Abstract
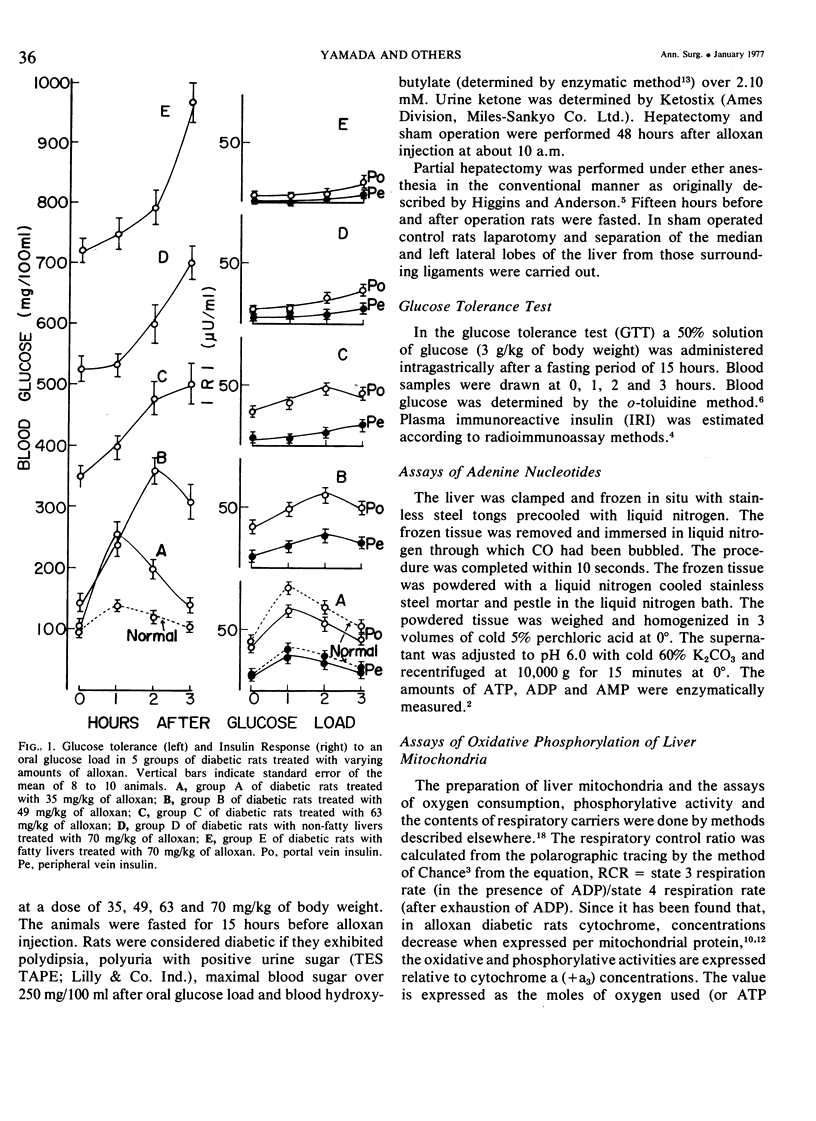
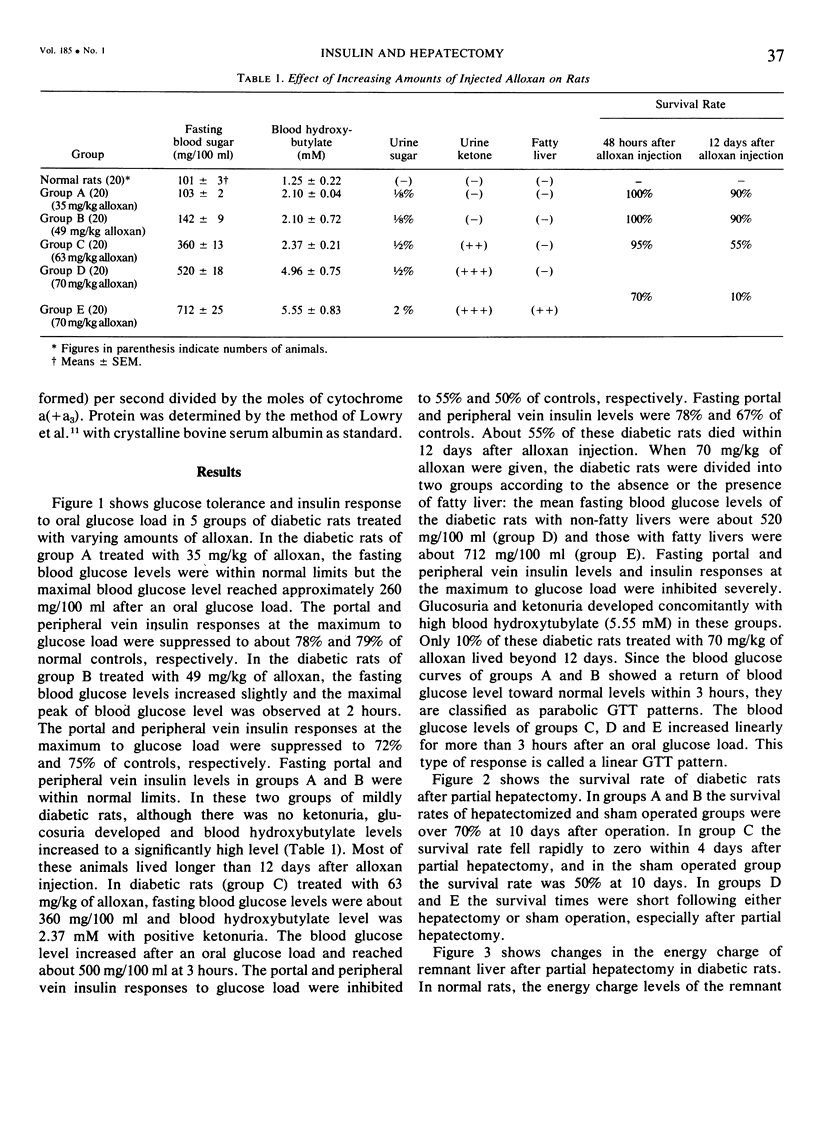
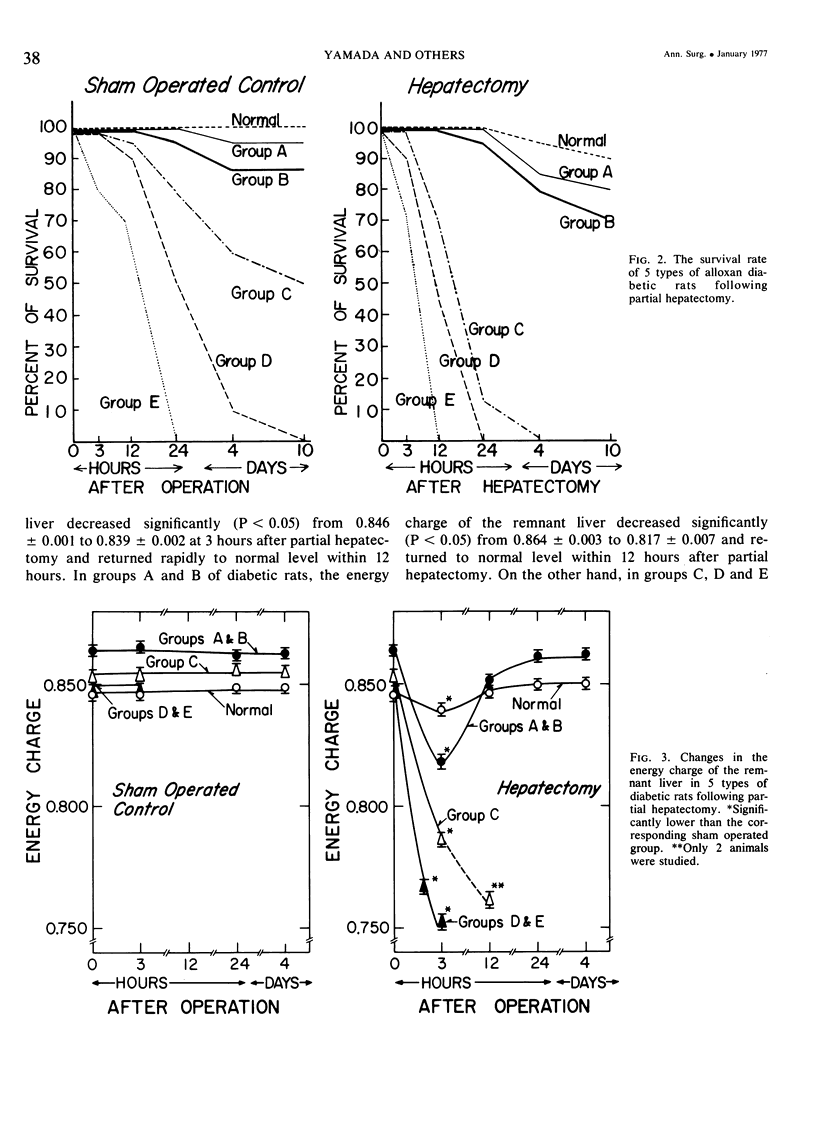
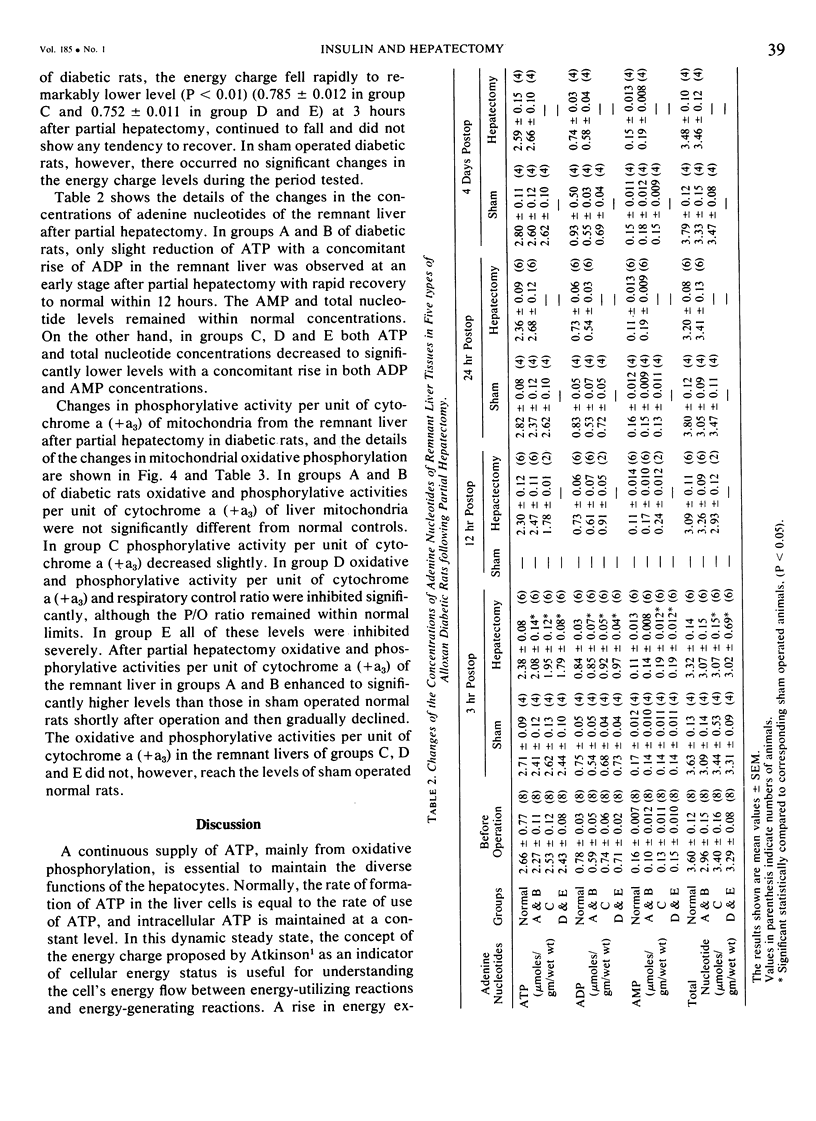
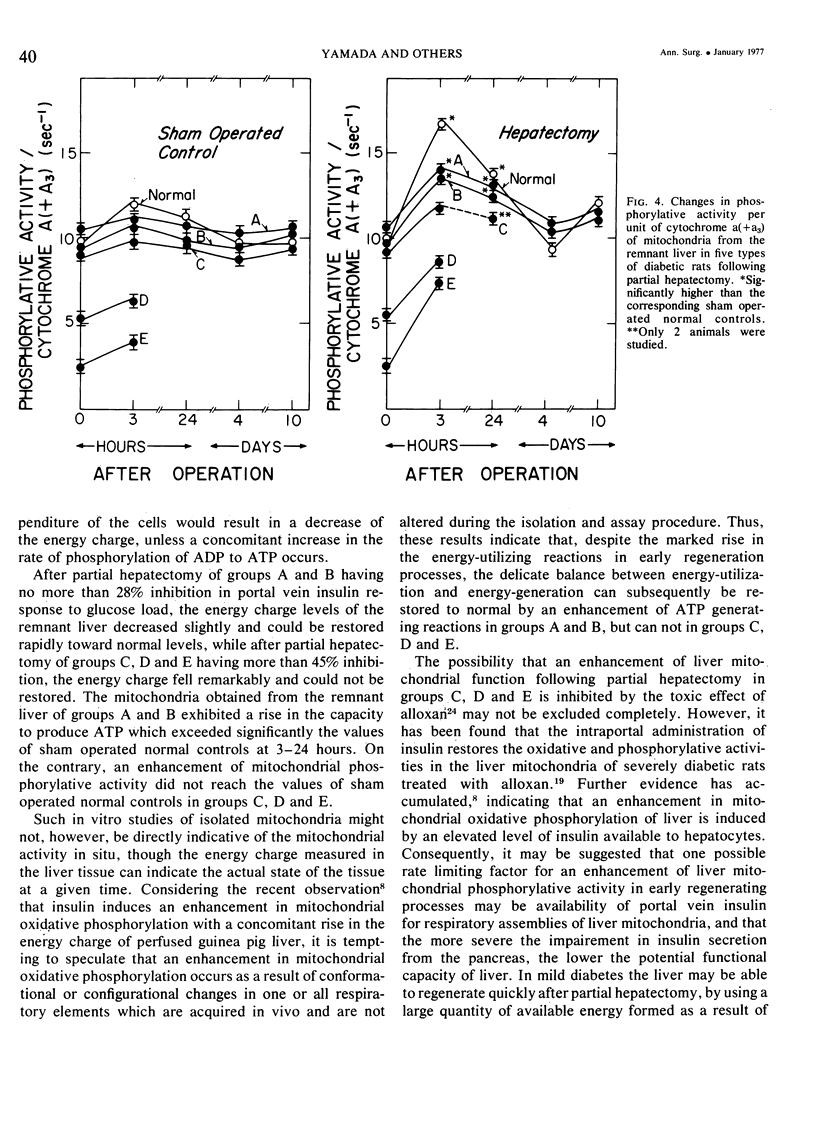
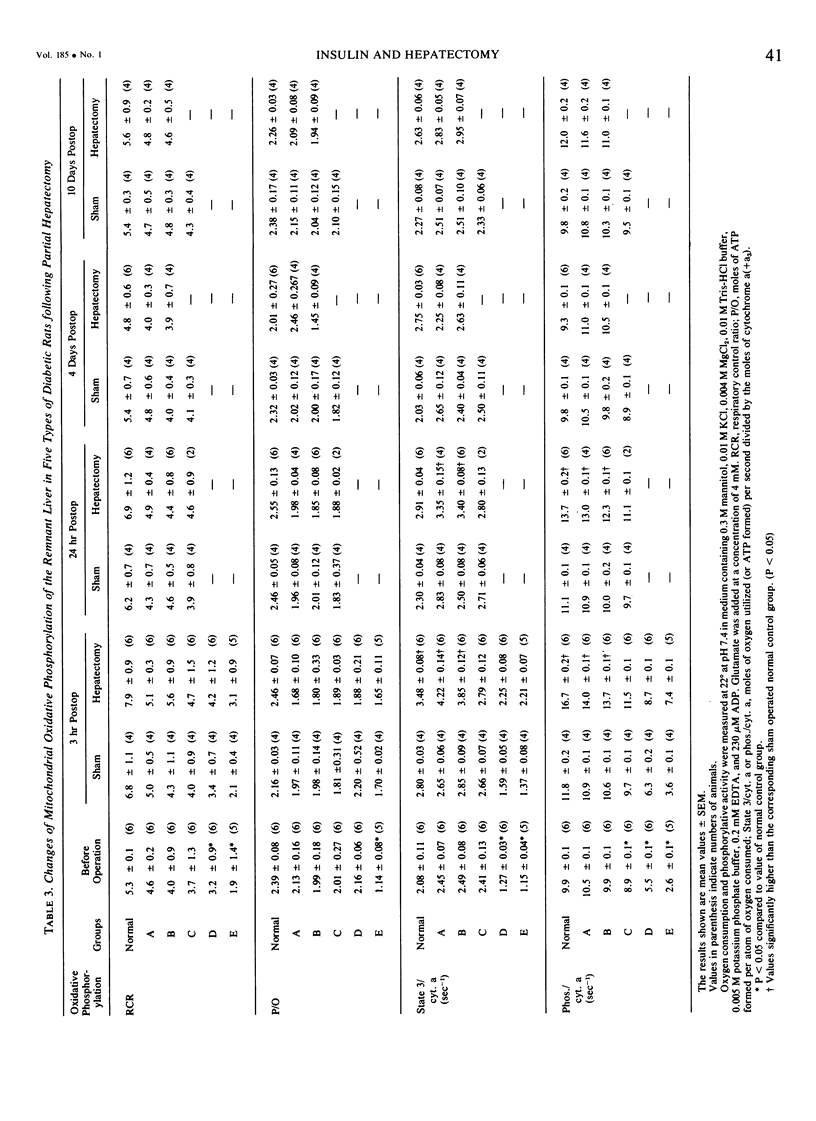
On the basis of changes in the adenine nucleotide the mitochondrial metabolism of the remnant liver, insulin requirements for hepatic regeneration were studied in diabetic rats treated with varying amounts of alloxan. Mildly diabetic rats with less than 30% inhibition in maximal portal insulin response to oral glucose load, showed a parabolic glucose tolerance pattern and could tolerate partial hepatectomy. Whereas, severely diabetic rats with more than 45% inhibition showed a linear glucose tolerance pattern and died within 24 hours after partial hepatectomy. In the former rats, the energy charge (ATP + 1/2ADP/ATP + ADP + AMP) levels of the remnant liver decrease slightly at an early period after partial hepatectomy but could be restored rapidly to normal levels with a concomitant rise of oxidative phosphorylation in remnant liver mitochondria. In contrast, the energy charge levels in the latter groups fell more markedly and could not be restored, because of insufficient enhancement of mitochondrial oxidative phosphorylation. It is suggested that an enhancement in mitochondrial phosphorylative activity of the remnant liver following partial hepatectomy is inhibited in proportion to the severity of impaired insulin secretion, resulting in a decrease of the potential functional capacity of liver.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HULTMAN E. Rapid specific method for determination of aldosaccharides in body fluids. Nature. 1959 Jan 10;183(4654):108–109. doi: 10.1038/183108a0. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Ida T., Ozawa K., Honjo I. Glucose intolerance after massive liver resection in man and other mammals. Am J Surg. 1975 May;129(5):523–527. doi: 10.1016/0002-9610(75)90310-4. [DOI] [PubMed] [Google Scholar]
- Ida T., Sato M., Yamaoka Y., Takeda H., Kamiyama Y. Effect of insulin on mitochondrial oxidative phosphorylation and energy charge of the perfused guinea pig liver. J Lab Clin Med. 1976 Jun;87(6):925–933. [PubMed] [Google Scholar]
- Kimura K., Kamiyama Y., Ozawa K., Honjo I. Changes in adenylate energy charge of the liver after an oral glucose load. Gastroenterology. 1976 May;70(5 PT1):665–668. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lerner E., Shug A. L., Elson C., Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain fatty acyl coenzyme A esters in liver mitochondria of diabetic and hibernating animals. J Biol Chem. 1972 Mar 10;247(5):1513–1519. [PubMed] [Google Scholar]
- Ozawa K., Ida T., Yamada T., Honjo I. Significance of glucose tolerance as prognostic sign in hepatectomized patients. Am J Surg. 1976 May;131(5):541–546. doi: 10.1016/0002-9610(76)90006-4. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Kitamura O., Mizukami T., Yamaoka Y., Kamano T. Human liver mitochondria. Clin Chim Acta. 1972 May;38(2):385–393. doi: 10.1016/0009-8981(72)90130-1. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Kitamura O., Yamaoka Y., Mizukami T., Kamano T. Role of portal blood on the enhancement of liver mitochondrial metabolism. Am J Surg. 1972 Jul;124(1):16–20. doi: 10.1016/0002-9610(72)90157-2. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Takeda H., Yamaoka Y., Nambu H., Kamiyama Y. Adenine nucleotide metabolism in regenerative, atrophic, and necrotizing processes of the liver. Gastroenterology. 1974 Dec;67(6):1225–1230. [PubMed] [Google Scholar]
- Ozawa K., Yamada T., Honjo I. Role of insulin as a portal factor in maintaining the viability of liver. Ann Surg. 1974 Nov;180(5):716–719. doi: 10.1097/00000658-197411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Yamaoka Y., Nanbu H., Honjo I. Insulin as the primary factor governing changes in mitochondrial metabolism leading to liver regeneration and atrophy. Am J Surg. 1974 Jun;127(6):669–675. doi: 10.1016/0002-9610(74)90344-4. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Ohsawa T., Takasan H., Ozawa K. Energy requirement in regenerative and atrophic processes of the liver in man and other mammals. Surg Gynecol Obstet. 1974 Aug;139(2):234–240. [PubMed] [Google Scholar]
- Younathan E. S. Effect of alloxan on mitochondrial adenosinetriphosphatase activity and the ATP-ADP exchange reaction. Arch Biochem Biophys. 1966 Feb;113(2):439–443. doi: 10.1016/0003-9861(66)90211-6. [DOI] [PubMed] [Google Scholar]


