Abstract
A pronounced depletion of an opsonic protein for hepatic reticuloendothelial (RE) phagocytosis has been demonstrated in critically ill trauma patients. This opsonic α2 surface binding (SB) glycoprotein has immunologic identity and a similar amino acid composition to cold insoluble globulin (CIg). Since CIg can be concentrated in cryoprecipitate, it was utilized as a readily available source of opsonic α2SB glycoprotein for replacement therapy after injury with documented hypoopsonemia. Six septic patients (2 multiple trauma, 2 thermal burn, and 2 intra-abdominal abscess) were studied to test whether cryoprecipitate infusion would restore this humoral component. Pre- and posttherapy opsonin levels were determined by bioassay and electroimmunoassay. In all patients, severe opsonin depletion was reversed following cryoprecipitate infusion. All patients had a rapid improvement in febrile state, normalization of leukocyte levels, and improvement in pulmonary function as evidenced by decreasing requirements for end expiratory pressure at lowered levels of inspired oxygen. One patient was studied more extensively and demonstrated an increase in cardiac output, limb blood flow, total body and limb oxygen delivery, total body and limb oxygen consumption and a progressive decrease in pulmonary shunt fraction. Thus, opsonic α2SB glycoprotein deficiency can be reversed by cryoprecipitate infusion in critically ill septic injured patients. Replacement of this humoral factor may be an important therapeutic modality in prevention of multiple organ failure, but it should be administered only after documentation of hypoopsonemia in traumatized patients.
Full text
PDF



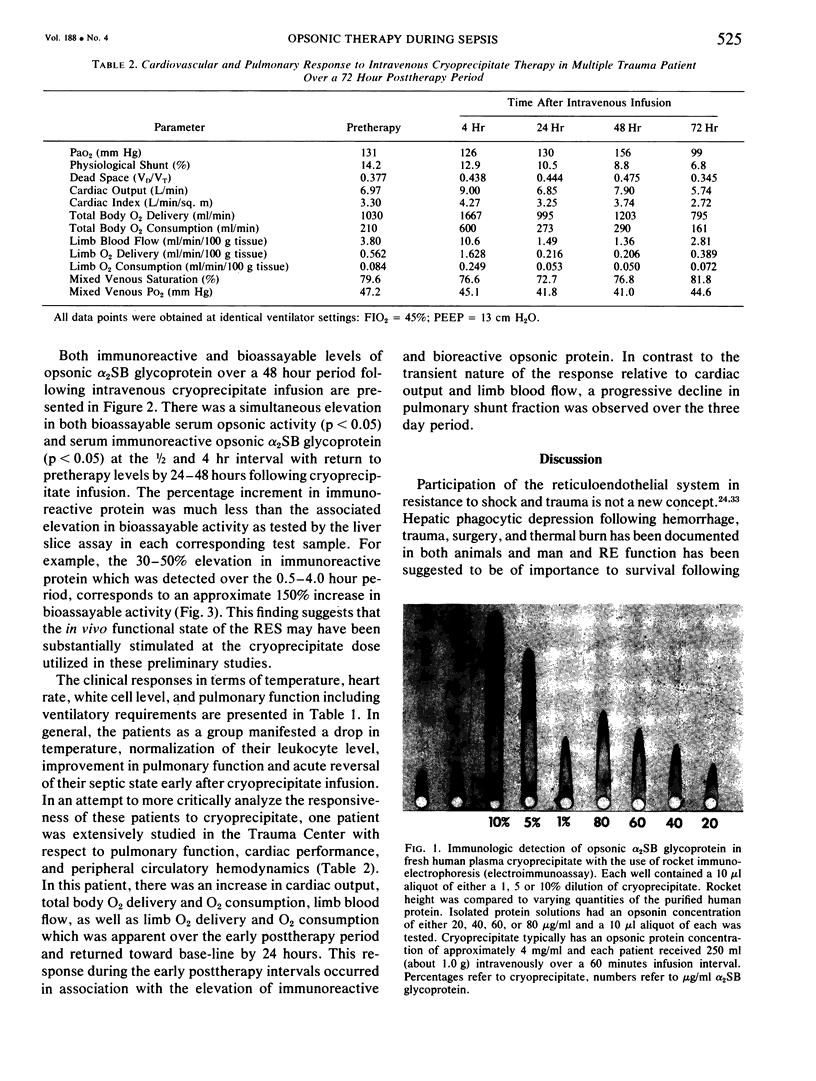
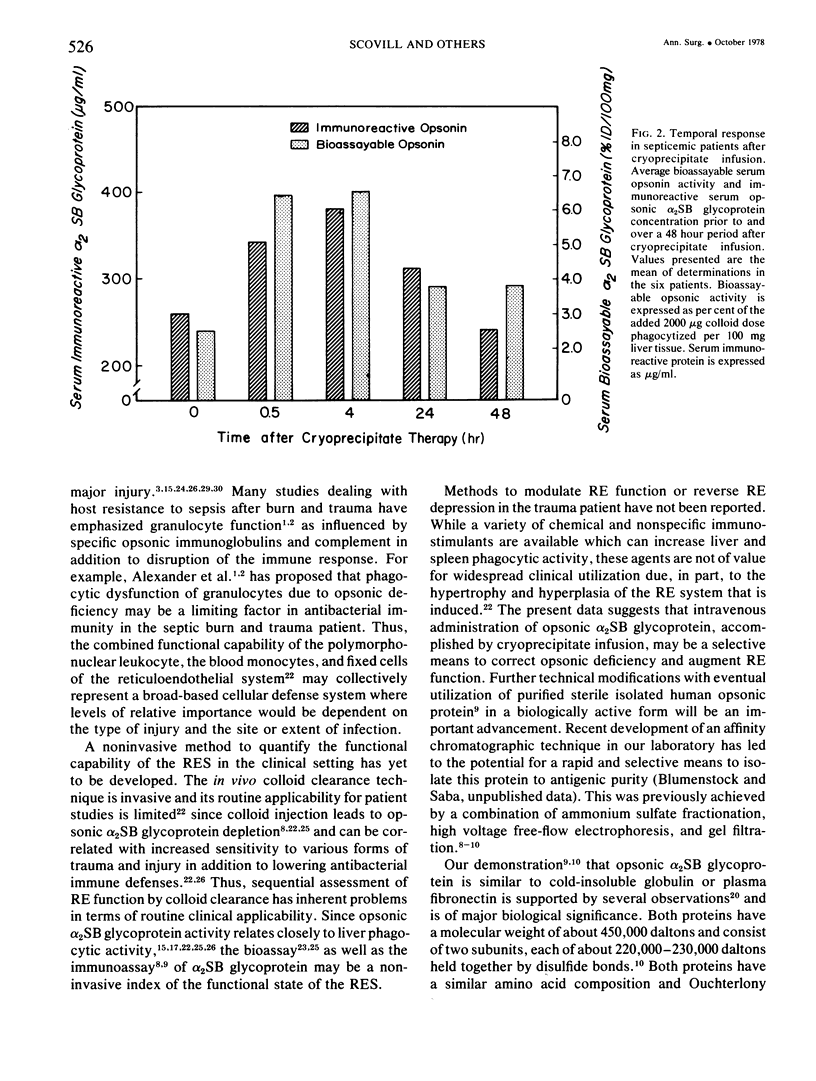
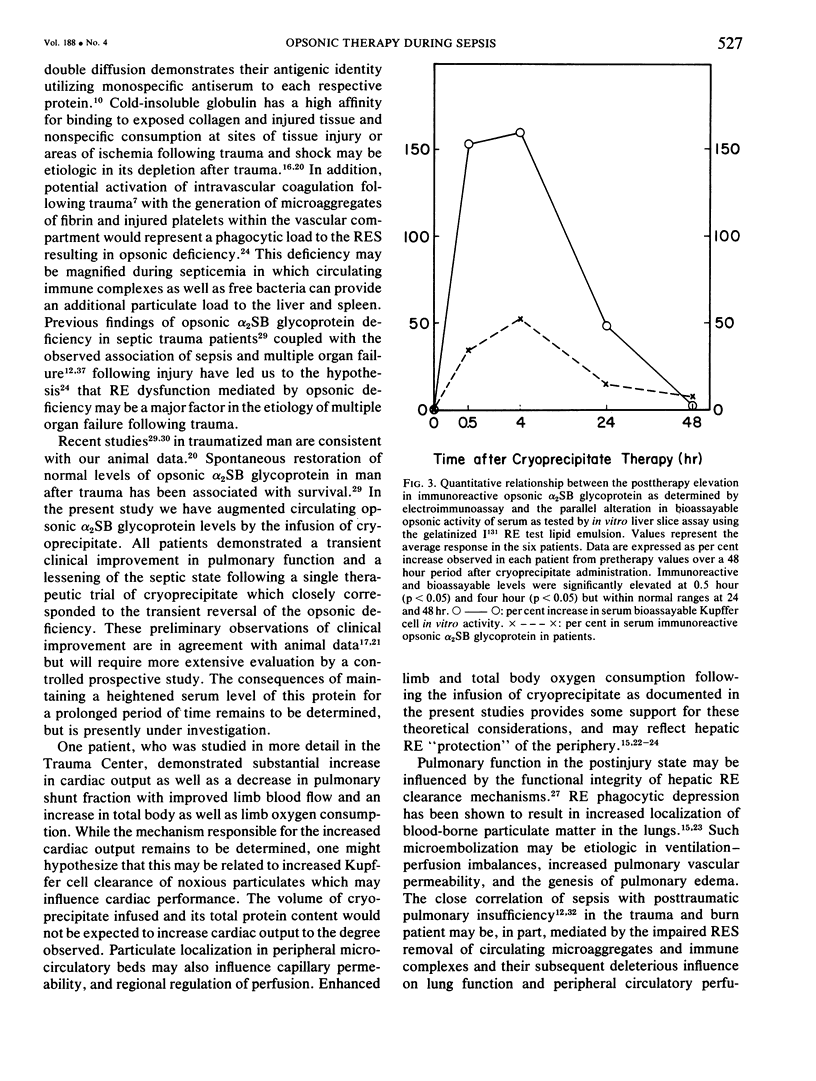


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Korelitz J., Alexander N. S. Prevention of wound infections. A case for closed suction drainage to remove wound fluids deficient in opsonic proteins. Am J Surg. 1976 Jul;132(1):59–63. doi: 10.1016/0002-9610(76)90291-9. [DOI] [PubMed] [Google Scholar]
- Alexander J. W., McClellan M. A., Ogle C. K., Ogle J. D. Consumptive opsoninopathy: possible pathogenesis in lethal and opportunistic infections. Ann Surg. 1976 Dec;184(6):672–678. doi: 10.1097/00000658-197612000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura B. M., Hershey S. G. Reticuloendothelial function in experimental injury and tolerance to shock. Adv Exp Med Biol. 1972;33(0):545–569. doi: 10.1007/978-1-4684-3228-2_57. [DOI] [PubMed] [Google Scholar]
- Berman I. R. Intravascular microaggregation and the respiratory distress syndromes. Pediatr Clin North Am. 1975 May;22(2):275–287. doi: 10.1016/s0031-3955(16)33129-7. [DOI] [PubMed] [Google Scholar]
- Berman I. R., Smulson M. E., Pattengale P., Schoenbach S. F. Pulmonary microembolism after soft tissue injury in primates. Surgery. 1971 Aug;70(2):246–253. [PubMed] [Google Scholar]
- Blaisdell F. W. Pathophysiology of the respiratory distress syndrome. Arch Surg. 1974 Jan;108(1):44–49. doi: 10.1001/archsurg.1974.01350250036009. [DOI] [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P. Purification of alpha-2-opsonic protein from human serum and its measurement by immunoassay. J Reticuloendothel Soc. 1978 Feb;23(2):119–134. [PubMed] [Google Scholar]
- Di Luzio N. R., Lindsey E. S. Surgery-induced alterations in plasma recognition factor activity in normal renal donors and renal recipients. Proc Soc Exp Biol Med. 1973 Jul;143(3):715–718. doi: 10.3181/00379727-143-37398. [DOI] [PubMed] [Google Scholar]
- Fulton R. L., Jones C. E., Jr The etiology of post-traumatic pulmonary insufficiency in man. Rev Surg. 1975 Mar-Apr;32(2):84–85. [PubMed] [Google Scholar]
- Gans H., Lowman J. T. The uptake of fibrin and fibrin-degradation products by the isolated perfused rat liver. Blood. 1967 Apr;29(4):526–539. [PubMed] [Google Scholar]
- Goldman A. S., Rudloff H. B., McNamee R., Loose L. D., DiLuzio N. R. Deficiency of plasma humoral recognition factor activity following burn injury. J Reticuloendothel Soc. 1974 Mar;15(3):193–198. [PubMed] [Google Scholar]
- Kaplan J. E., Molnar J., Saba T. M., Allen C. Comparative disappearance and localization of isotopically labeled opsonic protein and soluble albumin following surgical trauma. J Reticuloendothel Soc. 1976 Nov;20(5):375–384. [PubMed] [Google Scholar]
- LEE L., McCLUSKEY R. T. Immunohistochemical demonstration of the reticuloendothelial clearance of circulating fibrin aggregates. J Exp Med. 1962 Nov 1;116:611–618. doi: 10.1084/jem.116.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist O., Saldeen T., Sandler H. Pulmonary damage following pulmonary microembolism in the dog. Effect of various types of treatment. Acta Chir Scand. 1976;142(1):15–19. [PubMed] [Google Scholar]
- Saba T. M., Di Luzio N. R. Reticuloendothelial blockade and recovery as a function of opsonic activity. Am J Physiol. 1969 Jan;216(1):197–205. doi: 10.1152/ajplegacy.1969.216.1.197. [DOI] [PubMed] [Google Scholar]
- Saba T. M. Effect of surgical trauma on the clearance and localization of blood-borne particulate matter. Surgery. 1972 May;71(5):675–685. [PubMed] [Google Scholar]
- Saba T. M. Prevention of liver reticuloendothelial systemic host defense failure after surgery by intravenous opsonic glycoprotein therapy. Ann Surg. 1978 Aug;188(2):142–152. doi: 10.1097/00000658-197808000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Kaplan J. E., Bernard H. R., Powers S. R., Jr Disturbances in circulating opsonic activity in man after operative and blunt trauma. J Surg Res. 1977 Jun;22(6):709–716. doi: 10.1016/0022-4804(77)90113-5. [DOI] [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Kaplan J. E., Bernard H., Powers S., Jr Deficits in reticuloendothelial humoral control mechanisms in patients after trauma. J Trauma. 1976 Nov;16(11):898–904. doi: 10.1097/00005373-197611000-00008. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W. Blood gas calculator. J Appl Physiol. 1966 May;21(3):1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- Suwa K., Bendixen H. H. Change in PaCO2 with mechanical dead space during artificial ventilation. J Appl Physiol. 1968 Apr;24(4):556–562. doi: 10.1152/jappl.1968.24.4.556. [DOI] [PubMed] [Google Scholar]
- Walker L., Eiseman B. The changing pattern of post-traumatic respiratory distress syndrome. Ann Surg. 1975 May;181(5):693–697. doi: 10.1097/00000658-197505000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZWEIFACH B. W., BENACERRAF B., THOMAS L. The relationship between the vascular manifestations of shock produced by endotoxin, trauma, and hemorrhage. II. The possible role of the reticulo-endothelial system in resistance to each type of shock. J Exp Med. 1957 Sep 1;106(3):403–414. doi: 10.1084/jem.106.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]