Abstract
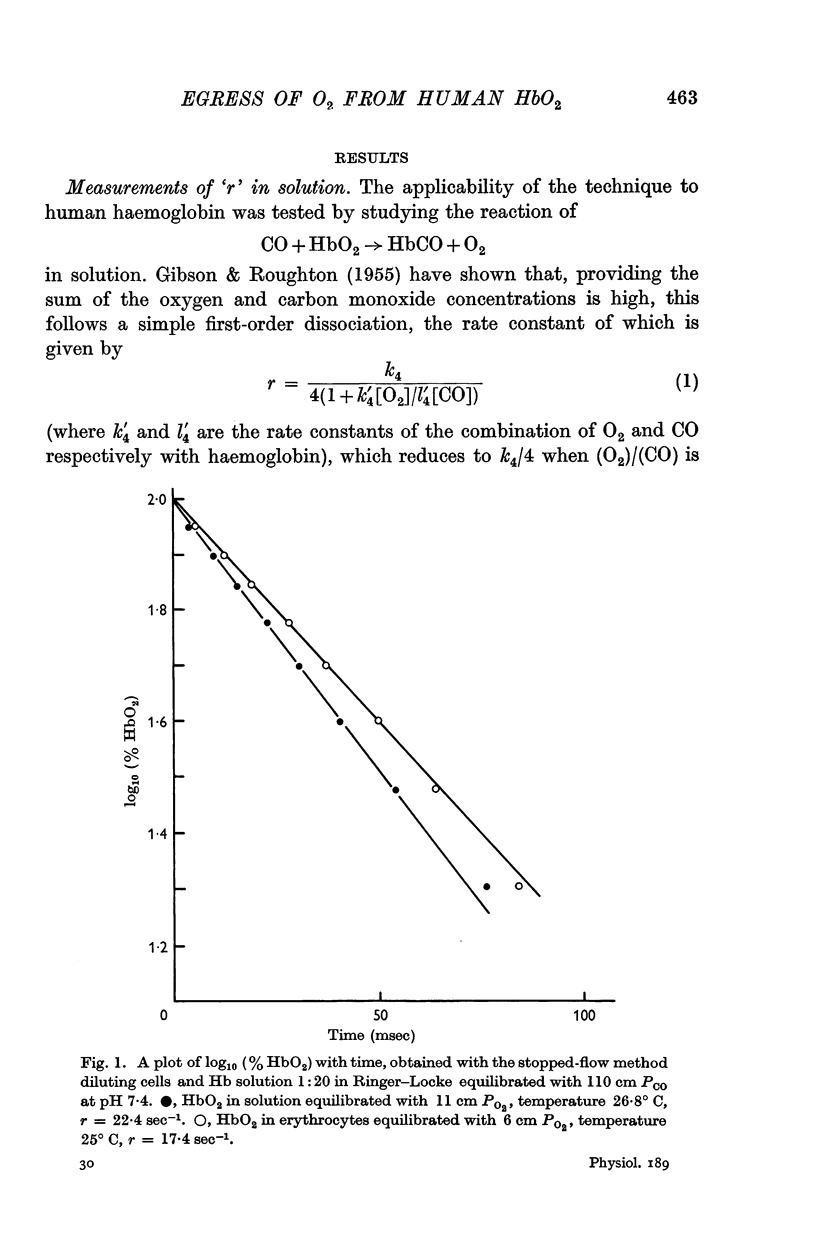
1. Spectrophotometric measurements, using the rapid-mixing constantflow and stopped-flow techniques, have been made of the rate of egress of oxygen from human HbO2 in solution and in erythrocytes.
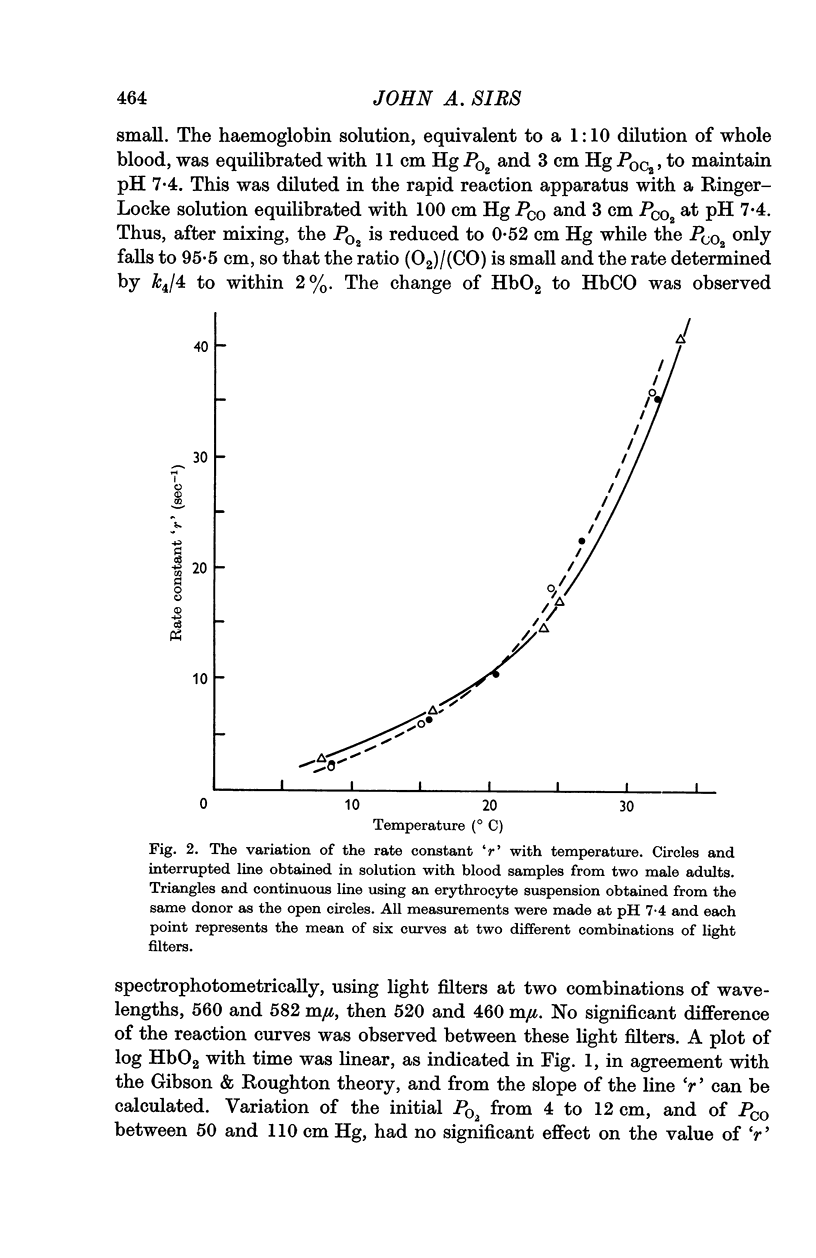
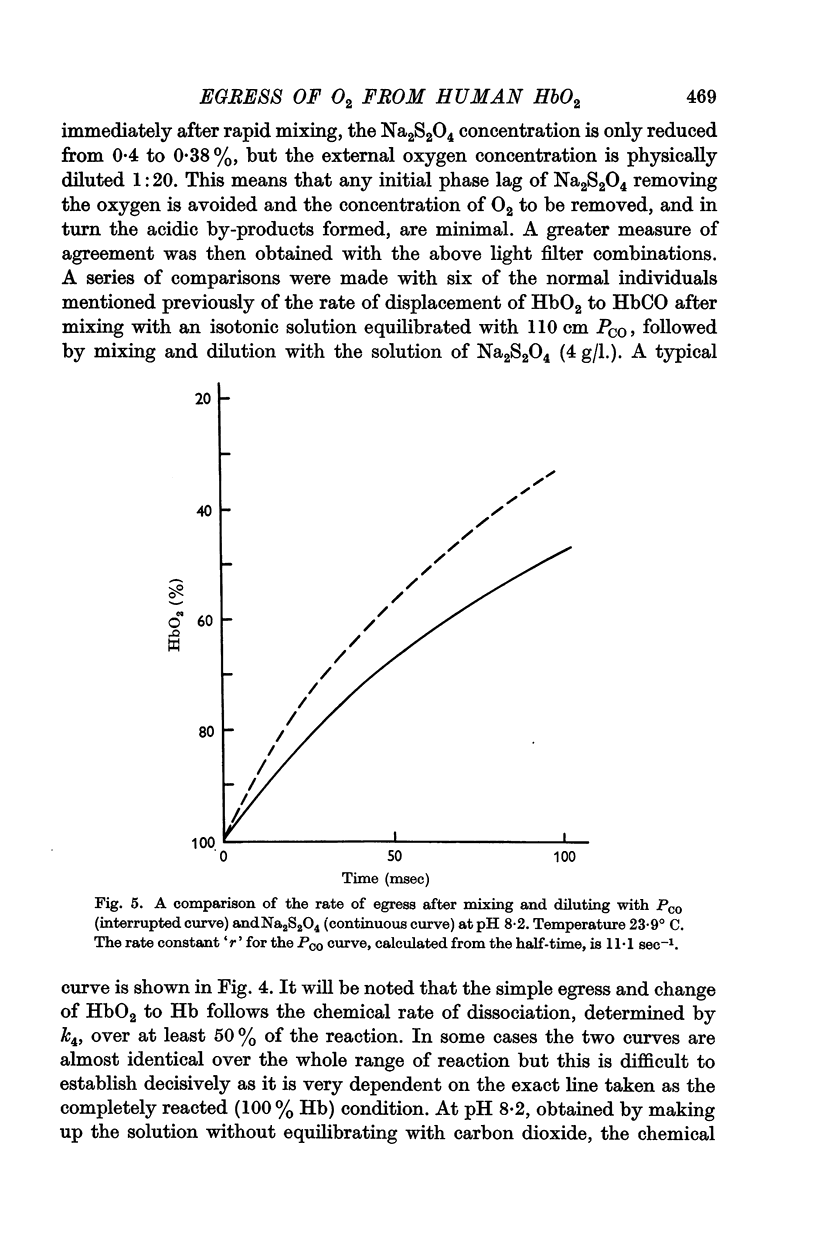
2. By 1:20 dilution in a medium containing a high concentration of carbon monoxide k4, the rate constant of the reaction Hb4O8 → Hb4O6+O2 has been determined. At pH 7·4 and 20° C, k4 in solution was 42·0 sec-1 and within the intact cell 41·4 sec-1. The respective activation energies were 19·3 and 18 kcal.
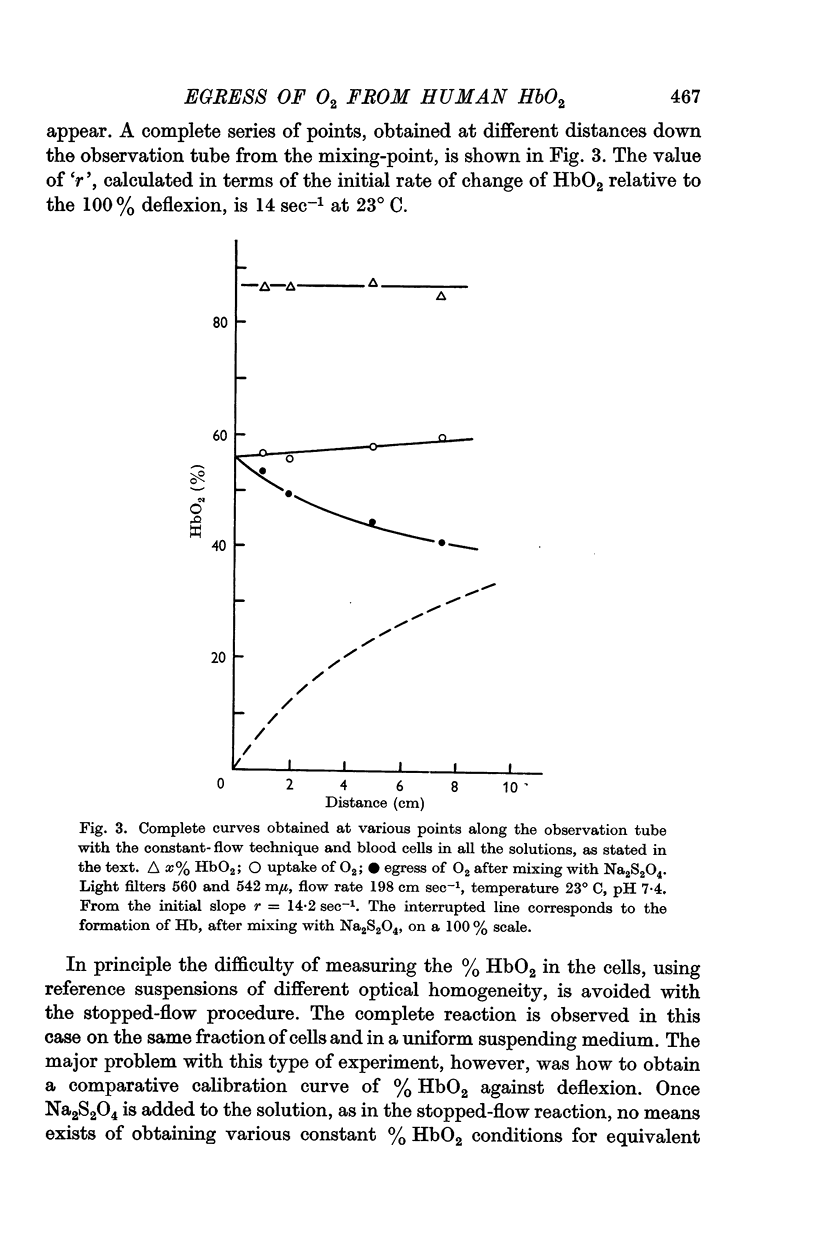
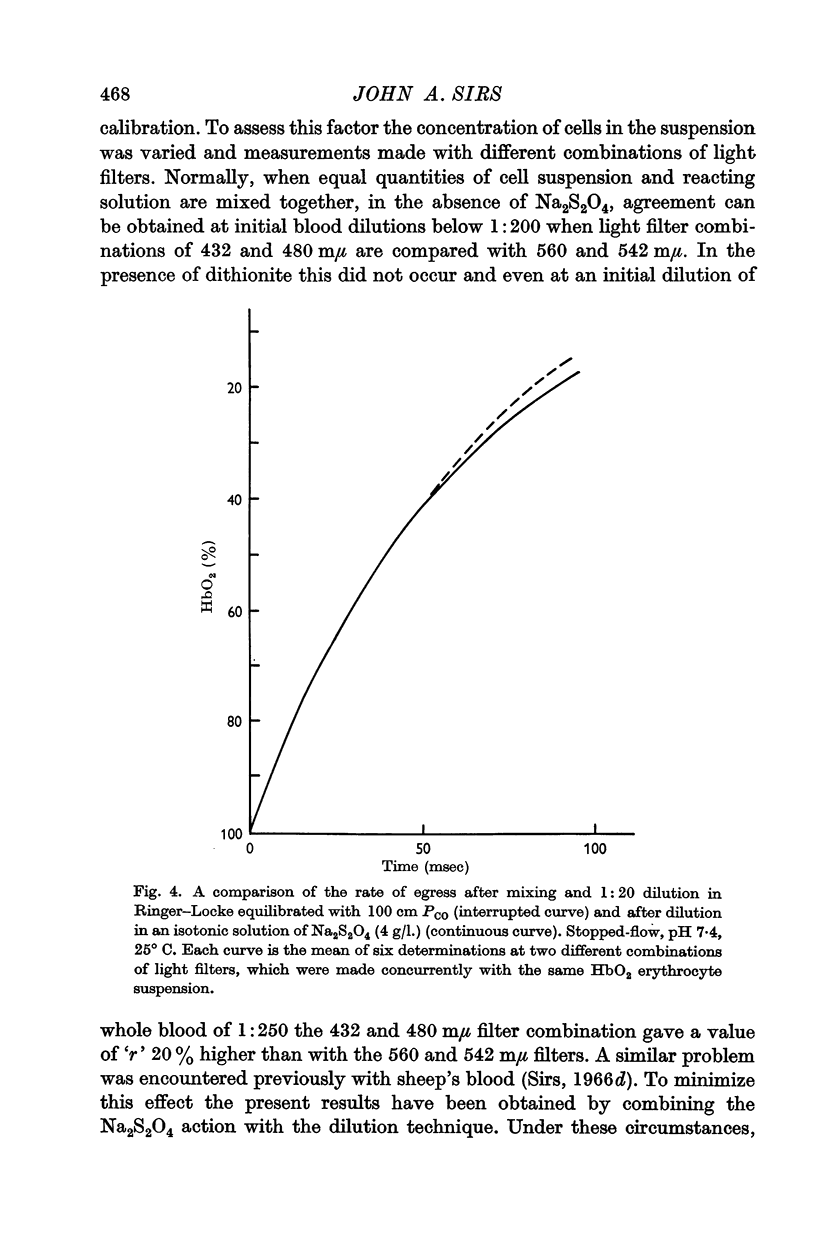
3. The rate of dissociation of HbO2 to Hb, after mixing an erythrocyte suspension with Na2S2O4, was identical, over at least 50% of the reaction, with the change of HbO2 to HbCO above.
4. An analysis of the data indicates that the cell membrane has little or no resistance to the passage of oxygen and the internal contents are effectively mixed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GIBSON Q. H., KREUZER F., MEDA E., ROUGHTON F. J. The kinetics of human haemoglobin in solution and in the red cell at 37 degrees C. J Physiol. 1955 Jul 28;129(1):65–89. doi: 10.1113/jphysiol.1955.sp005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., ROUGHTON F. J. The kinetics of dissociation of the first oxygen molecule from fully saturated oxyhaemoglobin in sheep blood solutions. Proc R Soc Lond B Biol Sci. 1955 Mar 15;143(912):310–334. doi: 10.1098/rspb.1955.0014. [DOI] [PubMed] [Google Scholar]
- LEGGE J. W., ROUGHTON F. J. W. Some observations on the kinetics of haemoglobin in solution and in the haemoglobin in solution and in the red blood corpuscle. Biochem J. 1950 Jun-Jul;47(1):43–52. doi: 10.1042/bj0470043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson W. H., Jr, Holland R. A., Forster R. E. Effect of temperature on deoxygenation rate of human red cells. J Appl Physiol. 1965 Sep;20(5):912–918. doi: 10.1152/jappl.1965.20.5.912. [DOI] [PubMed] [Google Scholar]
- NICOLSON P., ROUGHTON F. J. W. A theoretical study of the influence of diffusion and chemical reaction velocity on the rate of exchange of carbon monoxide and oxygen between the red blood corpuscle and the surrounding fluid. Proc R Soc Lond B Biol Sci. 1951 Jun;138(891):241–264. doi: 10.1098/rspb.1951.0020. [DOI] [PubMed] [Google Scholar]
- PROTHERO J., BURTON A. C. The physics of blood flow in capillaries. I. The nature of the motion. Biophys J. 1961 Sep;1:565–579. doi: 10.1016/s0006-3495(61)86909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUGHTON F. J. Kinetics of gas transport in the blood. Br Med Bull. 1963 Jan;19:80–89. doi: 10.1093/oxfordjournals.bmb.a070013. [DOI] [PubMed] [Google Scholar]
- SIRS J. A., ROUGHTON F. J. Stopped-flow measurements of CO and O2 uptake by hemoglobin in sheep erythrocytes. J Appl Physiol. 1963 Jan;18:158–165. doi: 10.1152/jappl.1963.18.1.158. [DOI] [PubMed] [Google Scholar]
- SIRS J. A. THE FACILITATED UPTAKE OF NITRIC OXIDE BY HAEMOGLOBIN IN ERYTHROCYTES. Biochim Biophys Acta. 1964 Jul 15;90:108–116. doi: 10.1016/0304-4165(64)90123-0. [DOI] [PubMed] [Google Scholar]
- Sirs J. A. The velocity constant, k4, of the reaction Hb4O8 plus CO, in sheep erythrocytes and dilute haemoglobin solutions. Biochim Biophys Acta. 1966 Sep 5;126(1):19–27. doi: 10.1016/0926-6585(66)90032-x. [DOI] [PubMed] [Google Scholar]


