Atopic eczema is a chronic, relapsing, inflammatory skin condition associated with epidermal barrier dysfunction. This article provides a summary of current knowledge on eczema and its management.
Sources and selection criteria
We used the following sources of information to write this review:
PubMed search using the key words “atopic eczema”, “atopic dermatitis”, “incidence”, “genetics”, “pathogenesis”, “treatment”, and “management”. We gave preference to original articles published in the past three years and recent review articles published in high impact journals
Search of the following Cochrane Library databases: Cochrane Database of Systemic Reviews; Database of Abstracts and Reviews of Effectiveness; Cochrane Central Register of Controlled Trials
Personal archive of references.
How do we define atopic eczema?
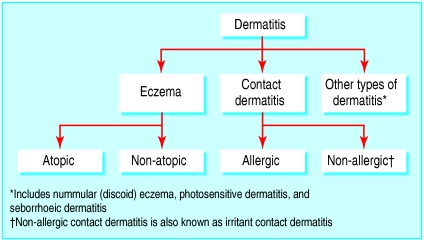
Atopic eczema and atopic dermatitis are terms that have been used synonymously (for a clinical definition see box), but a review committee by the World Allergy Organisation has published its recommended terminology (see fig 1).1
Fig 1.
Subgroups of dermatitis. Some patients may have a combination of subgroup types
Eczema is subdivided into atopic and non-atopic eczema because a proportion of patients exhibit eczema without atopic features. Children with atopic eczema are more likely than those with non-atopic eczema to develop asthma later in life, and their eczema more often persists into adulthood. However, atopic and non-atopic eczema have not been shown to respond differently to treatment, and patients with non-atopic eczema may subsequently develop atopic features.
Summary points
Atopic eczema is an itchy inflammatory skin condition with associated epidermal barrier dysfunction
The prevalence of atopic eczema seems to be rising, but the factors responsible for this rise are not fully understood
The pathophysiology of eczema involves systemic as well as cutaneous immune and epidermal dysfunction
Eczema is a complex trait with significant genetic and environmental influences
Emollients and topical steroids are the mainstay of treatment for mild to moderate eczema; moderate to severe eczema may require the addition of second line agents such as topical or systemic calcineurin inhibitors, ultraviolet phototherapy, or systemic azathioprine
A clearer understanding of the genetic basis and pathophysiology of eczema is expected to lead to new improved treatments
The results of future studies relating clinical features, pathogenesis, and molecular genetics may lead to a clearer understanding and more accurate subclassification of eczema.
Why is eczema important?
Itch as a symptom is often underestimated in terms of the problems that it can cause. Sleep disturbance and worries about appearance are often reported, as well as feelings of guilt and frustration as a consequence of a child's eczema.
Although the effects of total sleep deprivation on human health are well recognised, recent studies have highlighted that recurrent, partial sleep disturbance (often occurring in patients with atopic eczema) also results in considerable neurocognitive impairment.
When taken together, the social, emotional, and financial effects seem greater on a family looking after a child with moderate to severe eczema than on a family caring for a child with type 1 diabetes.3
Epidemiology
Prevalence and incidence of atopic eczema
The prevalence of atopic eczema varies widely between populations, with estimates of less than 2% in children in China and Iran but up to 20% in northern and western Europe, Australia, and the United States. Many studies have reported an increasing prevalence, but some recent research has suggested stable prevalence and incidence.4,5
Importance of environmental factors
Any recent increase in prevalence cannot be explained by genetic change, and environmental influences must therefore play a role in phenotypic expression. Migrant studies suggest a strong link between atopic eczema and environmental factors. A higher incidence of atopic eczema is associated with urban and industrial settings, higher socioeconomic status, and smaller family size.
These observations have contributed to the so called “hygiene hypothesis”—that is, infections early in life give protection against the development of atopic disease. Some but not all research findings support this hypothesis.6
Little is known about the role of dietary factors in the aetiology of eczema. A protective effect of breast feeding has been postulated, but the evidence is conflicting. A Cochrane review concluded that there is inadequate evidence to advise women to avoid specific foods during pregnancy or breast feeding with the aim of protecting their children from atopic diseases.7
Pathophysiology—what do we know?
The clinical phenotype of eczema has a multifactorial aetiology, and the pathophysiology is not fully understood.
Immune dysfunction
Many inter-related components of the immune system are known to be dysfunctional in the context of atopic eczema (table). There is cutaneous hyper-reactivity to environmental triggers that would be innocuous to non-atopic individuals, and barrier function is disturbed in the clinically uninvolved skin of patients with atopic eczema as well as within eczema lesions.
Table 1.
Some features of the innate and acquired immune systems that are thought to play a pathological role in atopic eczema
| Component of immune system | Mechanism/dysfunction |
|---|---|
| T lymphocytes |
Predominance of TH2 over TH1 subtypes in the systemic immune response |
| Regulatory T cells (Tregs) are also important | |
| Keratinocytes |
Keratinocytes produce cytokines and chemokines to induce cutaneous immune responses |
| Atopic keratinocytes have a reduced ability to synthesise antimicrobial peptides | |
| Keratinocytes produce ceramides, which are important in skin barrier function | |
| Antigen presenting cells |
Langerhans cells and inflammatory dendritic epidermal cells (specific to atopic eczema skin) play a role in T cell activation |
| Eosinophils and IgE | The roles of allergen specific IgE reactions and autoreactivity remain controversial |
Immunopathogenesis is discussed in more detail on the bmj.com web extra.
Are allergies important?
It is well established that patients with atopic eczema may become sensitised to common food and environmental allergens. However, whether these reactions are responsible for precipitating or maintaining atopic dermatitis remains controversial. For example, a placebo controlled double blind trial showed that various measures to reduce house dust mites could improve severe atopic eczema,8 but this finding was not reproduced in a more recent study with similar design.9
Clinical experience suggests that food allergy is not commonly an important factor in the relapse of atopic dermatitis; in the small number of patients in whom food allergy is important, this is usually obvious to the patient or their carers.
Definition of atopic dermatitis*
To qualify as having atopic dermatitis, an individual must have:
An itchy skin condition in the last 12 months
-
Plus three or more of the following:
Onset before 2 years of age (not applicable in child under 4 years)
History of flexural involvement
History of generally dry skin
History of other atopic disease (or history in first degree relative if child is under 4 years)
Visible flexural dermatitis
These diagnostic criteria have been validated in hospital and community settings and have used in many epidemiological research studies worldwide
*UK refinement of the Hanifin and Rajka Diagnostic Criteria.2
Non-allergic factors
Irritants such as detergents disrupt the barrier function of skin and may precipitate flare-ups of pre-existing dermatitis.
Pruritus results in scratching and causes additional damage to the skin barrier, predisposing to secondary infection. This perpetuates the so called “itch-scratch cycle.”
Staphylococcus aureus is often isolated from eczematous lesions as well as from uninvolved skin, and overgrowth is associated with relapse of atopic eczema.
Psychological stress may be an additional environmental stimulus, perhaps through neuroimmunoregulation.
Genetic factors in atopic eczema
Twin studies show that genetic factors are important in the predisposition to atopic eczema, with concordance rates of about 0.8 and 0.2 in monozygotic and dizygotic twin pairs respectively.
Eczema and other atopic disorders show clustering in families, and children whose parents have atopic eczema show a higher risk of developing eczema than children of parents with asthma or hay fever. This suggests that additional skin genes may influence the eczema phenotype.
Many different candidate genes have been studied because of their theoretical roles in the aetiology of atopic eczema. These include the high affinity IgE receptor (FCε RI-β), the mast cell chymase gene, the cytokine gene cluster on chromosome 5q31-33, and the SPINK5 gene (as mutations in this gene cause Netherton's syndrome,10 a rare genodermatosis characteristised by an eczematous rash and raised IgE levels).
Genome-wide screens in families with atopic eczema from four different populations have detected several regions showing linkage with atopic eczema. Interestingly, only two of these regions have shown a linkage to asthma or other atopic disorders in more than one study,11 which indicates that separate genes may be responsible for eczema. Furthermore, the regions on 1q21, 3q21, 17q25, and 20p linked to atopic eczema seem to overlap with known psoriasis susceptibility loci,12 although a recent investigation of the 17q25 locus failed to demonstrate variants in the known PSORS2 psoriasis locus in children with atopic eczema.13 The colocalisation of eczema and psoriasis genes supports the concept that specific skin genes may influence the eczema phenotype via control of epidermal function.
A recent large scale DNA microarray analysis has shown that four genes involved with epidermal differentiation (on chromosome 1q21) show different levels of expression in eczematous skin compared with controls.14
How to treat atopic eczema
The fluctuating course of atopic eczema and a substantial placebo response highlight the need for randomised controlled trials for the evaluation of treatments or interventions. Clinical practice indicates that the management of acute weeping eczema is rather different from chronic lichenified eczema, but these factors are not always discussed or included in the entry criteria of clinical trials.
Mild to moderate eczema: emollients and topical steroids
Mild to moderate eczema is very common and patients are usually managed in primary care. The mainstay of treatment remains the appropriate use of emollients and topical steroids. Good evidence exists to support the efficacy of topical steroids, which need only be applied once daily. Surprisingly, however, there is limited evidence to support the beneficial effects of emollients, although they have been shown to reduce the requirements for topical steroids by up to 50%.15 Short term bursts of potent topical steroid are equivalent to prolonged use of mild steroids,16 and prolonged use of intermittent potent steroids (two days a week) can reduce the frequency of flare-ups compared with emollient.17
The importance of a multidisciplinary approach is increasingly recognised, especially for patient and family education, including information about avoidance of irritants and allergens and about how to use topical treatments. Patient and carers are often anxious about the potential side effects of topical steroids, and appropriate education is important to avoid overtreatment or undertreatment.
It is important to recognise complications such as secondary bacterial infection, eczema herpeticum (herpes simplex virus infection), and skin atrophy induced by the overuse of topical steroids. Indications for referral to secondary care include concern over diagnosis, a poor response to first line treatment, and the development of complications.
Moderate to severe eczema: second line treatments
Second line treatments for patients whose eczema has not responded to adequate topical steroids include topical calcineurin inhibitors, ultraviolet (UV) phototherapy, and systemic agents. Good evidence exists for the efficacy of these treatments, but longer term studies to monitor safety are now required, as well as comparisons between the treatments.
Topical calcineurin inhibitors
The discovery that systemic ciclosporin is an effective treatment for atopic eczema led to the development of topical calcineurin inhibitors, and tacrolimus and pimecrolimus have been introduced into clinical practice.
A recent systematic review18 and meta-analysis19 found limited evidence that pimecrolimus is more effective than placebo in treating mild to moderate atopic eczema and that tacrolimus is more effective than placebo and mild topical steroids in treating moderate to severe atopic eczema. Since then, a randomised controlled trial involving 487 adult patients with moderate to severe atopic eczema has shown that 0.1% tacrolimus was more effective than moderately potent topical steroids at achieving at least a 60% improvement at three months (73% and 52% of patients respectively).20 Formal evidence is lacking, however, for the efficacy of these agents in patients who have failed to respond to topical steroids.
A sensation of skin burning may be troublesome during the first weeks of treatment; the long term safety profile of topical calcineurin inhibitors is currently unknown. Post-marketing reports of tumour development in patients using topical calcineurin inhibitors combined with reports of lymphoma in non-human primates treated with systemic pimecrolimus have raised concerns. Topical calcineurin inhibitors are therefore currently recommended as second line agents. According to their licence, they should be prescribed by doctors with a special interest and experience in skin diseases for short term or intermediate use in patients who have had atopic eczema for more than two years and are unresponsive to conventional treatment (www.fda.gov/cder/drug/advisory/elidel_protopic.htm) (press release No 038, 2004, National Institute for Health and Clinical Excellence (www.nice.org.uk)).
UV phototherapy: UVB v UVA
Patients with atopic eczema often report improvement after exposure to natural sunlight. A randomised controlled trial involving 73 patients with moderate to severe atopic eczema found that twice weekly narrow band (311 nm) UVB for 12 weeks induced a 28% mean reduction in disease activity compared with a 1.3% mean reduction from exposure to visible light (placebo).21 Significant improvement was maintained at three months. The treatment was well tolerated, but the potential increased risk of skin cancer needs to be explained to patients and monitored in the longer term.
In contrast, UV exposure from conventional sunbeds (predominantly UVA, 315-400 nm) seemed relatively ineffective. High dose UVA1 (340-400 nm) improves acute severe eczema, but specialist irradiation devices are required. A further comparison of narrow band UVB versus medium dose UVA1 showed that narrow band UVB was more effective.22
Systemic immunosuppressants: ciclosporin and azathioprine
Moderate to severe atopic eczema may be only partially responsive to topical agents and may relapse after phototherapy; systemic agents may therefore need to be considered. Moderate to severe disease is more common in adults than children, and ethical issues mean that the randomised controlled trials of systemic agents have principally involved adults.
The efficacy of ciclosporin in the treatment of moderate to severe atopic eczema is well established. An open study in adults found that one to two six-week courses of ciclopsorin resulted in long term remission of atopic eczema at one year,23 but open trials in children found that continuous rather than intermittent usage achieved better control.24
Careful monitoring is required with ciclosporin use. Documented side effects include nephrotoxicity, immunosuppression, and predisposition to cancer, particularly cutaneous non-melanoma skin cancer and lymphoma. Direct evidence with respect to atopic eczema is currently confined to case reports.
Several open studies found that azathioprine, a purine analogue, may be effective in moderate to severe disease, and this has been confirmed by two recent randomised controlled trials.25,26 The results of a crossover trial using 2.5 mg/kg azathioprine showed a 25% improvement in disease activity, but 35% of the participants withdrew owing to side effects. Using a parallel group design and using doses of azathioprine according to thiopurinemethyltransferase (TPMT), a key enzyme in thiopurine metabolism status, Meggitt et al observed a larger mean improvement in disease activity (37%) and a lower dropout rate of 15%. Sustained improvement for at least three months after stopping azathioprine may also occur.26 In a retrospective open study, Murphy et al found that azathioprine resulted in a “good” or “excellent” response in 41/48 (85%) children with atopic eczema.27
Dose dependent nausea is the commonest side effect of azathioprine, followed by bone marrow and hepatic toxicity. In the randomised controlled trials, no participants developed serious laboratory abnormalities, although hypersensitivity to azathioprine occurred in two patients.26
Measurement of TPMT is now recommended before azathioprine is started. The aim is (a) to exclude patients who have absent TPMT activity because they are at profound risk of developing myelotoxicity28 and (b) to facilitate lower dosing in patients who have low TPMT activity.26 Regular haematological monitoring is still required as polymorphisms in other enzymes may contribute up to 35% of episodes of neutropenia in patients receiving azathioprine.29
The longer term risks of azathioprine in atopic eczema are unknown, but data from patients with rheumatoid arthritis and inflammatory bowel disease suggest that any risk of malignancy is small.30
Other systemic agents
Various systemic immunosuppressives, immunomodulators, and antimetabolites have been reported in case series or uncontrolled trials to be of benefit in patients with moderate to severe atopic eczema.
Mycophenolate mofetil (MMF) is an immunosuppressive drug, and its active metabolite, mycophenolic acid, selectively inhibits de novo purine synthesis in lymphocytes through an effect on inosine monophosphate dehydrogenase. Two open trials using 2.0 g MMF daily reported improvement in disease activity (68% median reduction and 55% mean reduction),31,32 whereas a third study using 2.0-2.5 g daily reported no clinically relevant effect in five patients.
Clinical features of eczema
Atopic eczema is characterised by itching and ill defined areas of redness and scaling typically affecting the limb flexures. The face may also be affected, particularly in infants and adults, but eczema may affect any part of the skin surface. In the acute stage, vesiculation, weeping, and crusting may occur. Lichenification (skin thickening) may predominate in the chronic phase.
Adverse effects of MMF relate principally to its inhibitory effects on the haematopoietic and immune system—including leucopenia, lymphopenia, and anaemia and require appropriate monitoring—but symptomatic side effects include gastrointestinal disturbance. An increased risk of herpetic infections was reported when mycophenolic acid was originally introduced for treating psoriasis,33 which may be of concern for patients with atopic eczema, and indeed one patient developed herpes retinitis.
Do probiotics help to prevent atopic eczema?
Probiotics are live bacterial cultures of commensal gut microflora that if taken orally may modulate the immune system.
Fig 2.
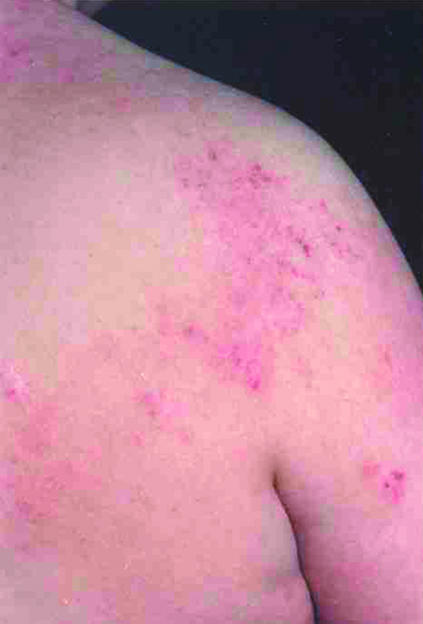
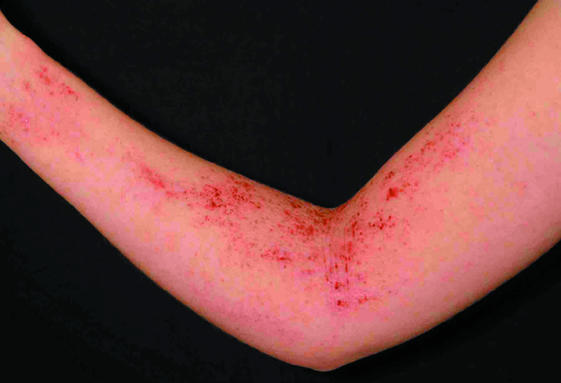

Left: Ill defined areas of erythema with multiple excoriations. Right (top): Flexural eczema showing crusting associated with secondary bacterial infection. Right (bottom): Erythema, scale, and lichenification on posterior neck
Additional educational resources
British Association of Dermatologists (www.bad.org.uk/healthcare/guidelines/)—Provides evidence based and up to date information for healthcare professionals
Online manual of the UK Diagnostic Criteria for atopic dermatitis (www.nottingham.ac.uk/dermatology/eczema/index.html)
National Institute for Health and Clinical Excellence (www.nice.org.uk/search.aspx?search-mode=simple&ss=eczema)—Provides national guidance in England and Wales on the treatment of eczema
Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines for care of atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines.” J Am Acad Dermatol 2004;50: 391-404.
Patient information and support groups
British Association of Dermatologists (www.bad.org.uk/public/leaflets)—Information on the diagnosis and treatment of eczema and other skin conditions
National Eczema Society (www.eczema.org)—This UK organisation provides information leaflets
American Academy of Dermatology's patient information service (www.aad.org/public/publications/pamphlets/eczemaatopicdermatitis.htm)
Further reading
Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest 2004;113: 651-7.
Williams HC. Atopic dermatitis. N Engl J Med 2005;352: 2314-24.
Hoffamn S, Epplen JT. The genetics of atopic dermatitis: recent findings and future options. J Mol Med 2005;83: 682-92.
Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol 2004;4: 978-88.
Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4(37)
A randomised placebo controlled trial found that when Lactobacillus GG was given prenatally to mothers for two to four weeks and to their infants (or breastfeeding mothers) at risk of atopic disease for six months, the frequency of eczema was reduced from 46% to 23% at two years. The severity of atopic eczema and IgE reactivity, however, was unchanged.34 A follow-up study found a persistent beneficial effect of Lactobacillus GG at four years with respect to frequency of eczema but not eczema severity. However, the prevalence of eczema in the placebo group was relatively high (46%). In contrast, no evidence exists to indicate that probiotics improve established atopic eczema.
Conclusion
Eczema is a common and distressing condition. The aetiology and immunopathogenesis are not fully understood, and therapeutic options are relatively limited. Developments in the rapidly advancing fields of research in eczema epidemiology, pathogenesis, and genetics will hopefully soon lead to management strategies that are more specifically targeted at pathogenic mechanisms.
Supplementary Material
 A more comprehensive, unedited version of this article is available on bmj.com
A more comprehensive, unedited version of this article is available on bmj.com
We thank W O C M Cookson and M Haniffa for critically reviewing the manuscript, and S J Meggitt and S Weatherhead for help in obtaining clinical photographs.
Contributors: SB and NJR contributed equally to the writing of this review.
Competing interests: The University of Newcastle and NJR's department have received financial support for atopic eczema research from SR Pharma and Fujisawa.
References
- 1.Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis? J Allergy Clin Immunol 2004;114: 150-8. [DOI] [PubMed] [Google Scholar]
- 2.Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The UK working party's diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131: 383-96. [DOI] [PubMed] [Google Scholar]
- 3.Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 2003;21: 105-13. [DOI] [PubMed] [Google Scholar]
- 4.Riedi CA, Rosario NA, Ribas LF, Backes AS, Kleiniibing GF, Popija M, et al. Increase in prevalence of rhinoconjunctivitis but not asthma and atopic eczema in teenagers. J Investig Allergol Clin Immunol 2005;15: 183-8. [PubMed] [Google Scholar]
- 5.Olesen AB, Bang K, Juul S, Thestrup-Pedersen K. Stable incidence of atopic dermatitis among children in Denmark during the 1990s. Acta Derm Venereol 2005;85: 244-7. [DOI] [PubMed] [Google Scholar]
- 6.Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the “hygiene hypothesis”: too clean to be true? Br J Dermatol 2005;152: 202-16. [DOI] [PubMed] [Google Scholar]
- 7.Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy and/or lactation for preventing or treating atopic disease in the child. Cochrane Database Syst Rev 2003(4): CD000133. [DOI] [PubMed]
- 8.Tan BB, Weald D, Strickland I, Friedmann PS. Double-blind controlled trial of effect of housedust-mite allergen avoidance on atopic dermatitis. Lancet 1996;347: 15-8. [DOI] [PubMed] [Google Scholar]
- 9.Gutgesell C, Heise S, Seubert S, Seubert A, Domhof S, Brunner E, et al. Double-blind placebo-controlled house dust mite control measures in adult patients with atopic dermatitis. Br J Dermatol 2001;145(1): 70-4. [DOI] [PubMed] [Google Scholar]
- 10.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet 2000;25: 141-2. [DOI] [PubMed] [Google Scholar]
- 11.Hoffjan S, Epplen JT. The genetics of atopic dermatitis: recent findings and future options. J Mol Med 2005;83: 682-92. [DOI] [PubMed] [Google Scholar]
- 12.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol 2004;4: 978-88. [DOI] [PubMed] [Google Scholar]
- 13.Morar N, Bowcock AM, Harper JI, Cookson WO, Moffatt MF. Investigation of the chromosome 17q25 PSORS2 locus in atopic dermatitis. J Invest Dermatol 2006;126: 603-6. [DOI] [PubMed] [Google Scholar]
- 14.Sugiura H, Ebise H, Tazawa T, Tanaka K, Sugiura Y, Uehara M, et al. Large-scale DNA microarray analysis of atopic skin lesions shows overexpression of an epidermal differentiation gene cluster in the alternative pathway and lack of protective gene expression in the cornified envelope. Br J Dermatol 2005;152: 146-9. [DOI] [PubMed] [Google Scholar]
- 15.Lucky AW, Leach AD, Laskarzewski P, Wenck H. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol 1997;14: 321-4. [DOI] [PubMed] [Google Scholar]
- 16.Thomas KS, Armstrong S, Avery A, Po AL, O'Neill C, Young S, et al. Randomised controlled trial of short bursts of a potent topical corticosteroid versus prolonged use of a mild preparation for children with mild or moderate atopic eczema. BMJ 2002;324: 768-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ 2003;326: 1367-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al. The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation. Health Technol Assess 2005;9(29): iii, xi-xiii, 1-230. [DOI] [PubMed] [Google Scholar]
- 19.Ashcroft DM, Dimmock P, Garside R, Stein K, Williams HC. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ 2005;330: 516-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Folster-Holst R, et al. A multicentre, randomized, double-blind, controlled study of longterm treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol 2005;152: 1282-9. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds NJ, Franklin V, Gray JC, Diffey BL, Farr PM. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001;357: 2012-6. [DOI] [PubMed] [Google Scholar]
- 22.Legat FJ, Hofer A, Brabek E, Quehenberger F, Kerl H, Wolf P. Narrowband UV-B vs medium-dose UV-A1 phototherapy in chronic atopic dermatitis. Arch Dermatol 2003;139: 223-4. [DOI] [PubMed] [Google Scholar]
- 23.Granlund H, Erkko P, Reitamo S. Long-term follow-up of eczema patients treated with cyclosporine. Acta Derm Venereol 1998;78(1): 40-3. [DOI] [PubMed] [Google Scholar]
- 24.Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol 2000;142: 52-8. [DOI] [PubMed] [Google Scholar]
- 25.Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol 2002;147: 324-30. [DOI] [PubMed] [Google Scholar]
- 26.Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet 2006;367: 839-46. [DOI] [PubMed] [Google Scholar]
- 27.Murphy LA, Atherton D. A retrospective evaluation of azathioprine in severe childhood atopic eczema, using thiopurine methyltransferase levels to exclude patients at high risk of myelosuppression. Br J Dermatol 2002;147: 308-15. [DOI] [PubMed] [Google Scholar]
- 28.Anstey AV, Wakelin S, Reynolds NJ. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol 2004;151: 1123-32. [DOI] [PubMed] [Google Scholar]
- 29.Evans WE, Hon YY, Bomgaars L, Coutre S, Holdsworth M, Janco R, et al. Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol 2001;19: 2293-301. [DOI] [PubMed] [Google Scholar]
- 30.Kwon JH, Farrell RJ. The risk of lymphoma in the treatment of inflammatory bowel disease with immunosuppressive agents. Crit Rev Oncol Hematol 2005;56: 169-78. [DOI] [PubMed] [Google Scholar]
- 31.Neuber K, Schwartz I, Itschert G, Dieck AT. Treatment of atopic eczema with oral mycophenolate mofetil. Br J Dermatol 2000;143: 385-91. [DOI] [PubMed] [Google Scholar]
- 32.Grundmann-Kollmann M, Podda M, Ochsendorf F, Boehncke WH, Kaufmann R, Zollner TM. Mycophenolate mofetil is effective in the treatment of atopic dermatitis. Arch Dermatol 2001;137: 870-3. [PubMed] [Google Scholar]
- 33.Epinette WW, Parker CM, Jones EL, Greist MC. Mycophenolic acid for psoriasis. A review of pharmacology, long-term efficacy, and safety. J Am Acad Dermatol 1987;17: 962-71. [DOI] [PubMed] [Google Scholar]
- 34.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357: 1076-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.