Abstract
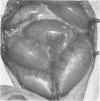
Two experiments were performed to determine the effect of heparin on experimental fibrinopurulent peritonitis in dogs. Peritonitis was induced by the creation of a 10 cm long isolated loop of terminal ileum. In a first experiment comprising 24 dogs the necrotic loop was removed 24 hours later without cleaning or irrigating the peritoneal cavity. All dogs showed fibrino-purulent peritonitis at that time. No antibiotics were given. All dogs received 500 ml of Ringer's lactate during surgery and were allowed p.o. fluids on the first postoperative day. At the time of excision the dogs were blindly randomized into a control group and two treatment groups receiving heparin 100 u/kg i.p. or s.c. respectively. Of the eight animals in the control group, five died of peritonitis and two showed residual intraperitoneal sepsis at the time of sacrifice 14 days after the initial surgery. Thus, only one dog cleared his peritoneal infection spontaneously. Of the heparin treated dogs six out of eight in the i.p. treated and seven out of eight in the s.c. treated group cleared their peritonitis spontaneously within 14 days (p ≤ 0.05 and 0.02 respectively). In a second experiment peritonitis was induced in 24 dogs as described above, but the necrotic loop was not removed. The dogs were blindly randomized to daily low dose heparin (50 u/kg s.c. b.i.d.) or no therapy. Only two out of 12 dogs of the control group survived the observation period of 14 days compared with eight out of 12 of the heparin treated group (p ≤ 0.05). However, in all dogs in this experiment residual i.p. sepsis was found. We conclude that heparin has a therapeutic effect in experimental canine peritonitis by preventing the additional apposition of fibrin and, thus, rendering the bacteria more susceptible to cellular and noncellular clearing mechanisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZER H., BLUEMEL G., PIZA F. UEBER ZUSAMMENHAENGE ZWISCHEN FIBRINOLYSE UND INTRAPERITONEALEN ADHAESIONEN. Wien Klin Wochenschr. 1963 Dec 6;75:881–883. [PubMed] [Google Scholar]
- BRYANT L. R. AN EVALUATION OF THE EFFECT OF FIBRINOLYSIN ON INTRAPERITONEAL ADHESION FORMATION. Am J Surg. 1963 Dec;106:892–897. doi: 10.1016/0002-9610(63)90152-1. [DOI] [PubMed] [Google Scholar]
- Beaufils M., Morel-Maroger L., Sraer J. D., Kanfer A., Kourilsky O., Richet G. Acute renal failure of glomerular origin during visceral abscesses. N Engl J Med. 1976 Jul 22;295(4):185–189. doi: 10.1056/NEJM197607222950402. [DOI] [PubMed] [Google Scholar]
- Buckman R. F., Jr, Bordos D., Bell W. R., Cameron J. L. Prevention of experimental postoperative adhesions by ancrod defibrinogenation. J Surg Res. 1975 Apr;18(4):377–384. doi: 10.1016/0022-4804(75)90097-9. [DOI] [PubMed] [Google Scholar]
- Buckman R. F., Woods M., Sargent L., Gervin A. S. A unifying pathogenetic mechanism in the etiology of intraperitoneal adhesions;. J Surg Res. 1976 Jan;20(1):1–5. doi: 10.1016/0022-4804(76)90075-5. [DOI] [PubMed] [Google Scholar]
- DAWSON J. L. A STUDY OF SOME FACTORS AFFECTING THE MORTALITY RATE IN DIFFUSE PERITONITIS. Gut. 1963 Dec;4:368–372. doi: 10.1136/gut.4.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervin A. S., Puckett C. L., Silver D. Serosal hypofibrinolysis. A cause of postoperative adhesions. Am J Surg. 1973 Jan;125(1):80–88. doi: 10.1016/0002-9610(73)90011-1. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. S. Radical surgical debridement in the treatment of advanced generalized bacterial peritonitis. Arch Surg. 1975 Oct;110(10):1233–1236. doi: 10.1001/archsurg.1975.01360160071012. [DOI] [PubMed] [Google Scholar]
- Huttunen R., Larmi T. K., Heikkinen E., Räsänen O. Free perforation of the colon. Etiology and treatment in 44 cases. Acta Chir Scand. 1974;140(7):535–541. [PubMed] [Google Scholar]
- JACKSON B. B. Observations of intraperitoneal adhesions; an experimental study. Surgery. 1958 Sep;44(3):507–514. [PubMed] [Google Scholar]
- James D. C., Ellis H., Hugh T. B. The effect of streptokinase on experimental intraperitoneal adhesion formation. J Pathol Bacteriol. 1965 Jul;90(1):279–287. doi: 10.1002/path.1700900132. [DOI] [PubMed] [Google Scholar]
- Jaques L. B., Waters E. T. The identity and origin of the anticoagulant of anaphylactic shock in the dog. J Physiol. 1941 Jun 30;99(4):454–466. doi: 10.1113/jphysiol.1941.sp003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIGHTLY J. J., AGOSTINO D., CLIFFTON E. E. The effect of fibrinolysin and heparin on the formation of peritoneal adhesions. Surgery. 1962 Jul;52:250–258. [PubMed] [Google Scholar]
- Lehman E. P., Boys F. THE PREVENTION OF PERITONEAL ADHESIONS WITH HEPARIN: AN EXPERIMENTAL STUDY. Ann Surg. 1940 Mar;111(3):427–435. doi: 10.1097/00000658-194003000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. W., Jr, Wichern W. A., Jr Perforated sigmoid diverticulitis. Appraisal of primary versus delayed resection. Am J Surg. 1971 May;121(5):536–540. doi: 10.1016/0002-9610(71)90134-6. [DOI] [PubMed] [Google Scholar]
- Myrhe-Jensen O., Larsen S. B., Astrup T. Fibrinolytic activity in serosal and synovial membranes. Rats, guinea pigs, and rabbits. Arch Pathol. 1969 Dec;88(6):623–630. [PubMed] [Google Scholar]
- OPIE E. L. INFLAMMATION IN SEROUS CAVITIES; DEFINITION AND MEASUREMENT. Arch Pathol. 1964 Jul;78:1–10. [PubMed] [Google Scholar]
- RILEY J. F. Functional significance of histamine and heparin in tissue mast cells. Ann N Y Acad Sci. 1963 Feb 26;103:151–163. doi: 10.1111/j.1749-6632.1963.tb53695.x. [DOI] [PubMed] [Google Scholar]
- Rosato E. F., Oram-Smith J. C., Mullis W. F., Rosato F. E. Peritoneal lavage treatment in experimental peritonitis. Ann Surg. 1972 Mar;175(3):384–387. doi: 10.1097/00000658-197203000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch J. P., Donaldson G. A. Perforative carcinoma of colon and rectum. Ann Surg. 1974 Nov;180(5):734–740. doi: 10.1097/00000658-197411000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINSSER H. H., PRYDE A. W. Experimental study of physical factors, including fibrin formation, influencing the spread of fluids and small particles within and from the peritoneal cavity of the dog. Ann Surg. 1952 Nov;136(5):818–827. doi: 10.1097/00000658-195211000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]