Abstract
1. Heterosynaptic facilitation was defined as an increase of amplitude of a test excitatory post-synaptic potential (EPSP) after the activation of a pathway (heterosynaptic pathway) different from that which produced the test EPSP. This phenomenon has been studied in Aplysia central nervous system under conditions which excluded the participation of post-tetanic potentiation.
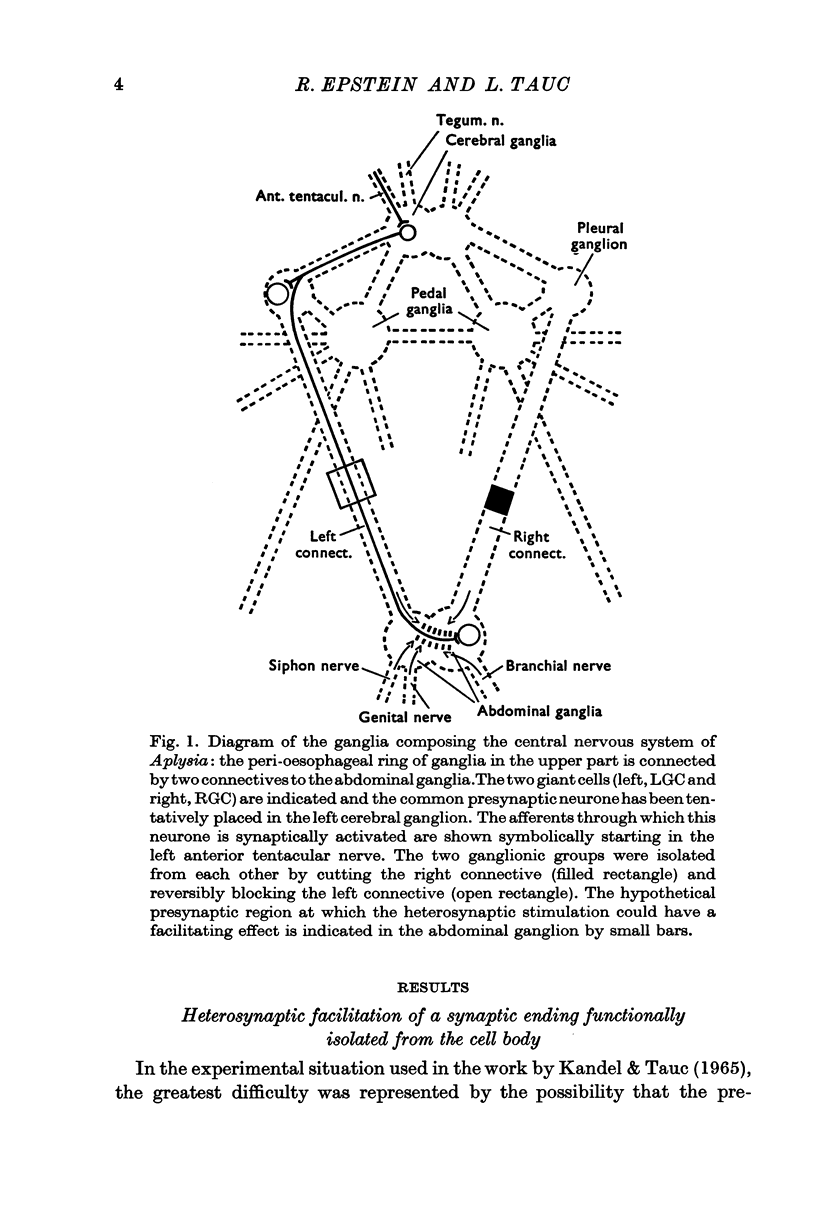
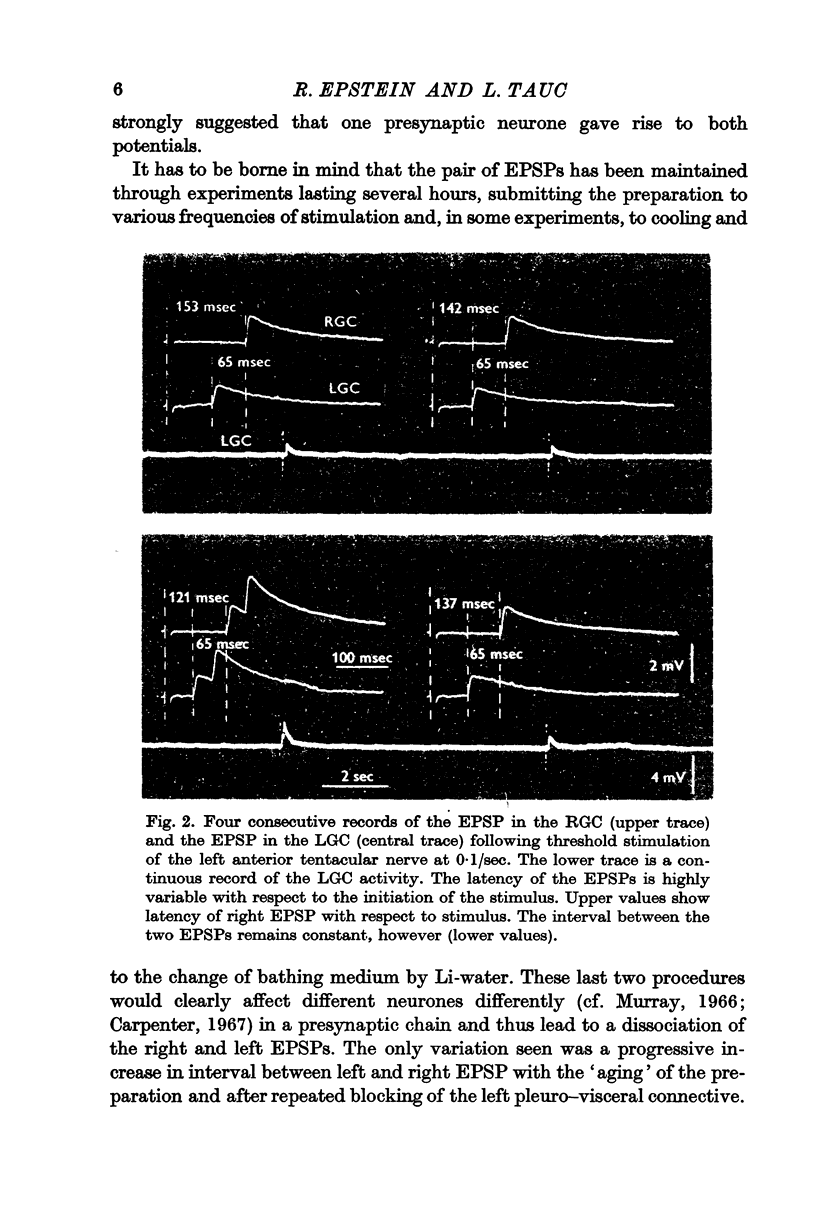
2. A unitary test was produced in the left and right giant cells, by indirect stimulation of an interneurone located in the peri-oesophageal ring.
3. During heterosynaptic stimulation, orthodromic and antidromic activation of the test interneurone was prevented by (1) isolating the synaptic afferent region of the test interneurone from the tested synapse on the right giant cell by a sucrose block applied on the left pleurovisceral connective, and (2) using physiological stimulation of a piece of skin as a heterosynaptic stimulus.
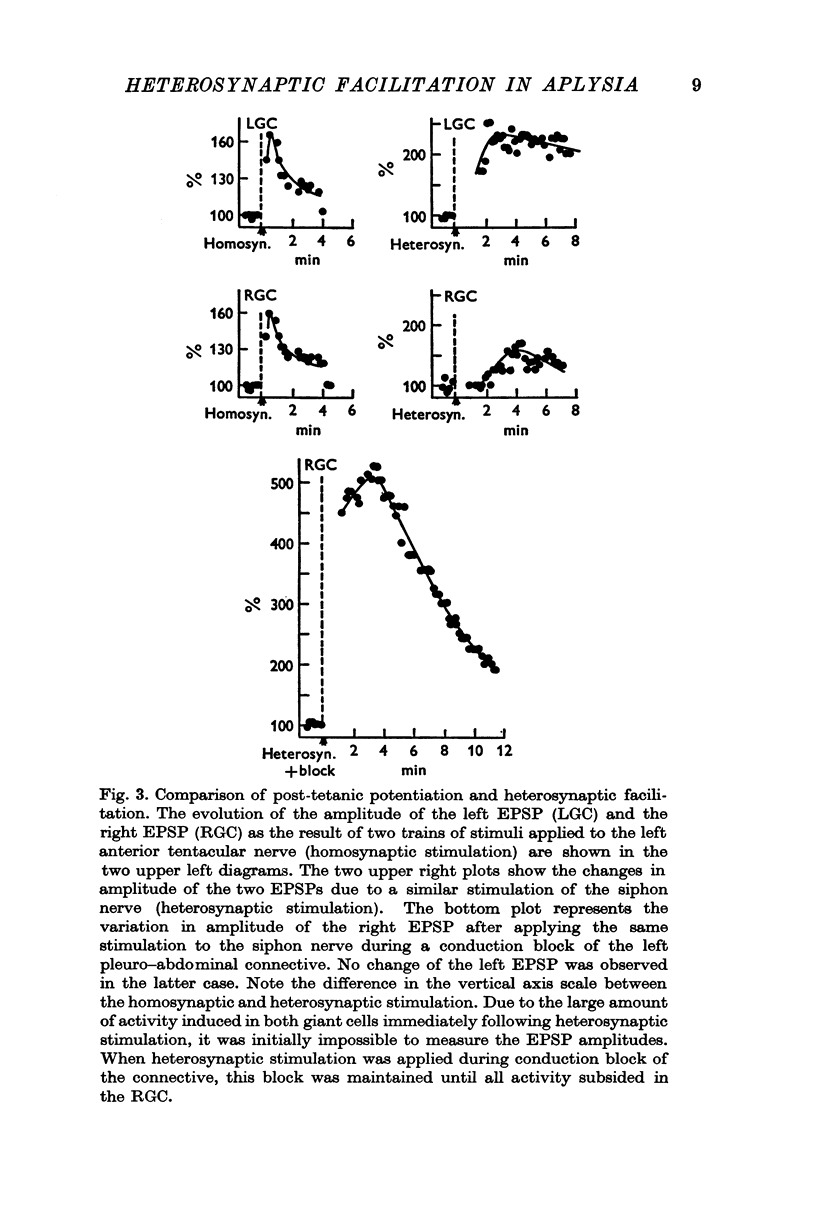
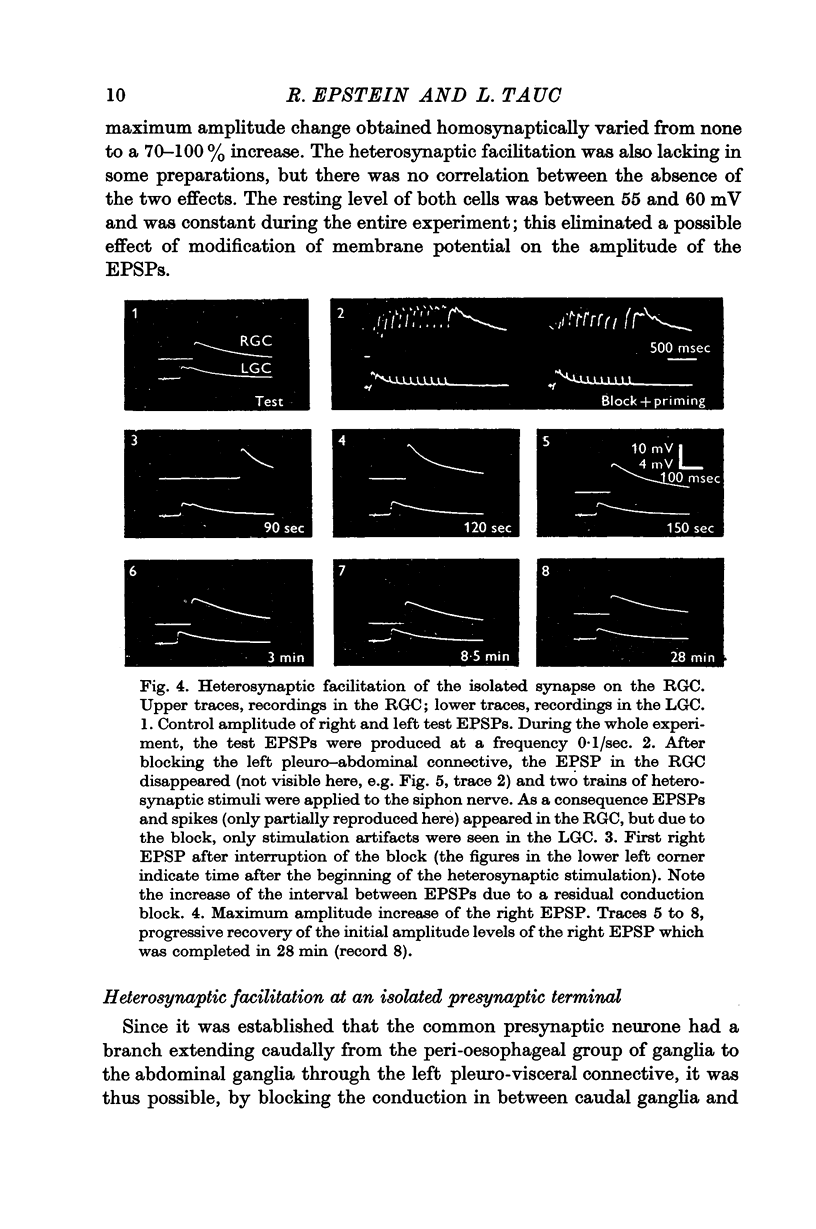
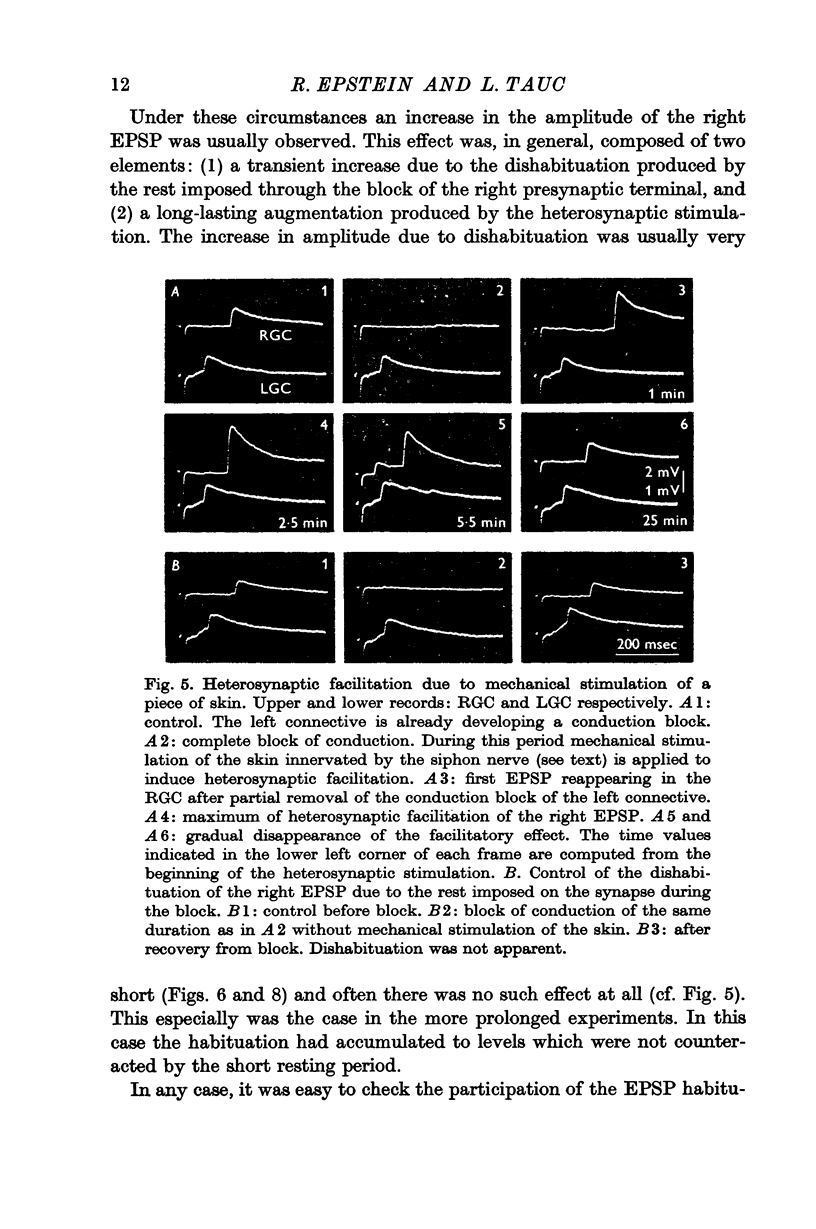
Under these conditions which prevented any firing in the test interneurone, heterosynaptic facilitation is observed as a 200% increase of amplitude of the test EPSP in the right cell which lasted more than 15 min.
When instead of the physiological stimulus a supramaximal electrical stimulation of the nerves afferent to the abdominal ganglion was used, the increase of amplitude of the test EPSP could reach as much as 500% of its original amplitude. The effectiveness of such heterosynaptic stimulus was smaller when it was applied in the absence of a block of the left pleuro-visceral connective.
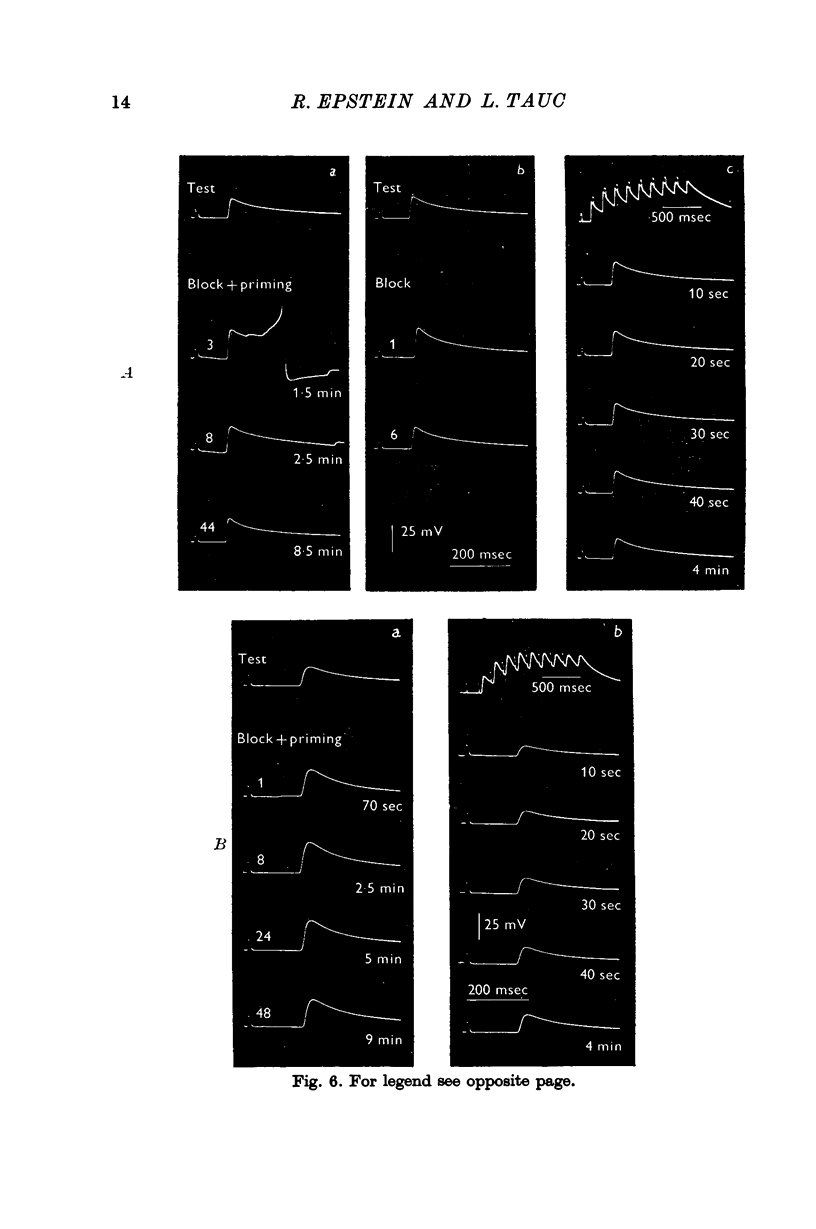
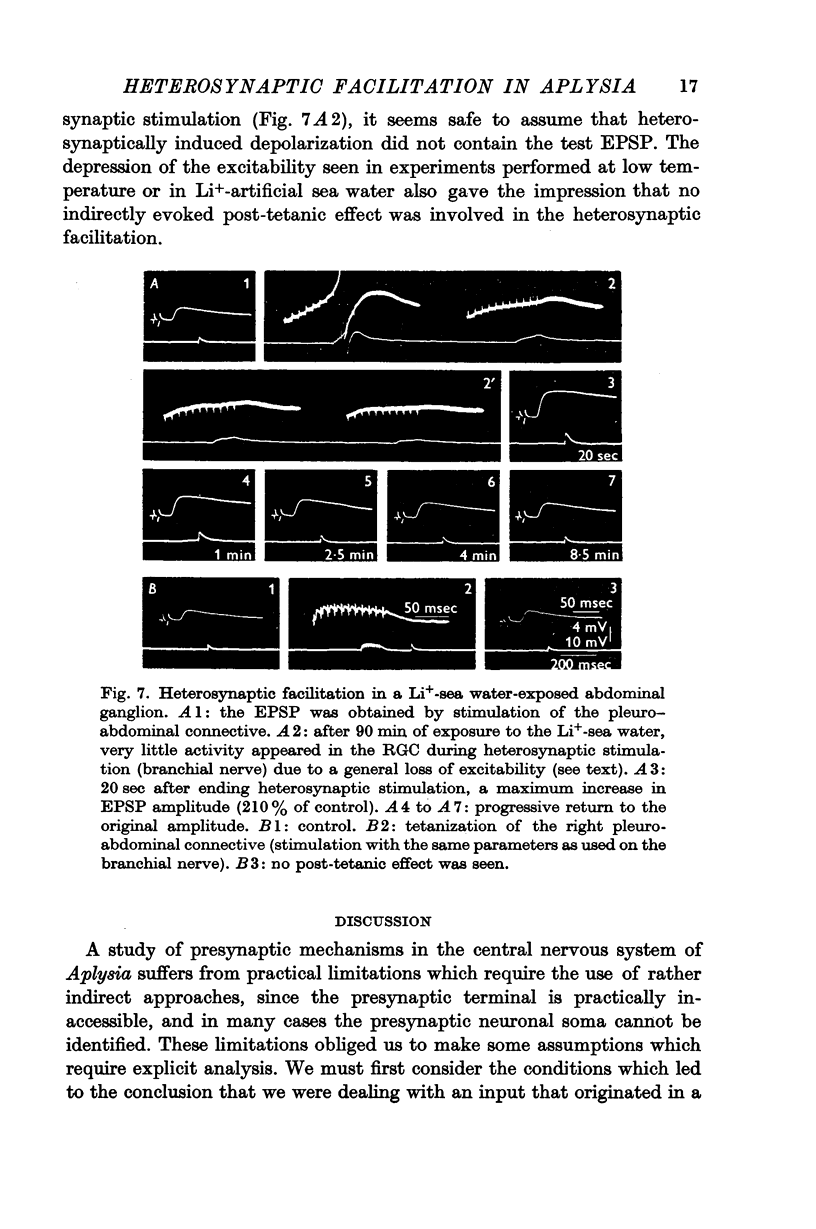
4. It was possible to produce heterosynaptic facilitation when the preparation was cooled to 7-9° C or if Li+ replaced Na+ in the medium. Both of these changes suppressed post-tetanic potentiation.
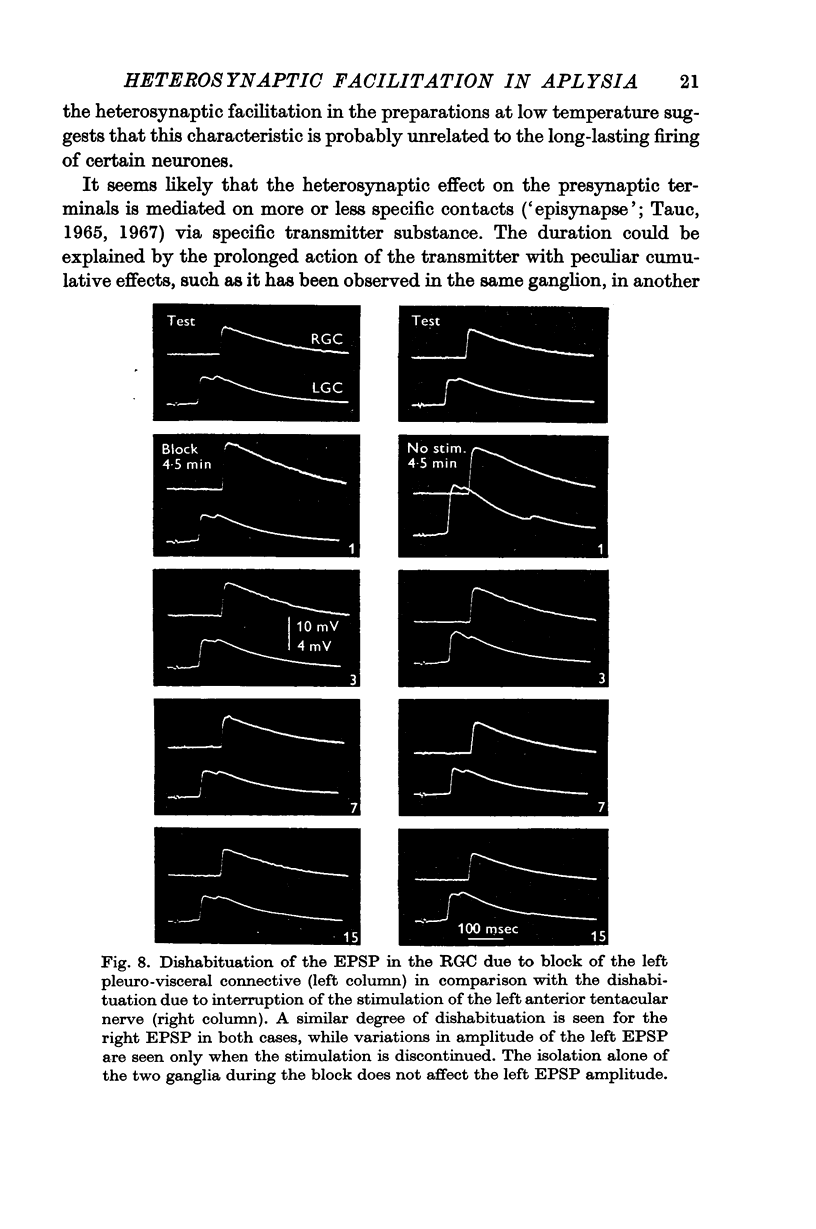
5. It was concluded that heterosynaptic facilitation is a phenomenon different from post-tetanic potentiation. Heterosynaptic facilitation is similar to heterosynaptic inhibition seen in other cells in the same preparation, except for the polarity of action. Both phenomena seem to result from comparable mechanisms, probably acting on the quantity of transmitter released.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruner J., Tauc L. Habituation at the synaptic level in Aplysia. Nature. 1966 Apr 2;210(5031):37–39. doi: 10.1038/210037a0. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O. Temperature effects on pacemaker generation, membrane potential, and critical firing threshold in Aplysia neurons. J Gen Physiol. 1967 Jul;50(6):1469–1484. doi: 10.1085/jgp.50.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J. FACILITATORY EFFECTS OF 5-HYDROXY-TRYPTAMINE ON THE CRAYFISH NEUROMUSCULAR JUNCTION. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1965 Jan 8;249:515–528. doi: 10.1007/BF00246558. [DOI] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958 Dec 30;144(3):442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Metabolic studies on the hyperpolarization following activity in mammalian non-myelinated nerve fibres. J Physiol. 1962 May;161:414–423. doi: 10.1113/jphysiol.1962.sp006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. An investigation of the post-tetanic potentiation of end-plate potentials at a mammalian neuromuscular junction. J Physiol. 1966 May;184(2):353–375. doi: 10.1113/jphysiol.1966.sp007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol. 1966 May;184(2):335–352. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES O. Effects of pH, changes in potassium concentration and metabolic inhibitors on the after-potentials of mammalian non-medullated nerve fibres. Arch Int Physiol Biochim. 1962 Mar;70:211–245. doi: 10.3109/13813456209092855. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I., GAGE P. W. ABOLITION OF POST-TETANIC POTENTIATION. Nature. 1964 Apr 18;202:299–300. doi: 10.1038/202299a0. [DOI] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. AN ELECTROPHYSIOLOGICAL STUDY OF THE ANATOMICAL RELATIONS OF TWO GIANT NERVE CELLS IN APLYSIA DEPILANS. J Exp Biol. 1963 Sep;40:469–486. doi: 10.1242/jeb.40.3.469. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Mechanism of heterosynaptic facilitation in the giant cell of the abdominal ganglion of Aplysia depilans. J Physiol. 1965 Nov;181(1):28–47. doi: 10.1113/jphysiol.1965.sp007743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELL L. M., WALL P. D. PRESYNAPTIC HYPERPOLARIZATION: A ROLE FOR FINE AFFERENT FIBRES. J Physiol. 1964 Aug;172:274–294. doi: 10.1113/jphysiol.1964.sp007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. W. The effect of temperature on the membrane properties of neurons in the visceral ganglion of Aplysia. Comp Biochem Physiol. 1966 Jun;18(2):291–303. doi: 10.1016/0010-406x(66)90188-5. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- TAUC L., HUGHES G. M. Modes of initiation and propagation of spikes in the branching axons of molluscan central neurons. J Gen Physiol. 1963 Jan;46:533–549. doi: 10.1085/jgp.46.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L. Site of origin and propagation in spike in the giant neuron of Aplysia. J Gen Physiol. 1962 Jul;45:1077–1097. doi: 10.1085/jgp.45.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauc L., Epstein R. Heterosynaptic facilitation as a distinct mechanism in aplysia. Nature. 1967 May 13;214(5089):724–725. doi: 10.1038/214724a0. [DOI] [PubMed] [Google Scholar]
- Tauc L. Presynaptic inhibition in the abdominal ganglion of Aplysia. J Physiol. 1965 Nov;181(2):282–307. doi: 10.1113/jphysiol.1965.sp007761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauc L. Transmission in invertebrate and vertebrate ganglia. Physiol Rev. 1967 Jul;47(3):521–593. doi: 10.1152/physrev.1967.47.3.521. [DOI] [PubMed] [Google Scholar]


