Abstract
We have developed a hexaplex PCR assay for rapid detection of the virulence and regulatory genes for cholera toxin enzymatic subunit A (ctxA), zonula occludens toxin (zot), accessory cholera enterotoxin (ace), toxin-coregulated pilus (tcpA), outer membrane protein (ompU), and central regulatory protein ToxR (toxR) in Vibrio cholerae and Vibrio mimicus. This hexaplex PCR proved successful in screening pathogenic-toxigenic and nonpathogenic-nontoxigenic V. cholerae and V. mimicus strains from both clinical and environmental sources.
Vibrio cholerae is an autochthonous inhabitant of brackish water and estuarine systems (2). V. cholerae O1 and O139 are currently believed to be the only serogroups causing epidemic cholera, yet non-O1 and non-O139 strains of V. cholerae and Vibrio mimicus have been associated with occasional outbreaks of cholera (9, 27). Conventional methods used to detect and classify cholera-causing vibrios isolated from clinical and environmental samples require several days to complete and involve culture in alkaline peptone water, thiosulfate citrate bile sucrose agar, slide agglutination with specific antisera, and assay for production of cholera toxin (26). However, V. cholerae O1 or O139 can be detected rapidly by using probes or primers employing the rfb region responsible for O-antigen biosynthesis (1, 8).
PCR and multiplex PCR including two genes have also been used to detect the presence of virulence-associated and regulatory genes (6, 11, 20, 25, 28-30). From a diagnostic point of view, toxigenic-pathogenic and nontoxigenic-nonpathogenic strains of V. cholerae can be differentiated by the presence of the cholera toxin and toxin-coregulated pilus genes. However, it is not known whether non-cholera toxin-producing strains possess other virulence genes, such as zot, ace, a part of the cholera toxin genetic element, and ompU. Although ompU has no role in the adhesion of bacteria (19, 22, 23), it can still serve as a useful marker for V. cholerae and V. mimicus. None of these methods can be relied on individually to identify all V. cholerae strains and detect virulence genes in a one-step PCR.
Recently, a PCR-based method targeted to the toxR gene was developed for species-specific identification of Vibrio parahaemolyticus (12). No information, however, is yet available in the literature on identification of V. cholerae with toxR primers, considering that toxR genes (showing sequence homology) are distributed among some other Vibrio species as well as V. cholerae (15, 21, 24, 25, 30). Since zot and ace are virulence factors and also play a role in the morphogenesis of the filamentous phage CTXφ (3), it is essential to look for a complete set of virulence genes comprising ctxA, zot, and ace, which are the core of the cholera toxin genetic elements, tcpA, and ompU. We therefore developed a hexaplex PCR for rapid detection of virulence and regulatory genes, including ctxA, zot, ace, tcpA, ompU, and toxR in V. cholerae and V. mimicus.
Bacterial strains.
A total of 91 V. cholerae and V. mimicus isolates were included in this study. Thirty-five strains were O1 serogroup strains (31 cholera toxin-producing strains and 4 non-cholera toxin-producing strains), 24 strains were serogroup O139 strains (23 cholera toxin-producing strains and 1 non-cholera toxin-producing strains), 21 were non-O1, non-O139 serogroup strains, and 11 strains were V. mimicus strains from laboratory stocks that had been identified previously by standard bacteriological methods (34). Details of the strains are listed in Table 1.
TABLE 1.
Results of analysis by hexaplex PCR with strains of V. cholerae O1, O139, non-O1, and non-O139 and V. mimicus
| Species and serotype | Cholera toxin production | Source | No. of strains tested | Gene present
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| ctxA | zot | ace | tcpAa | ompU | toxR | ||||
| V. cholerae | |||||||||
| O1 | Yes | Clinical | 31 | + | + | + | + | + | + |
| No | Clinical | 1 | − | − | − | − | + | + | |
| Environmental | 3 | − | − | − | − | + | + | ||
| O139 | Yes | Clinical | 13 | + | + | + | + | + | + |
| Environmental | 10 | + | + | + | + | + | + | ||
| No | Clinical | 1 | − | − | − | + | + | + | |
| Non-O1, non-O139 | No | Clinical | 2 | − | − | − | − | + | + |
| 6 | − | − | − | − | − | + | |||
| Animal | 1 | − | − | − | − | − | + | ||
| Environmental | 3 | − | − | − | − | + | + | ||
| 9 | − | − | − | − | − | + | |||
| V. mimicus | Yes | Environmental | 1 | + | + | + | + | + | + |
| No | Clinical | 3 | − | − | − | − | − | + | |
| Environmental | 6 | − | − | − | − | − | + | ||
| Environmental | 1 | − | − | − | + | + | + | ||
Strain(s) amplified the El Tor-specific tcpA gene.
V. cholerae O1 biotype El Tor strain 20 and biotype classical strain 569B and V. cholerae O139 strain ATCC 51394 were used as the PCR-positive controls for ctxA, zot, ace, tcpA, ompU, and toxR. Non-cholera toxin-producing V. cholerae O1 strains X-392, 2740-80, and 1074-78 and a ctxA-negative strain, CVD-103HgR, obtained from J. B. Kaper, Center for Vaccine Development, University of Maryland School of Medicine, Baltimore, Md., and a toxR-negative strain, O395-12 (18), and tcpA-negative strain, O395 RT 110-12 (32), provided by J. J. Mekalanos, Department of Microbiology and Molecular Genetics, Harvard Medical School, Boston, Mass., were used as negative controls. All strains were stored in 20% glycerol-Luria-Bertani broth at −70°C. Before use, identification of each culture was confirmed by selected biochemical tests and serology (34).
Hexaplex PCR.
V. cholerae and V. mimicus strains grown overnight at 37°C in Luria-Bertani broth (USB, Amersham Life Technology) were boiled and stored at −20°C until use. The bacterial cell lysate was used as the source of template DNA. Oligonucleotide sequences for the virulence and regulatory genes are shown in Table 2.
TABLE 2.
Sequences of primers used for detection of virulence and regulatory genes
| Targeta | Nucleotide sequence (5′-3′) | Amplicon size (bp) | Reference |
|---|---|---|---|
| ctxA-F | CGGGCAGATTCTAGACCTC CTG | 564 | 6 |
| ctxA-B | CGATGATCTTGGAGCATTC CCAC | ||
| zot-F | TCGCTTAACGATGGCGCGT TTT | 947 | 25 |
| zot-B | AACCCCGTTTCACTTCTACC CA | ||
| ace-F | TAAGGATGTGCTTATGATG GACACCC | 316 | 28 |
| ace-B | CGTGATGAATAAAGATACT CATAGG | ||
| ompU-F | ACGCTGACGGAATCAACCA AAG | 869 | 25 |
| ompU-B | GCGGAAGTTTGGCTTGAAG TAG | ||
| tcpA-F | CACGATAAGAAAACCGGTC AAGAG | ||
| tcpA-B/Clas | TTACCAAATGCAACGCCGA ATG | 620 | 25 |
| tcpA-B/∗ | CGAAAGCACCTTCTTTCACA CGTTG | 453 | 25 |
| toxR-F | CCTTCGATCCCCTAAGCAA TAC | 779 | 25 |
| toxR-B | AGGGTTAGCAACGATGCG TAAG |
F, forward; B, backward; Clas, classical specific; ∗, El Tor- and/or O139 serotype-specific amplification.
PCR amplification of the target DNA was carried out in a thermal cycler (ramping time 1o/s; Bio-Rad Inc.) with 200-μl PCR tubes with a reaction mixture volume of 50 μl. Each of the reaction mixtures contained 5 μl of 10× PCR amplification buffer [500 mM KCl, 100 mM Tris-HCl (pH 9.0), 1.0% Triton X-100] (Promega, Madison, Wis.), 5 μl of MgCl2 (25 mM), 5 μl each of 2.5 mM dATP, dCTP, dGTP, and dTTP (Amersham Pharmacia Biotech), 2 μl each of the forward and reverse primers for toxR (20 ng/μl), 0.5 μl of Taq DNA polymerase at 5 U/μl (Promega), and Milli-Q water to a final volume of 47.5 μl, and 2.5 μl of cell lysate (template DNA). PCR was programmed as follows: an initial denaturation at 94°C for 2 min, followed by 30 cycles consisting of 94°C for 1 min, 62°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 10 min.
A hexaplex PCR was carried out by simultaneous addition of primer pairs for ctxA, ompU, zot, ace, and tcpA (Table 2) in the same reaction mixture. In initial experiments, ctxA, ompU, zot, ace, and tcpA primer concentrations varied between 16 and 40 μM, keeping the toxR primer concentration fixed at either 40 or 50 μM in the final reaction mixture of 50 μl. Optimum results were obtained with a primer concentration of 16 μM for ctxA and ompU, 30 μM for zot, ace, and tcpA, and 50 μM for toxR. PCR conditions for amplification remained the same except for annealing of template DNA at 62°C for 1 min for 20 cycles and 54°C for 1 min for 10 cycles. Amplified products were separated by agarose (1.8%, wt/vol) gel electrophoresis in Tris-borate buffer (0.5× TBE), stained in ethidium bromide, and visualized with a Fluoro-S-MultiImager (Bio-Rad, Inc.). PCR-negative strains were verified by Southern blot hybridization.
Probes and hybridization.
Colony blots were prepared with nylon filters (Hybond; Amersham International, London, United Kingdom) and processed by a standard method (16). Briefly, colonies were lysed with denaturing solution (0.5 M NaOH, 1.5 NaCl) and neutralized in neutralizing solution (0.5 M Tris-HCl [pH 8.0], 1.5 M NaCl), and the liberated DNA was fixed to nylon membranes by exposure to UV light for 2 min, in accordance with the manufacturer's instructions.
PCR-amplified products of the ctxA, tcpA, and ompU genes (25) were used as probes, randomly labeled (5) with [α-32P]dCTP (3,000 Ci/mmol; BARC, Bombay, India) and hybridized at 65°C in phosphate buffer containing 500 mM Na2HPO4 (pH 7.2), 7% (wt/vol) sodium dodecyl sulfate, 1 mM EDTA, and 1% (wt/vol) bovine serum albumin. Hybridized blots were washed once in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 5 min at room temperature, two times in 2× SSC-0.1% sodium dodecyl sulfate for 10 min at 65°C, and once in 0.1× SSC-0.1% sodium dodecyl sulfate for 15 min at 65°C. Autoradiographs were developed from the hybridized filters with the Bio-Rad PhosphorImager screen and visualized in a PhosphorImager (Bio-Rad).
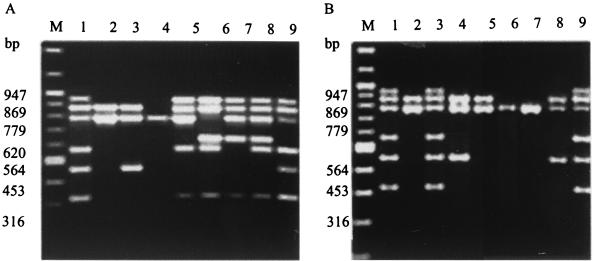
V. cholerae strains belonging to serogroups O1, O139, non-O1, and non-O139 and V. mimicus isolates of both clinical and environmental origin were screened for the presence of virulence and regulatory genes. Thirty-one isolates of V. cholerae O1 and 23 isolates of V. cholerae O139 gave positive results for the ctxA, zot, ace, tcpA (El Tor specific), ompU, and toxR genes. The fragment sizes were 564 bp for ctxA, 947 bp for zot, 316 bp for ace, 453 bp for El Tor-specific tcpA, 620 bp for classical specific tcpA, 869 bp for ompU, and 779 bp for toxR (Fig. 1A). Four isolates of V. cholerae O1 and one isolate of V. cholerae O139 gave positive results for the ompU and toxR genes. However, the V. cholerae O139 isolate also gave positive results for the El Tor-specific tcpA gene (Table 1).
FIG. 1.
(A) Ethidium bromide-stained agarose gel electrophoresis of hexaplex PCR products from Vibrio cholerae strains. Lane M, 100-bp DNA ladder (NEB); lanes 1, 8 and 9, cholera toxin-producing V. cholerae O1 El Tor strain 20, classical strain 569B, and V. cholerae O139 strain ATCC 51394, respectively; lanes 2 through 4, non-cholera toxin-producing V. cholerae O1 El Tor strains X392, 274-80, and 1074-78, respectively; lane 5, tcpA-negative V. cholerae O1 classical strain O395 RT 110-12; lane 6, toxR-negative V. cholerae O1 classical strain O395-12; lane 7, ctxA-negative V. cholerae O1 classical strain CVD 103-HgR. (B) Hexaplex PCR products of representative V. cholerae O1, O139, non-O1, and non-O139 and V. mimicus strains in an ethidium bromide-stained agarose gel. Lane M, 100-bp DNA ladder (NEB); lane 1, cholera toxin-producing V. cholerae O1 El Tor strain KO63 (diarrhea isolate from Kerala, India, 2000); lane 2, non-cholera toxin-producing V. cholerae O1 El Tor strain GS2 (isolate from Gerris spinolae, Varanasi, India, 1987); lane 3, cholera toxin-producing V. cholerae O139 strain VO522 (diarrhea isolate from Varanasi, India, 1994); lane 4, non-cholera toxin-producing V. cholerae O139 strain CO788 (diarrhea isolate from Kolkata, India, 1992); lanes 5 and 6, non-cholera toxin-producing V. cholerae non-O1, non-O139 strains 12475 and 13094, respectively (diarrhea isolates from Varanasi, India, 1979); lanes 7 and 8, non-cholera toxin-producing V. mimicus strains WM18 (water isolate from the River Ganges, Varanasi, India, 1988) and VM2 (water isolate from the River Ganges, Varanasi, India, 1988), respectively; and lane 9, cholera toxin-producing V. mimicus strain WM8 (water isolate from the River Ganges, Varanasi, India, 1986).
All of the non-O1, non-O139 V. cholerae strains yielded positive results by hexaplex PCR for the gene encoding the central regulatory protein ToxR. However, five of the isolates also yielded positive results for the ompU gene. None were positive for the ctxA, zot, ace, and tcpA genes (Table 1).
All of the V. mimicus strains were positive for the gene encoding the central regulatory protein ToxR (Table 1). Two environmental V. mimicus strains were positive for the genes encoding tcpA (El Tor specific) and ompU, and one strain was positive for the ctxA, zot, and ace genes. Results of the hexaplex PCR of representative strains of V. cholerae O1, O139, non-O1, and non-139 and V. mimicus possessing various combinations of virulence and regulatory genes are shown in Fig. 1B.
All of the V. cholerae O1, O139, non-O1, and non-O139 and V. mimicus strains that showed negative results either for ctxA, tcpA, and/or ompU or for all genes by hexaplex PCR were also negative in the colony blot assay (data not shown). These observations therefore confirm the absence of these genes in V. cholerae and V. mimicus.
The pathogenesis of cholera is a complex process, involving a number of factors which help the pathogen colonize the epithelium of the small intestine and produce enterotoxins that disrupt ion transport. Although production of cholera toxin by V. cholerae, encoded by the ctxAB genes, is directly responsible for the manifestation of diarrhea, cholera pathogenesis relies on the synergistic action of a number of other genes and part of the cholera toxin genetic elements comprising zonula occludens toxin, which increases the permeability of the small-intestinal mucosa by affecting the structure of the tight junction (4), and accessory cholera enterotoxin, which is capable of causing fluid accumulation in rabbit ileal loops (33).
In addition, the toxin-coregulated pilus (TCP) gene cluster is also required for pathogenesis of cholera. V. cholerae strains not carrying and carrying the ctx, zot, ace, and tcpA genes either alone or in combination have been reported (9, 10, 13, 14). Moreover, V. cholerae O1 and O139 strains isolated from the aquatic environment have been reported to be non-cholera toxin-producing, i.e., lacking the ability to produce cholera toxin (9, 10). However, we have demonstrated that environmental isolates, like clinical isolates, possess the ctxA, zot, ace, tcpA, and ompU genes, comprising the virulence gene cassette, and genes encoding surface organelles required for intestinal adherence and colonization (30). It has also been reported that V. cholerae strains devoid of the virulence gene cassette can cause diarrhea by a mechanism different from that of cholera toxin-producing V. cholerae (7, 14, 30).
The results obtained in this study by hexaplex PCR corroborate the finding that environmental isolates of V. cholerae O1 and O139 can be cholera toxin producing and pathogenic. A number of O1 and O139 V. cholerae, V. mimicus, and all of the non-O1, non-O139 strains lacked either the core of the cholera toxin genetic element and/or the tcpA or ompU gene or both, suggesting that none of the described genes alone or in combination are good indicators of pathogenicity.
We exploited the sequence homology of the toxR gene (17) among V. cholerae O1, O139, non-O1, and non-O139 and V. mimicus strains, both for screening and for determining sequence differences between the classical and El Tor biotype tcpA genes (11, 31). However, we could not differentiate strains belonging to serogroups O1 and O139. From the results of this study, it is concluded that the presence of virulence genes comprising cholera toxin genetic elements, outer membrane proteins, and TCP in V. cholerae O1, O139, non-O1, and non-O139 and V. mimicus can be readily determined. A one-step hexaplex PCR can therefore be used for rapid detection of virulence and regulatory genes, a valuable step in screening for both toxigenic-pathogenic and nontoxigenic-nonpathogenic V. cholerae and V. mimicus strains isolated from clinical and environmental sources.
Acknowledgments
This research was supported by a grant to D.V.S. (SP/SO/BO6/99) from the Department of Science and Technology, New Delhi, India, and funds contributed by the Department of Biotechnology, New Delhi, to the Rajiv Gandhi Centre for Biotechnology.
We thank Gopal Nath and B. N. Shukla, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, and A. C. P. Vicente, Department of Genetics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, for providing Vibrio cholerae and Vibrio mimicus strains and G. B. Nair, National Institute of Cholera and Enteric Diseases, Beliaghata, Kolkata, India, for comments on the manuscript.
REFERENCES
- 1.Albert, M. J., D. Islam, S. Nahar, F. Qadri, S. Falklind, and A. Weintraub. 1997. Rapid detection of Vibrio cholerae O139 Bengal from stool specimens by PCR. J. Clin. Microbiol. 35:1633-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colwell, R. R., J. B. Kaper, and S. W. Joseph. 1977. Vibrio cholerae, Vibrio parahaemolyticus and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394-396. [PubMed] [Google Scholar]
- 3.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasano, A., B. Baudry, D. W. Pumplin, S. S. Wasserman, B. D. Tall, J. M. Ketley, and J. B. Kaper. 1991. Vibrio cholerae produces second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 88:5242-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinberg, A., and B. Voglestein. 1984. A technique for rapid labeling of DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 137:266-267. [DOI] [PubMed] [Google Scholar]
- 6.Fields, P. I., T. Popovic, K. Wachsmuth, and Ø. Olsvik. 1992. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J. Clin. Microbiol. 30:2118-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino, K., S. Yamasaki, A. K. Mukhopadhyay, S. Chakraborty, A. Basu, S. K. Bhattacharya, G. B. Nair, T. Shimada, and Y. Takeda. 1998. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol. Med. Microbiol. 20:201-207. [DOI] [PubMed] [Google Scholar]
- 9.Kaper, J. B., J. G. Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaper, J. B., S. L. Moseley, and S. Falkow. 1981. Molecular characterization of environmental and nontoxigenic strains of Vibrio cholerae. Infect. Immun. 32:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keasler, S. P., and R. H. Hall. 1993. Detecting and biotyping Vibrio cholerae O1 with multiplex polymerase chain reaction. Lancet 341:1661.. [DOI] [PubMed] [Google Scholar]
- 12.Kim, Y., J. Okuda, C. Matsumoto, N. Takahashi, S. Hashimoto, and M. Nishibuchi. 1999. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 37:1173-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurazono, H., A. Pal, P. K. Bag, G. B. Nair, T. Karasawa, T. Mihara, and Y. Takeda. 1995. Distribution of genes encoding cholera toxin, zonula occludens toxin, accessory cholera enterotoxin, and El Tor hemolysin in Vibrio cholerae of diverse origins. Microb. Pathol. 18:231-235. [DOI] [PubMed] [Google Scholar]
- 14.Levine, M. M., R. E. Black, M. L. Clements, L. Cisnors, A. Saah, D. R. Nalin, D. M. Gill, J. P. Craig, C. R. Young, and P. Ristaino. 1982. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J. Infect. Dis. 145:296-299. [DOI] [PubMed] [Google Scholar]
- 15.Lin, Z., K. Kumagi, K. Baba, J. J. Mekalanos, and M. Nishibuchi. 1993. Vibrio parahaemolyticus has a homologue of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. J. Bacteriol. 175:3849-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 17.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48:271-279. [DOI] [PubMed] [Google Scholar]
- 18.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naksone, N., and M. Iwanaga. 1998. Characterization of outer membrane protein OmpU of Vibrio cholerae O1. Infect. Immun. 66:4726-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandi, B., R. K. Nandi, S. Mukhopadhyay, G. B. Nair, T. Shimada, and A. C. Ghose. 2000. Rapid detection of species-specific identification of Vibrio cholerae with primers targeted to the gene of outer membrane protein OmpW. J. Clin. Microbiol. 38:4145-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osorio, C. R., and K. E. Klose. 2000. A region of transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among Vibrio species. J. Bacteriol. 182:526-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provenzano, D., and K. E. Klose. 2000. Altered expression of toxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. USA 97:10220-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provenzano, D., C. M. Lauriano, and K. E. Klose. 2001. Characterization of the role of toxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence. J. Bacteriol. 183:3652-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reich, K. A., and G. K. Schoolnik. 1994. The light organ symbiont Vibrio fisheri possesses a homologue of the Vibrio cholerae transmembrane transcriptional activator ToxR. J. Bacteriol. 176:3085-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera, I. N. G., J. Chun, A. Huq, R. B. Sack, and R. R. Colwell. 2001. Genotype associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 67:2421-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakazaki, R. 1992. Bacteriology of Vibrio and related organisms, p. 37-55. In D. Barua and W. B. Greenough III (ed.), Cholera. Plenum Medical Book Company, New York, N.Y.
- 27.Sanyal, S. C., M. I. Huq, P. K. B. Neogy, K. Alam, M. I. Kabir, and A. S. M. H. Rahman. 1983. Vibrio mimicus as an aetiologic agent of diarrhoea and its pathogenesis. Indian J. Med. Microbiol. 1:1-12. [Google Scholar]
- 28.Shi, L., S. Miyoshi, M. Hiura, K. Tomochika, T. Shimada, and S. Shinoda. 1998. Detection of genes encoding cholera toxin (cholera toxin), zonula occludens toxin (ZOT), accessory cholera enterotoxin (ACE) and heat stable enterotoxin (ST) in Vibrio mimicus clinical strains. Microbiol. Immunol. 42:823-828. [DOI] [PubMed] [Google Scholar]
- 29.Shirai, H., M. Nishibuchi, T. Ramamurthy, S. K. Bhattacharya, S. C. Pal, and Y. Takeda. 1991. Polymerase chain reaction of the cholera enterotoxin operon of Vibrio cholerae. J. Clin. Microbiol. 29:2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, D. V., M. H. Matte, G. R. Matte, S. Jiang, F. Sabeena, B. N. Shukla, S. C. Sanyal, A. Huq, and R. R. Colwell. 2001. Molecular analysis of Vibrio cholerae O1, O139, non-O1, and non-O139 strains: clonal relationships between clinical and environmental isolates. Appl. Environ. Microbiol. 67:910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor, R. K., C. Shaw, K. Peterson, P. Spears, and J. J. Mekalanos. 1987. Safe, live Vibrio cholerae vaccines? Vaccine 6:151-154. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusion to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trucksis, M., J. E. Galen, J. Michalski, A. Fasano, and J. B. Kaper. 1993. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc. Natl. Acad. Sci. USA 90:5267-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 1987. Manual for laboratory investigation of acute infection. Publication W.H.O. CDD/83.3. Program for Control of Diarrhoeal Diseases. World Health Organization, Geneva, Switzerland.