Abstract
The temporal and spatial behavior of a number of mutants of the photosynthetic, facultative anaerobe Rhodobacter sphaeroides to both step changes and to gradients of oxygen was analyzed. Wild-type cells, grown under a range of conditions, showed microaerophilic behavior, accumulating in a 1.3-mm band about 1.3 mm from the meniscus of capillaries. Evidence suggests this is the result of two signaling pathways. The strength of any response depended on the growth and incubation conditions. Deletion of either the complete chemosensory operons 1 and 2 plus the response regulator genes cheY4 and cheY5 or cheA2 alone led to the loss of all aerotactic responses, although the cells still swam normally. The Prr system of R. sphaeroides responds to electron flow through the alternative high-affinity cytochrome oxidase, cbb3, controlling expression of a wide range of metabolic pathways. Mutants with deletions of either the complete Prr operon or the histidine kinase, PrrB, accumulated up to the meniscus but still formed a thick band 1.3 mm from the aerobic interface. This indicates that the negative aerotactic response to high oxygen levels depends on PrrB, but the mutant cells still retain the positive response. Tethered PrrB− cells also showed no response to a step-down in oxygen concentration, although those with deletions of the whole operon showed some response. In gradients of oxygen where the concentration was reduced at 0.4 μM/s, tethered wild-type cells showed two different phases of response, with an increase in stopping frequency when the oxygen concentration fell from 80 to 50% dissolved oxygen and a decrease in stopping at 50 to 20% dissolved oxygen, with cells returning to their normal stopping frequency in 0% oxygen. PrrB and CheA2 mutants showed no response, while PrrCBA mutants still showed some response.
Tactic responses are a common feature of motile bacteria. A sensory system converts environmental signals into a change in the direction of rotation of the flagellar motor, resulting in the accumulation of microbial populations in microenvironments optimal for growth (1).
Taxis towards oxygen (aerotaxis) has been identified in several bacterial species (45). It allows bacteria to migrate to concentrations of dissolved oxygen ideal for their current metabolism. In many species investigated, an active respiratory chain and Che proteins, such as CheA, CheW, and CheY (20, 21, 35), are essential for responses. Recent reports have identified a methyl-accepting chemotaxis protein (MCP)-like oxygen receptor, Aer, in Escherichia coli (2, 31) and Pseudomonas putida (26), whereas myoglobin-like aerotaxis transducers have been identified in Halobacterium salinarum and Bacillus subtilis (16) and putative oxygen transducers with a c-type heme-sensing domain have been identified in other species (4, 8), suggesting that several independent systems have evolved to control tactic behavior in response to changes in oxygen level.
Rhodobacter sphaeroides is a facultative non-sulfur-photosynthetic bacterium belonging to the α-subgroup of the Proteobacteria. It has an extremely versatile metabolism, being able to grow photoheterotrophically and chemoheterotrophically to ferment and utilize a wide range of organic acids for growth and to fix nitrogen and CO2. It swims using a single, subpolar flagellum that rotates clockwise, stopping periodically. Stops are the equivalent of tumbles in E. coli, reorienting the cell for the next period of smooth swimming. The R. sphaeroides sensory system is very complex, with several copies of che genes organized in multiple operons and up to 13 mcp-like genes scattered throughout the genome (23). Membrane-bound and cytoplasmic receptors are induced under different growth conditions (15). The expression of the two major operons is environmentally regulated (38) and growth phase dependent (S. Romagnoli and J. P. Armitage, unpublished data). The dominant stimuli depend on the growth conditions, and transport and partial metabolism of the attractant are required (17). The second chemosensory operon is responsible for wild-type responses under laboratory conditions (13), although a complex balance of che products is likely to occur to fine-tune chemotactic responses under different growth conditions (38). Responses to redox stimuli, such as light or the terminal electron acceptors oxygen or dimethyl sulfoxide, depend on the relative activities of the different branches of the electron transport chains (10), and the signal is fed into the second chemosensory pathway (34).
Although several strategies have been tried to find a homologue of the Aer protein in R. sphaeroides, none has been identified and the recent sequencing of the R. sphaeroides genome has not revealed any obvious aer homologues (http://www.rhodobacter.org). The difficulty in identifying an aerotactic receptor in R. sphaeroides, together with its complex sensory system and extremely versatile metabolism, encouraged us to consider other well-characterized mechanisms involved in redox sensing in R. sphaeroides and examine their possible role in motility. Since recent reports showed the interaction, in vivo and in vitro, of ArcB, the E. coli anaerobic sensor kinase, and CheY, the chemosensory response regulator (47, 19), we examined the role of the Prr system in aerotaxis. Prr is the analogue of the E. coli Arc system, and its activities as a membrane-bound sensor kinase, PrrB, and transcriptional response regulator, PrrA, are well documented in Rhodobacter species (3, 6, 24, 25, 42,). PrrB is a membrane-bound histidine protein kinase which responds, probably via another membrane protein, PrrC, to a change in the rate of electron flow through the high-affinity, constitutive alternative cytochrome oxidase, cbb3. PrrB controls the activity of the transcriptional activator PrrA. PrrA functions as a global anaerobic regulator for the induction of the photosynthetic genes (6, 7, 24, 28, 29) as well as genes involved in carbon dioxide fixation and nitrogen assimilation (18) and genes coding for components of the respiratory electron transport chain (44). Moreover, the sensor and response regulator functional domain show significant homology with the CheA-CheY couple (11, 46), pointing to a possible cross talk between sensory systems.
In this work, we examined the aerotactic behavior of Prr and Che mutants under three sets of conditions: large step changes in oxygen concentration, naturally generated oxygen gradients in capillaries, and reducing oxygen gradients in flow cells. We identified two aerotactic systems, both signaling through the chemosensory system: a positive signal which remains to be characterized and a negative PrrB-dependent system.
MATERIALS AND METHODS
Strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Subscript numbers on che genes indicate different che homologues (38).
TABLE 1.
Bacterial strains and plasmids used in this study
| Name | Genotype | Reference |
|---|---|---|
| E. coli strains | ||
| DH5α | Nalr Δ(lacZYA-argF). General cloning strain | 14 |
| S17-1 λpir | Conjugative strain containing λpir | 39 |
| R. sphaeroides strains | ||
| WS8N | Wild type strain, Nalr | 41 |
| JPA211 | ΔcheA2 | 13 |
| JPA420 | ΔcheOp1 (cheY1 cheA1 cheW1 cheR1 cheY2) ΔcheOp2 (cheY3 cheA2 cheW2 cheW3 cheR2 cheB1 tlpC) ΔcheY4 | 38 |
| JPA441 | ΔcheOp1 (cheY1 cheA1 cheW1 cheR1 cheY2) ΔcheOp2 (cheY3 cheA2 cheW2 cheW3 cheR2 cheB1 tlpC) ΔcheY4 ΔcheY5 | This study |
| SR11 | ΔprrB | This study |
| SR4 | ΔprrBCA | This study |
| Plasmids | ||
| pUC18 | General cloning vector Apr | Gibco BRL |
| pUI1653 | pBs derivative containing the whole prr locus from R. sphaeroides 2.4.1. The prrBgene was deleted and replaced with a 2.2-kbp Sm/Sp cartridge. This plasmid was used to construct a chromosomal deletion of the prrB gene in R. sphaeroides WS8N. Tcr Sm/Spr | 6 |
| pUI1659 | pBs derivative containing the prr system from R. sphaeroides 2.4.1. The prrBCA loci were deleted and replaced with a 2.2-kbp Sm/Sp cartridge. This plasmid was used to construct a chromosomal deletion of the prr system in R. sphaeroides WS8N. Tcr Sm/Spr | 6 |
| pPrrB | 1.4-kbp BamHI/KpnI prrB PCR product cloned into pUC18 | This study |
| pPrrB0.9 | 0.9-kbp BamHI/EcoRI fragment of pPrrB cloned into pUC18 | This study |
| pPrrB0.5 | 0.5-kbp EcoRI fragment of pPrrB cloned into pUC18 | This study |
| PPrrA | 0.5-kbp BamHI/HindIII prrA PCR product cloned into pUC18 | This study |
| PSRDY5N | 1.039-kbp EcoRI/PstI PCR product containing the upstream region of cheY5 cloned into pUC18 | This study |
| PSRDY5C2 | 921-bp PstI/HindIII PCR product containing the downstream region of cheY5 cloned into pUC18 | This study |
| pSR22.1 | 784-bp EcoRI/SmaI fragment of pSRDY5N cloned into pUC18 | This study |
| pSR22.6 | 248-bp SmaI/PstI fragment of pSRDY5N cloned into pUC18 | This study |
| pK18mobsacB | Mobilizable vector for allelic exchange; Kmr | 37 |
| pSRK18.1 | Derivative of pK18mobsacB containing the flanking regions of cheY5, pSRDY5N, and pSRDY5C | This study |
R. sphaeroides strains, all derivatives of WS8N, a spontaneous nalidixic-resistant strain of wild-type strain WS8, were grown aerobically in the dark and anaerobically in the light as previously described (38). For aerobic growth, R. sphaeroides was grown using either succinate (sodium salt) or M22 medium (40) supplemented with 5 mM fructose and 0.1% Casamino Acids at 30°C with vigorous shaking. For photosynthetic growth, succinate medium was used and the cultures were incubated with constant light (25 W/m2) at 30°C (38). The prr mutants were grown in the presence of streptomycin at 25 μg/ml.
E. coli strains used for standard cloning procedures and conjugation were grown as described elsewhere (38). One hundred micrograms of ampicillin/ml, 25 μg of kanamacin/ml, 25 μg of streptomycin/ml, and 25 μg of tetracycline/ml were added as needed.
DNA manipulations.
Plasmid and chromosomal DNA extraction, restriction enzyme digests, DNA ligation, and Southern blotting analysis were performed by using standard procedures (36). The oligonucleotide primers used for PCRs were purchased from Genosys Biotechnologies Ltd. (Cambridge, United Kingdom) and are summarized in Table 2.
TABLE 2.
Oligonucleotides used for PCR
| Primer | Sequence (5′ to 3′) | Restriction sitea |
|---|---|---|
| Primers used for deleting cheY5 | ||
| ΔY5NF | GCGCGAATTCAGGAGCCTGCGCCCCTCCTC | EcoRI |
| ΔY5NR | GCGCCTGCAGTCATCCCTGCGCCACTCCGA | PstI |
| ΔY5CF | AAAACTGCAGCTGAGGCAAGGCCCCGCCGG | PstI |
| ΔY5CR | GCGCAAGCTTTCGTCGGCGCCTGCGGCCACG | HindIII |
| Primers used for confirming the presence of the prr system | ||
| prrBfor | CGCGGATCCATACTCGGTCCCGACGGCATTC | BamHI |
| prrBrev | CGGGGTACCTCAGGTCTGGATCAGGACGTTC | KpnI |
| prrAfor | GGGGGGATCCGCTGAGGATCTGGTATTCG | BamHI |
| prrArev | GGGGAAGCTTTCAGCGCGGGCTGCGCTTCGCCAG | HindIII |
Underlined in the sequence.
Production of deletion mutants.
The in-frame deletion of cheY5 was achieved by PCR amplification and subsequent cloning of the flanking regions of cheY5 into the allelic exchange suicide vector pK18mobsacB, following the protocol previously described (38).
The vectors pUI1653 and pUI1659 (a generous gift from J. Eraso, University of Texas) were used for in-frame, partial replacement of the coding region of prrB and prrBCA with a streptomycin cartridge, respectively, as described previously (6). Since these vectors were constructed using R. sphaeroides 2.4.1, the presence of the prr system in R. sphaeroides WS8N was confirmed by PCR amplification of the prrB and prrA genes. The sequencing of these PCR products revealed a 99.5% identity. DNA sequencing was carried out by the Automated Sequencing Service, Department of Biochemistry, University of Oxford, using Thermosequenase (Amersham) dye terminators on an ABI 377 sequencer.
Measurement of accumulation patterns in microcapillaries.
Flat microslide capillaries (0.9 by 3.6 mm; Camlab, Cambridge, United Kingdom) were filled with a mid-log-phase cell suspension (optical density at 600 nm [OD600] = 0.4) withdrawn directly from the growing culture and incubated at 30°C in the dark for at least 15 min. Dark-field microscopic observations were carried out using a 4X magnification lens. Photographs were taken with a Nikon FX-35A camera, using TMZ p3200 KODAK film, with an exposure times between 2 and 0.2 seconds.
Measurements of oxygen diffusion in microcapillaries.
The oxygen concentrations in the cell suspensions in the microcapillaries were measured using a miniaturized Clark-type oxygen electrode (Unisense, Aarhus, Denmark) (32). Calibrations were carried out from readings in media saturated with either air (100% dissolved oxygen tension [DOT]; 260 μM) or oxygen-free nitrogen (0% DOT) (25°C; salinity ≤ 1‰). The microelectrode (tip diameter ≤ 10 μm; stirring sensitivity ≤ 2%) was positioned at the meniscus and carefully advanced inwards, with a manual micromanipulator, progressing by 100-μm steps every 60 s. The microelectrode was connected to a picoAmmeter (Unisense), and measurements were recorded on a strip chart recorder. The analog output resulting from the difference between the rate of oxygen diffusion and the rate of oxygen respiration by the cells was converted to atmospheric partial pressure according to the manufacturer's instructions.
Tethered cell responses to step changes.
To measure the responses of cells to large changes in oxygen concentration, cells grown to mid-log phase (OD700 = 0.4) were harvested and resuspended in 10 mM Na-HEPES, pH 7.2, containing chloramphenicol at 50 μg/ml (to inhibit de novo protein synthesis) and either 2 mM succinate (sodium salt) or 5 mM fructose. A 10-μl aliquot of cells was tethered to a coverslip by using 1 μl of antiflagellar antibody, incubated for 15 min in a humidity chamber, and then placed in a flow chamber mounted on the microscope stage. After a brief period of equilibration, an appropriate field of rotating cells was chosen. A 10 mM concentration of Na-HEPES, pH 7.2, containing chloramphenicol at 50 μg/ml and either 2 mM succinate (sodium salt) or 5 mM fructose was flowed through the chamber. Step changes were created by switching to either buffer that had been sparged for 45 min with oxygen-free nitrogen or buffer that was completely oxygenated. A Nikon Optiphot phase-contrast microscope was used with a 100× objective and a 5× projection lens, and results were recorded on videotape and analyzed with the Hobson Bactracker (Hobson Tracking Systems, Sheffield, United Kingdom) and the program Arot7, as described elsewhere (38).
Tethered cell behavior in linear oxygen gradients.
Smooth linear gradients were produced using the principle of gradient formation used for centrifugation, as described previously (30). The gradients were formed by mixing 100% DOT buffer (10 mM Na-HEPES [pH 7.2] containing 50 μg of chloramphenicol/ml), which was produced by vigorous shaking, with buffer with 0% DOT, which was produced by sparging with oxygen-free nitrogen for at least 45 min immediately before use. The gradients were formed with nitrogen flowing gently over the apparatus. Cells were tethered by their flagella in a flow chamber as described above, and their responses to a gradient decreasing from 100% DOT to 0% DOT at a rate of 0.2% DOT/s (0.4 μM/s) were measured by motion analysis and analyzed as previously described (30).
RESULTS
Accumulation pattern in capillaries.
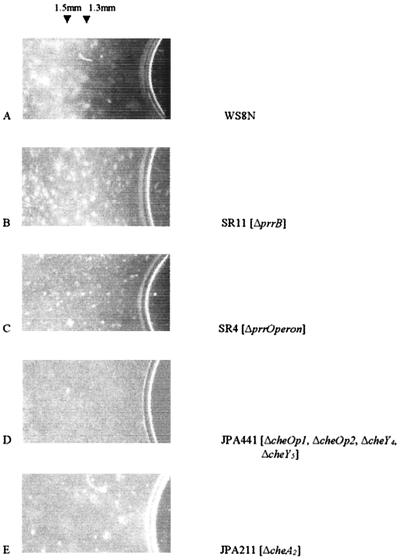
Aerobic cells of R. sphaeroides WS8N formed a distinct band of highly motile cells approximately 1.5 mm from the atmospheric interface (Fig. 1A). The position of the band was stable, and cells within the band (about 1.5 mm wide) remained motile for more than 24 h. Behind the band (i.e., over 3 mm from the aerobic interface), cells showed little or no motility. Cells of the strain with deletion of PrrB (SR11) accumulated up to the meniscus but also gradually spread into a band, broader than that of the wild type, starting about 1.3 mm from the meniscus, while the strain with the complete Prr operon deleted (SR4) showed an intermediate phenotype (Fig. 1B and C). Wild-type or prr mutant cells grown on sodium succinate required only 15 to 20 min of incubation before forming a tactic band, while cells grown on fructose, although equally motile, required 45 to 60 min before a clear band became visible (data not shown). It seemed possible that PrrB could be directly controlling the phosphorylation state of one of the CheY proteins. We therefore produced a strain lacking the known CheY proteins. Cells with deletions of cheOp1, cheOp2, cheY4, and cheY5 (JPA441) were uniformly distributed, but when cheA2 (JPA211) was deleted (and therefore the CheYs were all present at wild-type levels), the cells were also uniformly distributed and did not form a band (Fig. 1D and E, respectively). Both these strains swam normally. This suggests that CheA2 and not any other Che protein is required for the aerotactic response. Wild-type cells grown anaerobically in the light formed a band of motile cells about 2 mm from the meniscus (data not shown). Unfortunately, microscopic observations and measurements of the oxygen gradient within the phototrophic cell suspension were not possible, as both wild-type and the prr mutant accumulated very rapidly in the microscope light beam.
FIG. 1.
Distributions of cell suspensions in the capillary assay. Microcapillary slides (0.9 by 3.6 mm; Camlab) were filled with bacterial culture removed directly from the growing medium and incubated at 30°C in the dark for 15 min. Cells appear as bright areas against the dark background. The meniscus at the air interface is visible at the right side of each photo.
Measurement of the oxygen concentration in the capillaries.
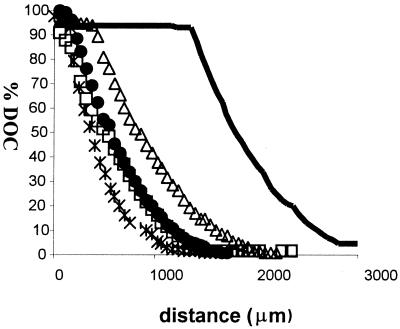
The oxygen concentration within the microcapillary slides was measured after a clear tactic band had formed with the wild-type strain (about 20 min). The profile of oxygen concentrations (Fig. 2) reflected the pattern of cell distribution shown in Fig. 1. With wild-type cells, a measurable drop in oxygen concentration started at 1.5 mm from the meniscus, at the edge of the band, and a concentration equivalent to 2.5% DOT (6.5 μM) was reached at 2.8 mm from the meniscus, at the distant edge of the band.
FIG. 2.
Measurements of oxygen concentrations in microcapillary slides filled with wild-type and mutant R. sphaeroides cells removed directly from the growing culture. A microelectrode oxygen probe (Unisense) was used as described in Materials and Methods. Oxygen concentrations were measured after 20 min of incubation. −, WS8N (wild type); ×, JPA441 (ΔcheOp1 cheOp2 cheY4 cheY5); Δ, JPA211 (ΔcheA2); □, SR11 (ΔprrB); •, SR4 (ΔprrOperon).
The pattern of oxygen concentrations in microcapillaries containing mutant cells reflected their more homogeneous cell distribution. The steepest decrease in oxygen concentration was shown by the strain lacking cheOp1, cheOp2, cheY4, and cheY5 (JPA441). An immediate reduction in oxygen tension started beneath the meniscus and reached a concentration of oxygen lower than 2.5% DOT (6.5 μM) at 1 mm from the aerobic interface. A similar profile was seen in JPA211 (ΔcheA2), although the decrease in oxygen concentration consistently started at 0.5 mm from the meniscus, and a concentration of about 2.5% oxygen was reached at about 1.7 mm from the meniscus. No obvious differences were seen with the two chemosensory strains in terms of cell distributions within the capillaries, with both of these strains uniformly distributed up to the meniscus.
The profile shown by the prr mutants was consistent with the cell distribution presented in Fig. 1. The decrease in oxygen started at the meniscus, with a concentration lower than 2.5% being reached at 1.3 mm from the meniscus where an aerotactic band formed, although it was less defined and broader than that with the wild type. The concentration of oxygen within the tactic band formed by the prr mutants was less than 10 μM.
Behavior of tethered cells in response to step changes in oxygen concentration.
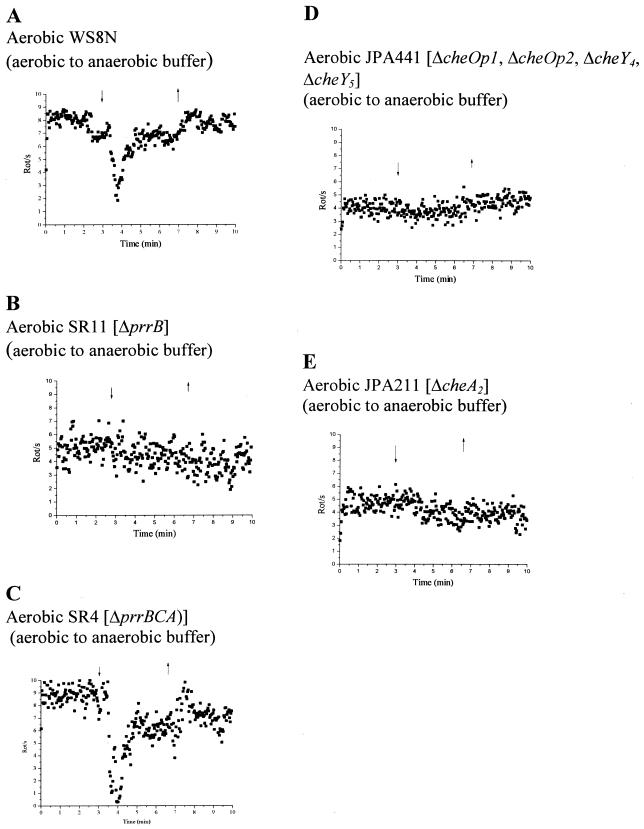
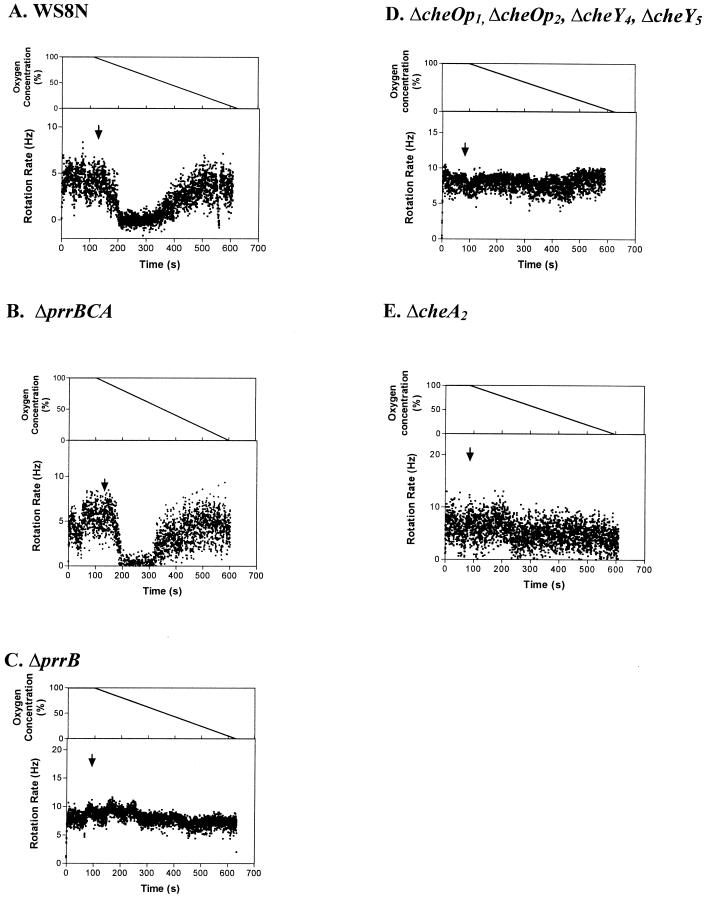
The changes in rotational behavior of tethered wild-type and mutant cells to a step-down in oxygen concentration are shown in Fig. 3. R. sphaeroides WS8N cells grown aerobically using fructose as a carbon source and incubated in aerobic buffer plus fructose showed a transient stop when challenged with anaerobic buffer (Fig. 3A). Within 30 s of the buffer shift, cells stopped transiently before adapting, as expected for a tactic response. Cells with deletion of the redox sensor kinase PrrB (SR11) showed no response to step changes in oxygen (Fig. 3B). Interestingly, however, when the whole prr operon was deleted, the mutant responded to the shift to anaerobic buffer (Fig. 3C). Mutants in the chemosensory genes, JPA211 (ΔcheA2) and JPA 441 (ΔcheOp1 ΔcheOp2 ΔcheY4 ΔcheY5), showed no response (Fig. 3D and E).
FIG. 3.
Tethered cells analysis of aerobically grown cells to oxygen step changes in a background of 5 mM fructose. (A) Wild type; (B) SR11 (ΔprrB); (C) SR4 (ΔprrBCA); (D) JPA441 (ΔcheOp1 ΔcheOp2 ΔcheY4 ΔcheY5); (E) JPA211 (ΔcheA2). Each graph represents the average behavior of at least 20 cells. The arrows indicate addition (downward arrowhead) and removal (upward arrowhead) of oxygenated buffer.
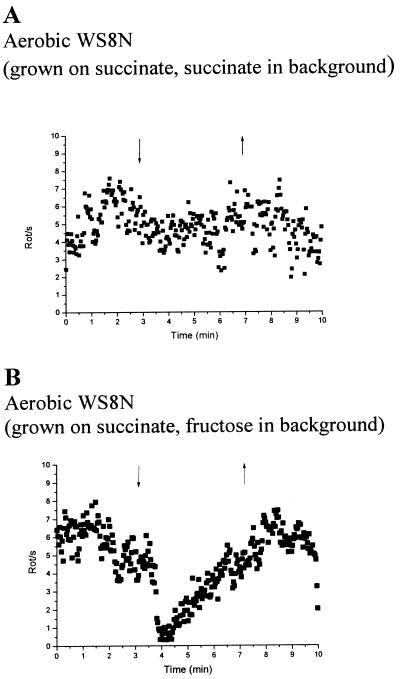
Cells grown aerobically with sodium succinate as the carbon source showed little or no response to a transient step-down in oxygen in a background of sodium succinate (Fig. 4A). However, when the same population of cells was challenged with a transition from aerobic to anaerobic buffer in the presence of fructose, the cells stopped and adapted (Fig. 4B).
FIG. 4.
Tethered cells analysis of wild-type cells grown aerobically on sodium succinate medium to oxygen step changes. Each graph represents the average behavior of at least 20 cells. The arrows indicate addition (downward arrowhead) and removal (upward arrowhead) of oxygenated buffer. Either 2 mM sodium succinate (A) or 5 mM fructose (B) was used as steady background during buffer transition.
Wild-type cells grown photoheterotrophically on succinate stopped and then adapted when switched from anaerobic to aerobic buffer (Fig. 5A). Photoheterotrophic chemosensory mutants JPA211 (ΔcheA2) and JPA441 (ΔcheOp1 ΔcheOp2 ΔcheY4 ΔcheY5) gave no response (data not shown), nor did the mutant with deletion of PrrB (Fig. 5B).
FIG. 5.
Tethered analysis of photoheterotrophically grown wild-type (A) and SR11 (ΔprrB) (B) cells. Each graph represents the average behavior of at least 20 cells in response to transition from anaerobic to aerobic buffer. The arrows indicate addition (downward arrowhead) and removal (upward arrowhead) of deoxygenated buffer during the transition.
Behavior of tethered cells in a continuous, decreasing oxygen gradient.
The responses of tethered cells to a continuously decreasing concentration of oxygen (i.e., gradients) were measured for R. sphaeroides cells grown aerobically with fructose as the main carbon source. Responses to the oxygen gradients were found for R. sphaeroides WS8N (Fig. 6A) and for cells with deletions of the prr operon (SR4) (Fig. 6B), but no response was seen for ΔprrB (SR11) (Fig. 6C) or for the chemotaxis deletion mutants JPA211 (ΔcheA2) and JPA441 (ΔcheOp1 ΔcheOp2 ΔcheY4 ΔcheY5) (Fig. 6D and E, respectively). The threshold responses for WS8N occurred at about 85% DOT (221 μM) and for the Prr operon deletion (SR4) at approximately 90% DOT (234 μM). Once the threshold concentration of oxygen was reached, the WS8N population progressively slowed rotation as the concentration decreased. An increasing percentage of the population stopped over a period of 45 s as the DOT fell. The population remained stopped for a period of about 150 s as the DOT fell from 80% to about 50% (from 208 to 130 μM). The population then underwent a transition phase of slow rotation and frequent stops (about 50 to 20% DOT; 130 to 52 μM) before returning, in about 150 to 180 s, to the prestimulus behavior at 10 to 0% DOT (26 to 0 μM). SR4 (ΔprrBCA) showed a similar behavior, although the recovery time was more rapid, with the whole population returning to prestimulus behavior in about 100 to 120 s (Fig. 6B). Under these conditions cells were in a constantly changing environment; the individual cells during the period when the oxygen concentration changed at 0.4 μM/s between 208 and 130 μM remained stopped and did not show adaptation to the changing environment, perhaps suggesting saturation of the sensing system.
FIG. 6.
Responses to the artificial oxygen gradient of wild-type and mutant tethered cells. (A) R. sphaeroides WS8N; (B) SR4 (ΔprrBCA); (C) SR11 (ΔprrB); (D) JPA441 (ΔcheOp1 ΔcheOp2 ΔcheY4 ΔcheY5); (E) JPA211 (ΔcheA2). The arrow shows when the gradient starts. The upper trace shows the change in oxygen concentration over time. The concentration of oxygen was changed at 0.4 μM/s (0.2 DOT/s).
DISCUSSION
These data offer a new perspective on oxygen sensing and tactic responses in R. sphaeroides and suggest that R. sphaeroides prefers microaerophilic conditions whether grown photoheterotrophically or aerobically. They also suggest that the histidine protein kinase, PrrB, which is responsible for controlling the phosphorylation state of the response regulator PrrA and, thus, expression of a very wide range of metabolic pathways, has an additional role in aerotaxis. Both the PrrB-dependent signal and a second as-yet-unidentified aerotactic signal require CheA2 to signal to the flagellar motor. The prr mutant strains did not lose the ability to form a tactic band, unlike the chemosensory mutants; nevertheless, their distribution was different from that of the wild type. This suggests two different aerotaxis signals producing a complex tactic response consisting of (i) a microaerophilic response, attracting cells to a low concentration of oxygen; and (ii) an aerophobic response to atmospheric oxygen concentrations controlled by the Prr system and therefore ultimately sensitive to the activity of high-affinity cytochrome c oxidase cbb3. Both systems are coordinated by the Che system. A similar complex behavior has been reported for B. subtilis and H. salinarum, where aerophilic and aerophobic responses coexist and can be discriminated only in specific mutants (16). Indeed, in E. coli and P. putida, where a dedicated aerotactic receptor (Aer) has been identified, aer strains are only partially defective for aerotaxis, suggesting a second sensory pathway also requiring respiratory electron transport, and complementation studies have shown that a delicate balance of receptor(s) activity and protein copy number is involved in this kind of response (2, 26).
Data produced in this laboratory in recent years suggested a model of sensing and integration of multiple signals in R. sphaeroides. In particular, it has been proposed (10, 13) that R. sphaeroides controls its swimming direction to accumulate in microenvironments in which the rate of electron transport is maximal, as has been suggested for other facultative species (45). In addition, stimuli affecting the electron flow produce different responses under different growth conditions (13, 34), suggesting that these responses may depend upon specific metabolic activity (13, 33). Taxis towards oxygen by R. sphaeroides is a good example of a complex behavior linked to both energy level and metabolic rate. The response of aerobically grown tethered wild-type cells to a transient drop in oxygen concentration and gradient of oxygen was clearly observed only when cells were analyzed in the presence of a neutral carbon source (i.e., fructose). When the carbon source was also an electron donor, e.g., sodium succinate, only minor changes in the swimming behavior were detected, suggesting that the redox state or metabolic activity affected the tactic response.
The behavior of cells grown anaerobically in the light responding to a step-up in oxygen is consistent with data previously produced in this laboratory (9, 10, 12). The transient stop shown by photoheterotrophically grown cells to the addition of oxygenated buffer suggests that the diversion of electrons from the photosynthetic cyclic electron flow to a high-affinity oxidase (5) causes the response to oxygen. This finding, combined with the data presented here, indicates that taxis to oxygen in R. sphaeroides depends upon respiration and that changes in the oxidase activity trigger a response. One of the most intriguing results is the difference in behavior of cells with deletions of the complete PrrBCA operon versus that in the strain lacking only PrrB. PrrC transduces the change in electron flow through the CcoQ subunit of the constitutive, high-affinity cytochrome oxidase cbb3. PrrC controls the phosphorylation state of PrrB, which in turn controls the activity of the transcriptional activator, PrrA, that controls the expression of a very wide range of metabolic pathways.
We have recently shown by lacZ fusions that deleting the whole prr system induces a 50% increase in the level of expression of cheOp2 in cells in mid-log phase, while deletion of prrB has less of an effect (S. Romagnoli and J. P. Armitage, unpublished data). Deletion of the whole operon will result in a change in expression level of a wide range of pathways, including the chemosensory pathway encoding CheA2. It is possible that the effect of PrrB deletion is indirect and that it controls the activity of another protein which interacts with CheA2, or that increased CheA2 can overcome, to some extent, the loss of PrrB (unfortunately, the lack of controllable expression vectors in R. sphaeroides makes this difficult to test experimentally). A recent report has shown that deletion of RegA, the R. capsulatus transcriptional regulator homologue of PrrA, altered the pattern of expression of the high-affinity cbb3 cytochrome oxidase and other components of the respiratory electron transport chain, particularly under aerobic growth conditions (44). Deletion of the complete operon may result in a greater change in the relative concentrations of the terminal oxidases. Since R. sphaeroides has a complex interaction between taxis and metabolism, alterations of the respiratory electron flow may also affect the response to oxygen.
As the environmental concentration of oxygen falls, respiratory electron flow is primarily sustained by the high-affinity cytochrome oxidase cbb3. This readjustment of the respiratory activity may result in the complex response seen to the changing gradient of oxygen. In the continuously decreasing gradient of O2, the pattern of behavior shown under constant stimulation may reflect the activity of two different oxygen sensing systems: a low-affinity system inducing the cells to increase their stopping frequency when oxygen falls from 80 to 50% DOT, and a high-affinity oxidase allowing the cells to regain motility in spite of the further reduction in oxygen, returning to their prestimulus rotation rate from 20% DOT downwards. The lack of any response by the chemosensory mutants clearly shows that the Che system is involved in both of these responses. The sustained period of about 2 min when all the cells in the population remained stopped is interesting and may reflect saturation of the sensory system or the effect of conflicting signals from the two systems.
Deletion of cheA2 alone or of the whole second che operon caused loss of all oxygen responses, as seen for chemotaxis to weak organic acids (13) and for photoresponses (34), showing that the products of the second chemosensory operon, and CheA2 in particular, are necessary for all wild-type responses tested. How CheA2 transduces variations of oxygen concentration from either PrrB or the second aerotactic system remains unclear. So far, none of the identified MCP-like receptors of R. sphaeroides has been shown to be involved in aerotaxis.
Does PrrB directly interact with CheA2 in the R. sphaeroides aerotactic responses? There are several suggested examples of cross talk between two-component systems in bacteria (43), which may be important for integrating multiple signals in a complex, ever-changing environment. Indeed, studies carried out in E. coli have shown how responses that are not mediated by MCPs can feed into the Che system. For example, (i) taxis towards sugars transported by the phosphoenolpyruvate-phosphotransferase system [PTS] depends on the phosphotransfer activity of enzyme I and phosphohistidine carrier protein of the PTS and directly controls the phosphorylation state of CheA (22); (ii) several data show the interaction in vivo and in vitro between ArcB, the E. coli anaerobic sensor kinase, and CheY. Indeed, ArcB mutants have reduced chemotaxis under microaerobic conditions (47). (iii) Mutual phosphotransfer has been show between the nitrogen regulation (NR) system controlling the transcription of nif genes and Che proteins, directly linking nitrogen assimilation to chemotaxis, with overexpression of the transcriptional regulator NRII suppressing the smooth swimming of an E. coli ΔcheA strain (27), although neither of the latter two have been shown to operate in vivo with normal levels of expression.
In conclusion, the response to oxygen in R. sphaeroides seems to depend upon the synergy of two sensing systems, i.e., the Prr causing a negative response to atmospheric concentrations of oxygen and a second system causing a positive response to low oxygen concentrations, both feeding through the chemosensory protein CheA2. PrrB may provide a molecular link between the activity of the respiratory chain and Che proteins, with the activity of cbb3 cytochrome oxidase affecting R. sphaeroides tactic behavior.
Acknowledgments
During this project, S.R. was a European Commission TMR Marie Curie Research Fellowship holder (ERB FMB ICT 982883). H.L.P. thanks the NERC for their support. Work in this laboratory is supported by the BBSRC.
We also thank S. Kaplan and J. Eraso for providing us with plasmids.
REFERENCES
- 1.Armitage, J. P. 1999. Bacterial tactic responses. Adv. Microb. Physiol. 41:229-289. [DOI] [PubMed] [Google Scholar]
- 2.Bibikov, S. I., R. Biran, K. E. Rudd, and J. S. Parkinson. 1997. A signal transducer for aerotaxis in E. coli. J. Bacteriol. 179:4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird, T. D., S. Du, and C. E. Bauer. 1999. Autophosphorylation, phosphotransfer and DNA-binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. J. Biol. Chem. 274:16343-16348. [DOI] [PubMed] [Google Scholar]
- 4.Brooun, A. J., T. Bell, R. W. Freitas, A. Larsen, and M. Alam. 1998. An archaeal aerotaxis transducer combines subunit I core structures of eukaryotic cytochrome c oxidase and eubacterial methyl-accepting chemotaxis proteins. J. Bacteriol. 180:1642-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, J. C., J. T. Beatty, and J. L. Favinger. 1983. Increased activity of respiratory enzymes from photosynthetically grown Rhodopseudomonas capsulata in response to small amounts of oxygen. Arch. Microbiol. 134:324-328. [Google Scholar]
- 6.Eraso, J. M., and S. Kaplan. 1994. prrA, a putative response regulator involved in oxygen regulation of photosynthetic gene expression in Rhodobacter sphaeroides. J. Bacteriol. 176:32-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eraso, J. M., and S. Kaplan. 2000. From redox flow to gene regulation: role of PrrC protein of Rhodobacter sphaeroides 2.4.1. Biochemistry 39:2052-2062. [DOI] [PubMed] [Google Scholar]
- 8.Fu, R., J. D. Wall, and G. Voordouw. 1994. DcrA, a c-type heme containing methyl-accepting protein from Desulfovibrio vulgaris Hildenborough, senses the oxygen concentration of redox potential of the environment. J. Bacteriol. 176:344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauden, D. E. 1996. D.Phil. thesis. University of Oxford, Oxford, United Kingdom.
- 10.Gauden, D. E., and J. P. Armitage. 1995. Electron transport dependent taxis in Rhodobacter sphaeroides. J. Bacteriol. 177:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grebe, T. W., and J. B. Stock. 1999. The histidine kinase superfamily. Adv. Microb. Physiol. 41:139-227. [DOI] [PubMed] [Google Scholar]
- 12.Grishanin, R. I., D. E. Gauden, and J. P. Armitage. 1997. Photoresponses in Rhodobacter sphaeroides: role of photosynthetic electron transport. J. Bacteriol. 179:24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamblin, P. A., B. A. Maguire, R. N. Grishanin, and J. P. Armitage. 1997. Evidence for two chemosensory pathways in Rhodobacter sphaeroides. Mol. Microbiol. 26:1083-1096. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan, D. 1983. Transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-560. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, D. M., J. Skidmore, J. P. Armitage, and J. R. Maddock. 1999. Localization and environmental regulation of MCP-like proteins in Rhodobacter sphaeroides. Mol. Microbiol. 31:885-892. [DOI] [PubMed] [Google Scholar]
- 16.Hou, S., R. W. Larsen, D. Boudko, C. W. Riley, E. Karatan, M. Zimmer, G. W. Ordal, and M. Alam. 2000. Myoglobin-like aerotaxis transducer in Archaea and Bacteria. Nature 403:540-544. [DOI] [PubMed] [Google Scholar]
- 17.Jeziore-Sassoon, Y., P. A. Hamblin, C. A. Bootle-Willbraham, P. S. Poole, and J. P. Armitage. 1998. Metabolism is required for chemotaxis to sugars in Rhodobacter sphaeroides. Microbiology 144:229-239. [DOI] [PubMed] [Google Scholar]
- 18.Joshi, H. M., and F. R. Tabita. 1996. A global two-component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation and nitrogen fixation. Proc. Natl. Acad. Sci. USA 93:14515-14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, M., T. Mizuno, and T. Hakoshima. 1998. Crystallization of a complex between a novel C terminal transmitter, HPt domain, of the anaerobic sensor kinase ArcB and the chemotaxis response regulator CheY. Acta Crystallogr. D 54:140-142. [DOI] [PubMed] [Google Scholar]
- 20.Laszlo, D. J., and B. L. Taylor. 1981. Aerotaxis in Salmonella typhimurium: role of electron transport. J. Bacteriol. 145:990-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laszlo, D. J., M. Niwano, W. W. Goral, and B. L. Taylor. 1994. Bacillus cereus electron transport and proton motive force during aerotaxis. J. Bacteriol. 159:820-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lux, R., K. Jahreis, K. Bettenbrock, J. S. Parkinson, and J. W. Lengeler. 1995. Coupling the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:11583-11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenzie, C., M. Choundhary, F. W. Larimer, P. F. Predki, S. Stilwagen, J. P. Armitage, R. D. Barber, T. J. Donohue, J. P. Hosler, J. E. Newman, J. P. Shapleigh, R. E. Sockett, J. Zeilstra-Ryalls, and S. Kaplan. 2001. The home stretch, a first analysis of the nearly completed genome of Rhodobacter sphaeroides 2.4.1. Photosynth. Res. 70:19-41. [DOI] [PubMed] [Google Scholar]
- 24.Masuda, S., Y. Matsumoto, K. V. P. Nagashima, K. Shimada, K. Inoue, C. E. Bauer, and K. Matsuura. 1999. Structural and functional analysis of photosynthetic regulatory genes regA and regB from Rhodovulum sulfidophilum, Roseobacter denitrificans, and Rhodobacter capsulatus. J. Bacteriol. 181:4205-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosley, C. S., J. Y. Suzuki, and C. E. Bauer. 1994. Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J. Bacteriol. 176:7566-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols, N. N., and C. S. Harwood. 2000. An aerotaxis transducer gene from Pseudomonas putida. FEMS Microbiol. Lett. 182:177-183. [DOI] [PubMed] [Google Scholar]
- 27.Ninfa, A. J., E. Gottlin Ninfa, A. N. Lupas, A. Stock, B. Magasanik, and J. Stock. 1988. Cross talk between bacterial chemotaxis signal transduction proteins and regulators of transcription of the Ntr regulon: evidence that nitrogen assimilation and chemotaxis are controlled by a common phosphotransfer mechanism. Proc. Natl. Acad. Sci. USA 85:5492-5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh, J. I., and S. Kaplan. 1999. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry 38:2688-2696. [DOI] [PubMed] [Google Scholar]
- 29.Oh, J. I., and S. Kaplan. 2000. Redox signaling: globalization of gene expression. EMBO J. 19:4237-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Packer, H. L., and J. P. Armitage. 2000. Behavioral responses of Rhodobacter sphaeroides to linear gradients of the nutrients succinate and acetate. Appl. Environ. Microbiol. 66:5186-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebbapragada, A., M. S. Johnson, G. P. Harding, A. J. Zuccarelli, H. M. Fletcher, I. B. Zhulin, and B. L. Taylor. 1997. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox and energy signals for E. coli behavior. Proc. Natl. Acad. Sci. USA 94:10541-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Revsbech, N. P. 1989. An oxygen microelectrode with a guard cathode. Limnol. Oceanogr. 34:474-478. [Google Scholar]
- 33.Riondet, C., R. Cachon, Y. Wache, G. Alcaraz, and C. Divies. 2000. Extracellular oxidoreduction potential modifies carbon and electron flow in Escherichia coli. J. Bacteriol. 182:620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romagnoli, S., and J. P. Armitage. 1999. Role of chemosensory pathways in transient changes in swimming speed of Rhodobacter sphaeroides induced by changes in photosynthetic electron transport. J. Bacteriol. 181:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowsell, E. H., J. M. Smith, A. Wolfe, and B. L. Taylor. 1995. CheA, CheW, and CheY are required for chemotaxis to oxygen and sugar of the phosphotransferase system in Escherichia coli. J. Bacteriol. 177:6011-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pk18 and pk19: selection of defined deletions in the chromosome of Corynebacterium glutaminum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 38.Shah, D. S. H., S. L. Porter, A. C. Martin, P. A. Hamblin, and J. P. Armitage. 2000. Fine tuning bacterial chemotaxis: analysis of Rhodobacter sphaeroides behaviour under aerobic and anaerobic conditions by mutation of the major chemotaxis operons and cheY genes. EMBO J. 199:4601-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-794. [Google Scholar]
- 40.Sistrom, W. R. 1960. A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. J. Gen. Microbiol. 22:778-785. [DOI] [PubMed] [Google Scholar]
- 41.Sockett, R. E., J. C. A. Foster, and J. P. Armitage. 1990. Molecular biology of the Rhodobacter sphaeroides flagellum. FEMS Symp. 53:473-479. [Google Scholar]
- 42.Soufian, O., and S. Kaplan. 1999. Topological analysis of the membrane-localized redox responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 274:17290-17296. [DOI] [PubMed] [Google Scholar]
- 43.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 44.Swen, L. R., S. Elsen, T. H. Bird, D. L. Swen, H.-G. Koch, H. Myllykallio, F. Daldal, and C. E. Bauer. 2001. The RegB/RegA two component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J. Mol. Biol. 309:121-138. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, B. L., I. B. Zhulin, and M. S. Johnson. 1999. Aerotaxis and other energy-sensing behavior in bacteria. Annu. Rev. Microbiol. 53:103-128. [DOI] [PubMed] [Google Scholar]
- 46.Voltz, K. 1993. Structural conservation in the CheY superfamily. Biochemistry 32:11743-11753. [DOI] [PubMed] [Google Scholar]
- 47.Yaku, H., M. Kato, T. Hakishima, M. Tsuzuki, and T. Mizuno. 1997. Interaction between the CheY response regulator and the histidine-containing phosphotransfer (HPt) domain of the ArcB sensory kinase in Escherichia coli. FEBS Lett. 408:337-340. [DOI] [PubMed] [Google Scholar]