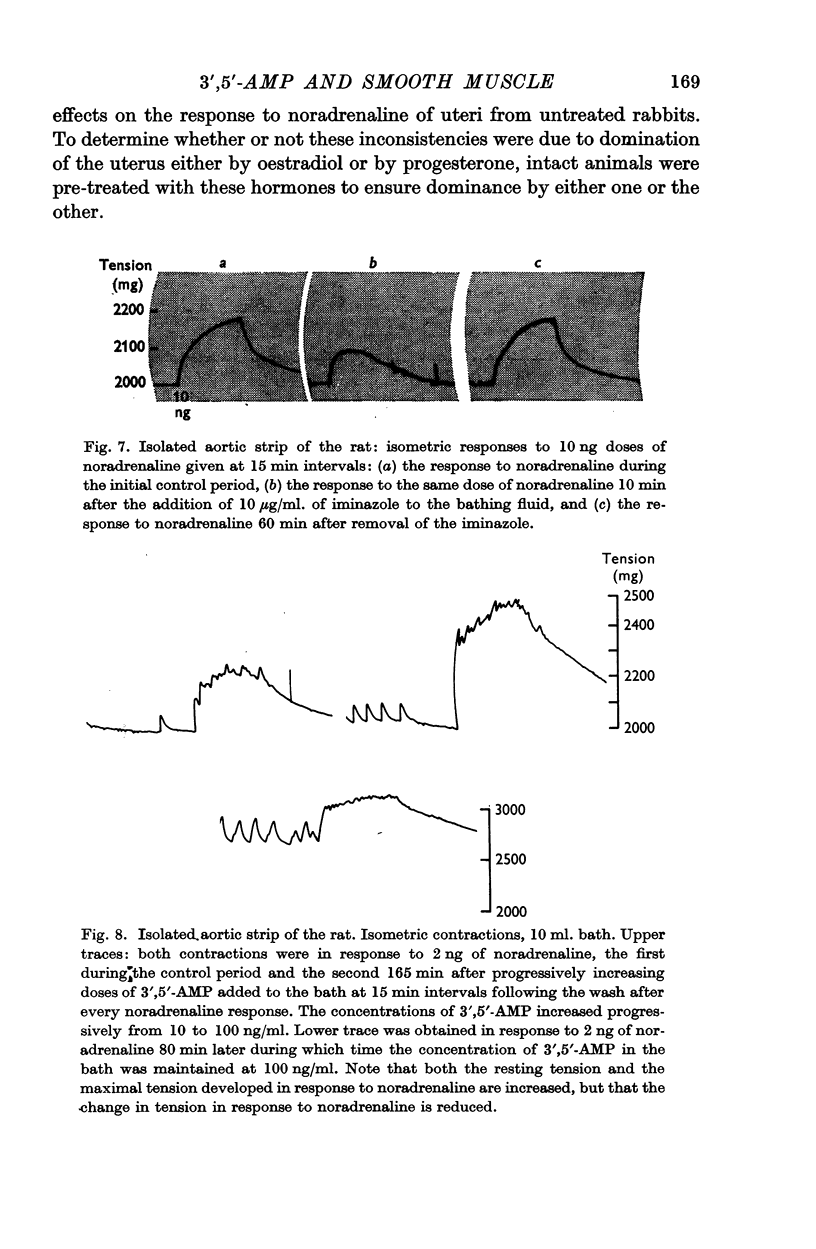
3. Intravenous infusions of theophylline and sodium fluoride potentiated pressor responses to catecholamines in the pithed rat. Infusions of iminazole depressed the responses in two animals and was without effect in two others.
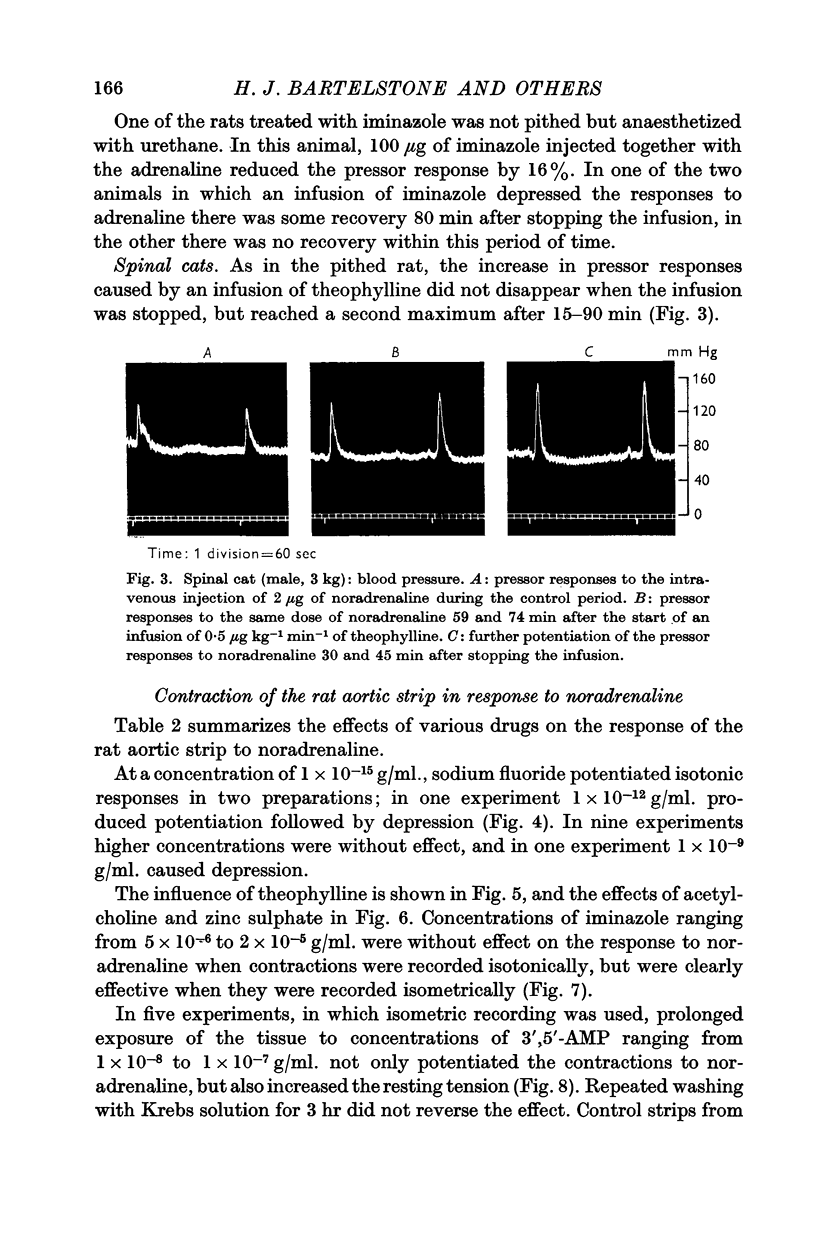
4. In spinal cats intravenous infusions of theophylline potentiated pressor responses to catecholamines, but sodium fluoride was without effect.
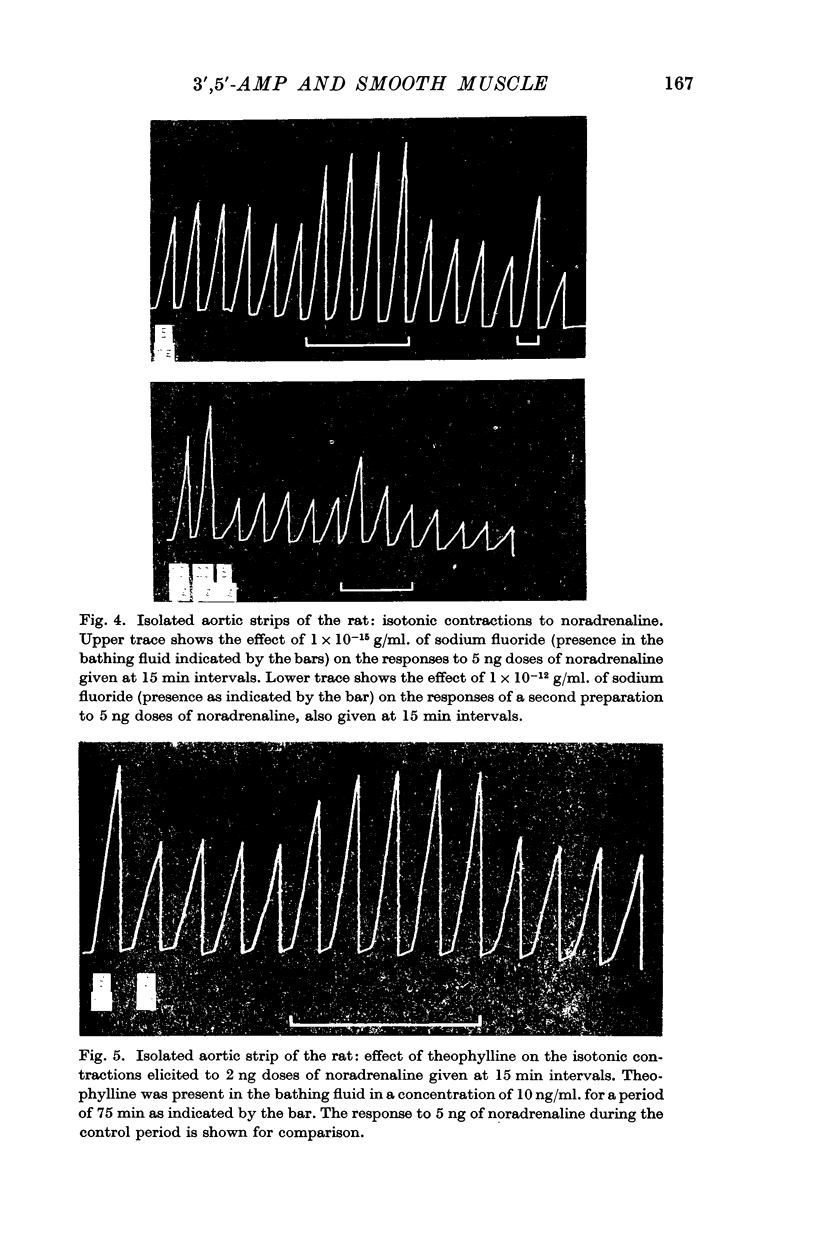
5. Contractions of the isolated rat aortic strip to noradrenaline were always potentiated by sodium fluoride and by theophylline, and depressed by iminazole, when they were recorded isometrically. Theophylline always potentiated the contractions, when they were recorded isotonically but sodium fluoride was mostly, and iminazole always, ineffective.
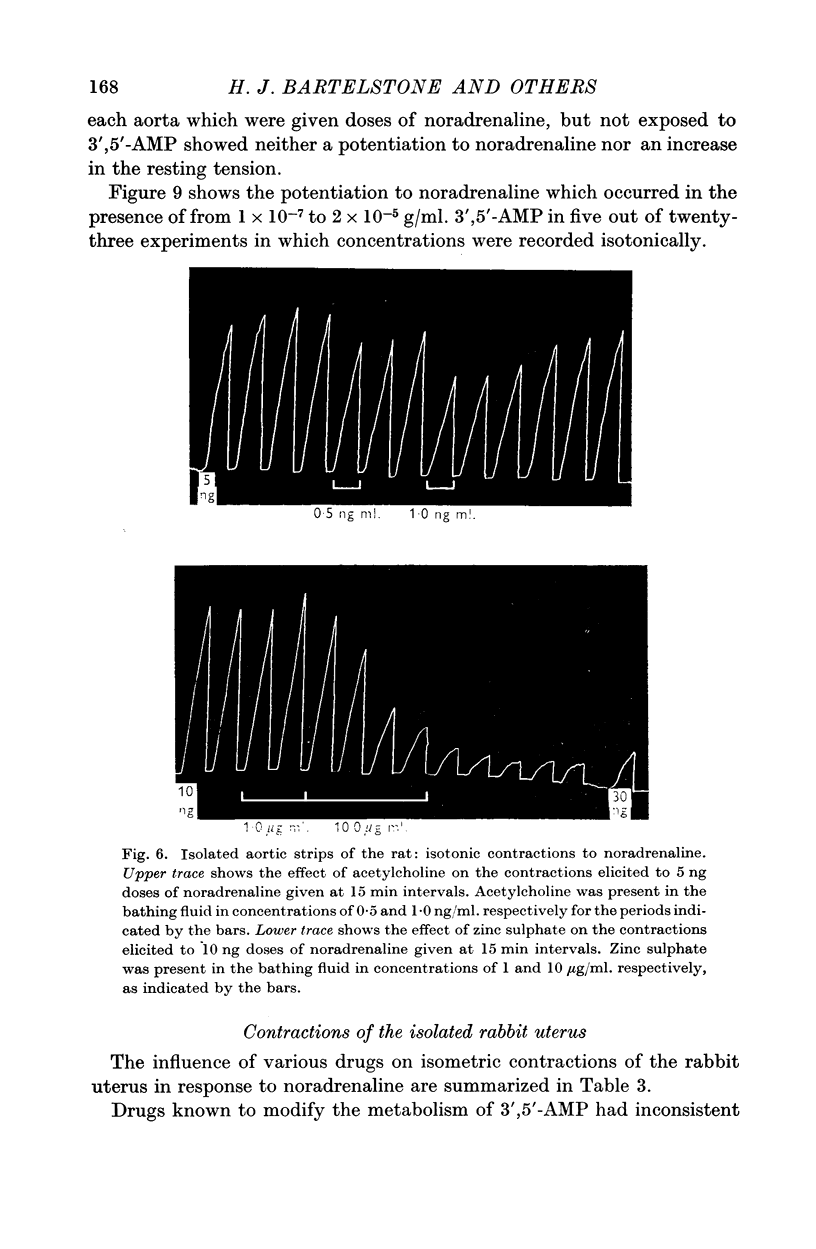
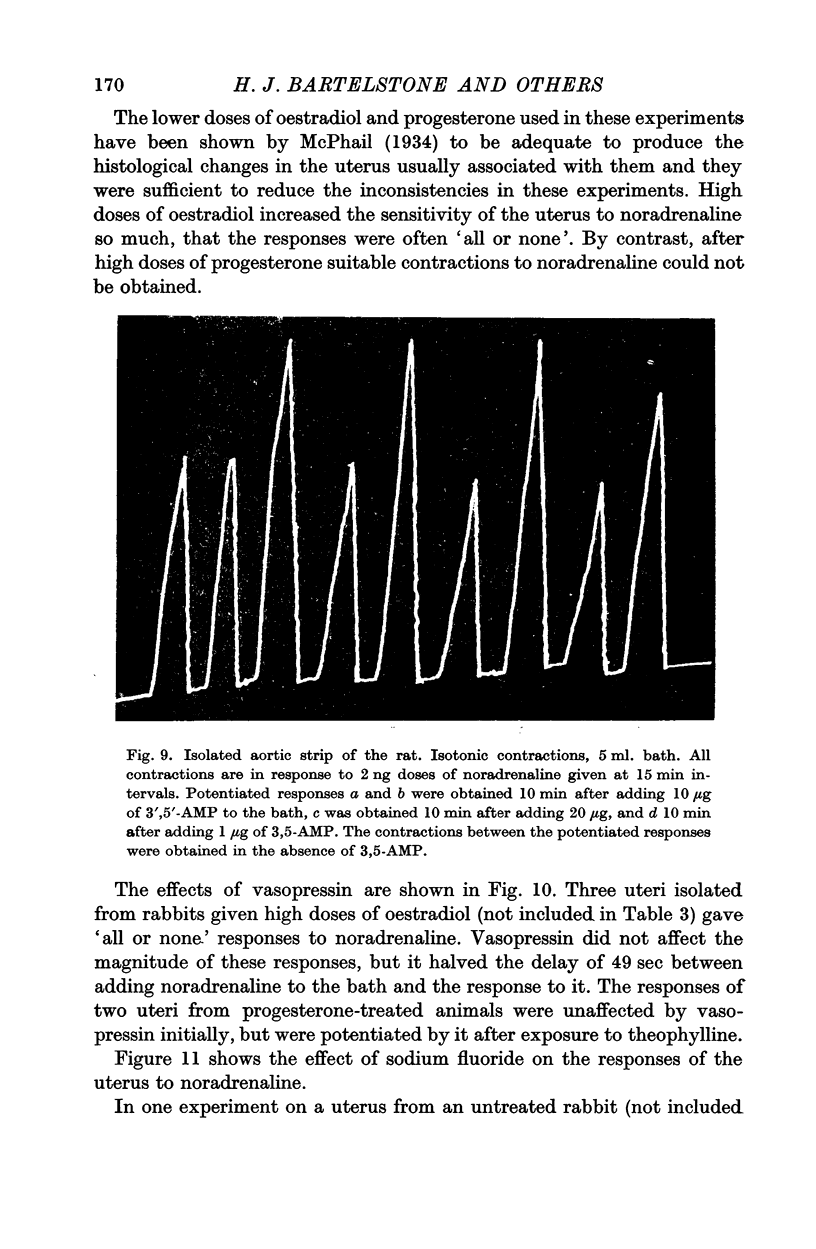
6. 3′,5′-AMP in concentrations from 0·1 to 20 μg/ml. potentiated the responses of the isolated rat aortic strip to noradrenaline in thirty-eight experiments out of hundred. Concentrations from 10 to 500 μg/ml. sometimes depressed contractions recorded isometrically. In five experiments, exposure to low concentrations for 3 hr increased the resting tension of the preparation.
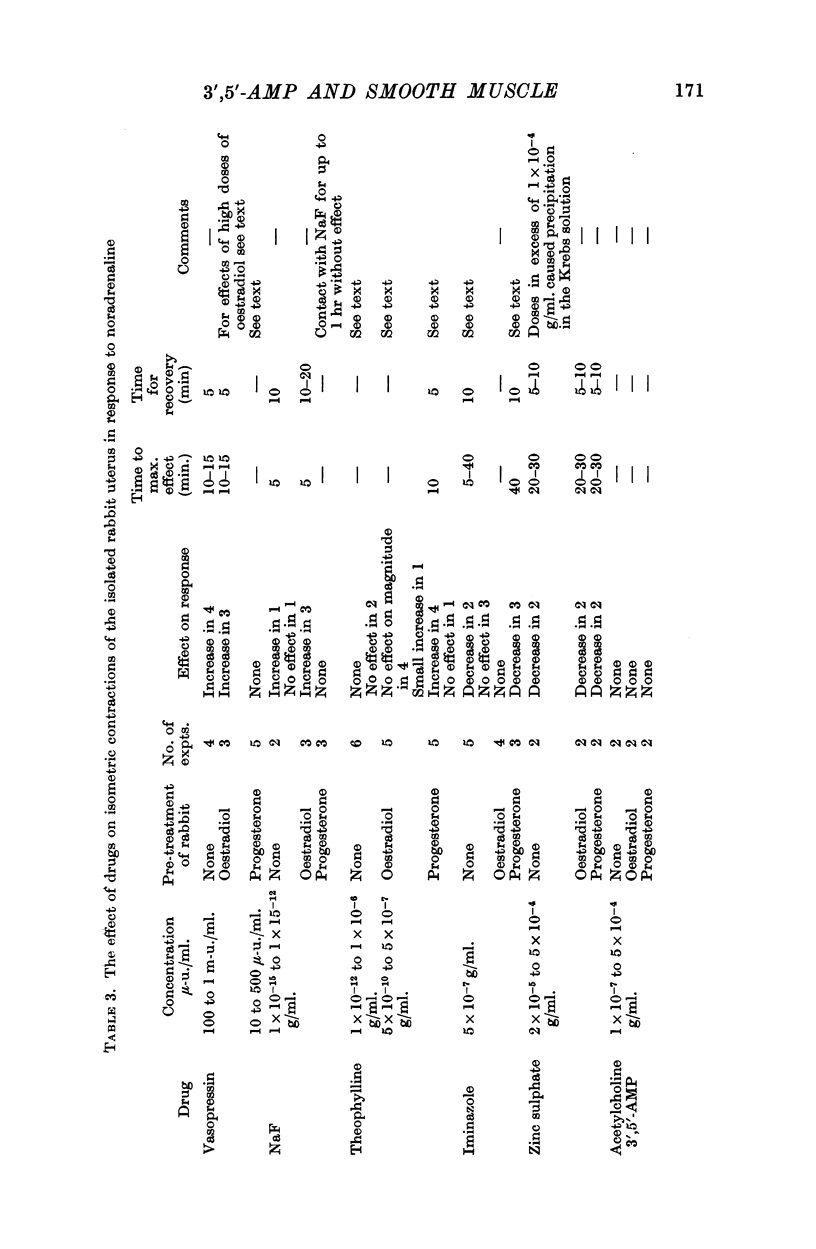
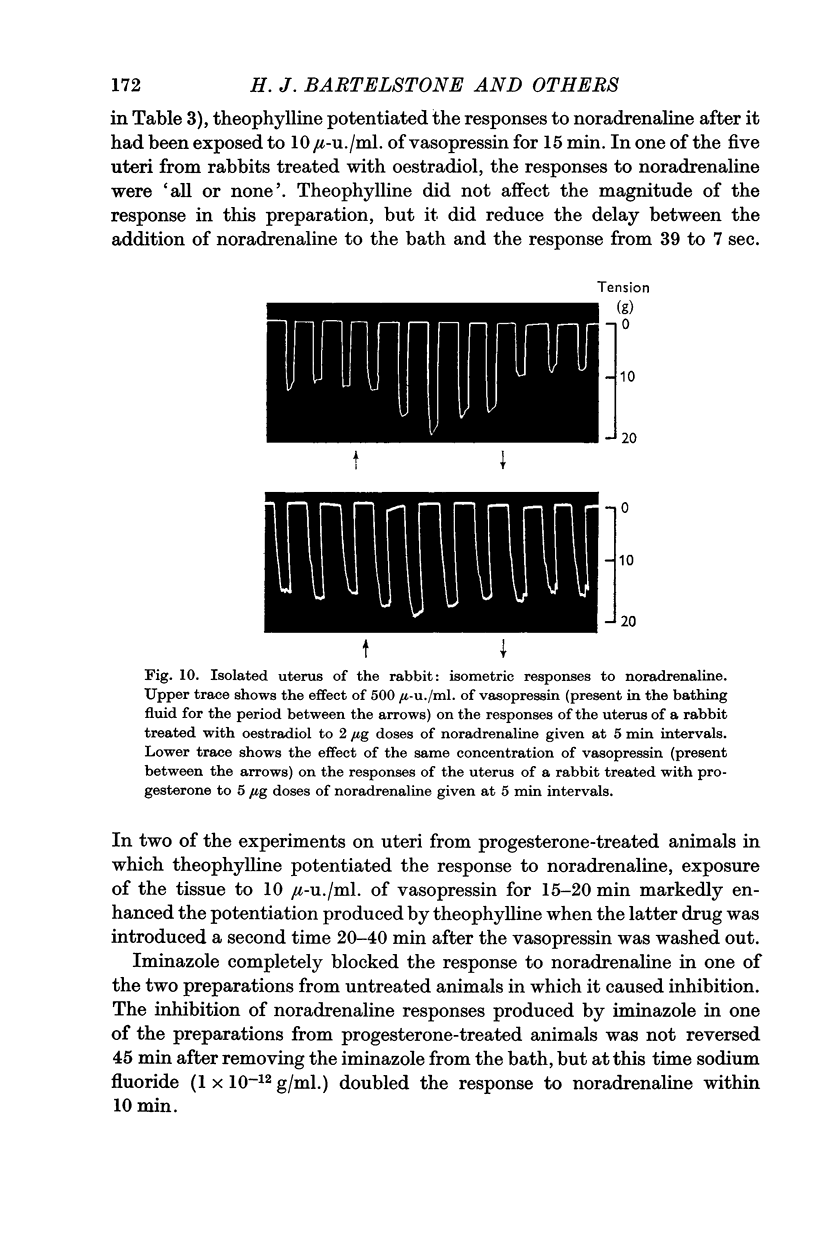
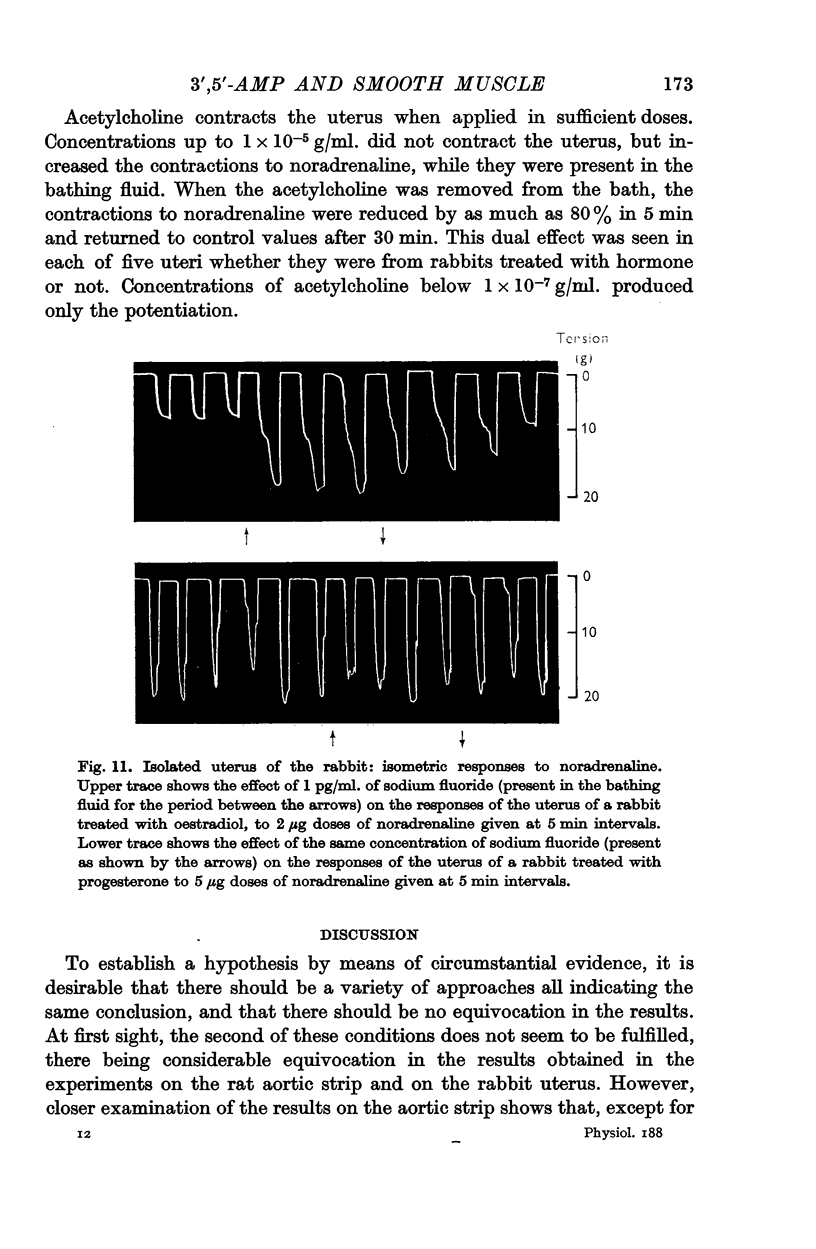
7. Responses to noradrenaline of uteri from oestradiol-treated rabbits were potentiated by vasopressin and by sodium fluoride, but not by theophylline or iminazole. In progesterone-treated animals the responses were unaffected by vasopressin and sodium fluoride, but potentiated by theophylline and depressed by iminazole. 3′,5′-AMP was without effect on the uterine responses.
8. It is concluded that the results support the view that an increase in the tissue content of 3′,5′-AMP potentiates the contraction of vascular and uterine smooth muscle in response to catecholamine. This view is supported by the observation that the nucleotide itself can potentiate the responses of the rat aortic strip to noradrenaline.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTELSTONE H. J., NASMYTH P. A. VASOPRESSIN POTENTIATION OF CATECHOLAMINE ACTIONS IN DOG, RAT, CAT, AND RAT AORTIC STRIP. Am J Physiol. 1965 Apr;208:754–762. doi: 10.1152/ajplegacy.1965.208.4.754. [DOI] [PubMed] [Google Scholar]
- BARTELSTONE H. J. Role of the veins in venous retunr. Circ Res. 1960 Sep;8:1059–1076. doi: 10.1161/01.res.8.5.1059. [DOI] [PubMed] [Google Scholar]
- BROWN E., CLARKE D. L., ROUX V., SHERMAN G. H. The stimulation of adenosine 3,5-monophosphate production by antidiuretic factors. J Biol Chem. 1963 Feb;238:852–853. [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- CSAPO A. Dependence of isometric tension and isotonic shortening of uterine muscle on temperature and on strength of stimulation. Am J Physiol. 1954 Jun;177(3):348–354. doi: 10.1152/ajplegacy.1954.177.3.348. [DOI] [PubMed] [Google Scholar]
- MURAD F., CHI Y. M., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3',5'-phosphate by preparations from cardiac muscle and liver. J Biol Chem. 1962 Apr;237:1233–1238. [PubMed] [Google Scholar]
- McPhail M. K. The assay of progestin. J Physiol. 1934 Dec 31;83(2):145–156. doi: 10.1113/jphysiol.1934.sp003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. Adenyl cyclase. II. The enzymatically catalyzed formation of adenosine 3',5'-phosphate and inorganic pyrophosphate from adenosine triphosphate. J Biol Chem. 1962 Apr;237:1228–1232. [PubMed] [Google Scholar]
- RALL T. W., WEST T. C. The potentiation of cardiac inotropic responses to norepinephrine by theophylline. J Pharmacol Exp Ther. 1963 Mar;139:269–274. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]