Abstract
Protein synthesis is regulated by the phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α) in response to different environmental stresses. One member of the eIF2α kinase family, heme-regulated inhibitor kinase (HRI), is activated under heme-deficient conditions and blocks protein synthesis, principally globin, in mammalian erythroid cells. We identified two HRI-related kinases from Schizosaccharomyces pombe which have full-length homology with mammalian HRI. The two HRI-related kinases, named Hri1p and Hri2p, exhibit autokinase and kinase activity specific for Ser-51 of eIF2α, and both activities were inhibited in vitro by hemin, as previously described for mammalian HRI. Overexpression of Hri1p, Hri2p, or the human eIF2α kinase, double-stranded-RNA-dependent protein kinase (PKR), impeded growth of S. pombe due to elevated phosphorylation of eIF2α. Cells from strains with deletions of the hri1+ and hri2+ genes, individually or in combination, exhibited a reduced growth rate when exposed to heat shock or to arsenic compounds. Measurements of in vivo phosphorylation of eIF2α suggest that Hri1p and Hri2p differentially phosphorylate eIF2α in response to these stress conditions. These results demonstrate that HRI-related enzymes are not unique to vertebrates and suggest that these eIF2α kinases are important participants in diverse stress response pathways in some lower eukaryotes.
An important mechanism regulating translation initiation in response to environmental stresses involves phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α) (11, 21, 38, 56, 80). A family of eIF2α kinases has been identified whose members share sequence and structural features in their catalytic domains but have unique flanking regulatory domains, allowing for their distinct control patterns. In mammals, four eIF2α kinases have been identified: double-stranded-RNA-dependent protein kinase (PKR), which is important for antiviral pathways mediated by interferon (37, 56); pancreatic eIF2α kinase (PEK)/Perk, which modulates gene expression in response to protein misfolding in the endoplasmic reticulum (27-30, 63, 65); GCN2, which is activated by nutritional stresses, including amino acid limitation (33, 80); and heme-regulated inhibitor kinase (HRI), which is mainly expressed in the erythroid lineage and couples protein synthesis, predominantly globin in these tissues, to the availability of heme (11, 12, 26).
In response to cellular stress, phosphorylation of eIF2α at Ser-51 reduces the activity of this initiation factor and modulates translational expression. For example, iron limitation and accompanying reductions in heme levels activate HRI through a mechanism involving autophosphorylation and an altered protein conformation (4, 11, 12). Distinct heme-binding sites were identified in the amino terminus of HRI and in the kinase insert region (58). Release of the heme association of HRI is proposed to elicit enhanced phosphorylation of eIF2α. eIF2, combined with initiator methionyl-tRNA and GTP, associates with the 40S ribosomal subunit and participates in the recognition of the start codon during initiation of translation (32). Upon recognition of the initiation codon, GTP complexed with eIF2 is hydrolyzed to GDP. Phosphorylation of eIF2α by HRI impedes the exchange of eIF2-GDP to the GTP-bound form that is catalyzed by the guanine nucleotide exchange factor eIF2B. The resulting reduction in eIF2-GTP levels impedes translation initiation in the cell, coupling a reduction of globin synthesis to the lowered levels of available heme (11, 12). Disruption of this eIF2α kinase in mice leads to aggregation of globins devoid of heme within the erythroid lineage, contributing to hyperchromic, hemolytic anemia with compensatory hyperplasia in the spleen and bone marrow (26). In reticulocytes and fetal liver nucleated progenitor cells, HRI was also observed to be activated by oxidative stress and heat shock, suggesting that this eIF2α kinase recognizes a broad spectrum of stress conditions (42, 72, 74, 87).
In nonvertebrate eukaryotes, translation is regulated by a more limited number of eIF2α kinases. In the well-characterized yeast Saccharomyces cerevisiae, phosphorylation of eIF2α is carried out only by the GCN2 protein kinase (33, 80). During nutrient limitation, GCN2 phosphorylation of eIF2α induces the translation of GCN4, a basic zipper (bZIP) transcriptional activator of genes involved in the metabolism of amino acids, nucleotides, and vitamins and the biogenesis of peroxisomes. That phosphorylation of eIF2α can lead to induction of gene-specific translation, even during the occurrence of a general reduction in translation, is also thought to be important in higher eukaryotes. Another bZIP protein, ATF4, is preferentially translated in response to phosphorylation of mammalian eIF2α by a mechanism involving upstream open reading frames, although it is uncertain whether the mechanism of leaky scanning described previously for GCN4 translation also functions in mammals (27). In invertebrate metazoans such as Drosophila melanogaster and Caenorhabditis elegans, the only eIF2α kinases present are GCN2 and PEK/Perk, which are proposed to be important for stresses impacting the cytoplasm and endoplasmic reticulum, respectively (52, 61, 65).
The apparent absence of HRI and PKR homologues in lower eukaryotes has led to speculation that these two eIF2α kinases are required for remediation of stresses specific to vertebrate organisms. In this report, however, we describe two HRI-related kinases in the fission yeast Schizosaccharomyces pombe. As with their mammalian counterparts, phosphorylation of the α subunit of eIF2 by either purified-yeast HRI-related enzyme is repressed by hemin, although with a higher apparent Ki value. Deletion of the HRI-related genes from S. pombe strains did not alter cell growth in nonstressed conditions, but their combined deletion blocked induction of eIF2α phosphorylation in response to heat shock or arsenic exposure and significantly reduced cell growth in response to these stress conditions. The present study demonstrates that HRI-related enzymes are not unique to vertebrates and suggests that this eIF2α kinase is important for resistance to diverse environmental stresses.
MATERIALS AND METHODS
Cloning hri1+ and hri2+and plasmid constructions.
HRI homologues were identified using the program BLAST and the S. pombe genome sequence (86). In the case of hri1+, the sum probability of random correspondence of the pairwise segments (i.e., the BLAST score) is 4.7 E−37. hri2+ also shared a high degree of similarity with rabbit HRI, with a score of 1.0 E−49, although the homology was fragmented, which was suggestive of the presence of multiple introns. This similarity extended to the N-terminal regulatory region of rabbit HRI, with a score of 4.0 E−5 between hri2+ and rabbit HRI. hri1+ is encoded on cosmid SPAC20G4 from nucleotide 6086 to 8200 (GenBank accession no. Z98600), and hri2+ is in cosmid SPAC222 from nucleotide 11617 to 13736 (acc. no. AL132798). To obtain hri1+ and hri2+ cDNAs, we isolated total RNA from S. pombe strain SP223 as previously described (62) and purified poly(A)+ RNA by using a MicroPoly(A) Pure isolation kit (Ambion, Austin, Tex.). Reverse transcription-PCR (RT-PCR) analysis was carried out using the Titan One Tube RT-PCR system (Roche, Indianapolis, Ind.) with the purified poly(A)+ RNA as the template. A 2,115-bp cDNA fragment of hri1+ and a 1,920-bp cDNA fragment of hri2+ were digested with NdeI and BamHI engineered at the 5′ and 3′ ends, respectively, and inserted between the unique NdeI and BamHI sites in E. coli expression vector pET15b (Novagen). The resulting plasmid, p555, encodes hri1+, and p598 contains hri2+. Cloned S. pombe cDNAs were sequenced by the dideoxy method and confirmed to be identical to those of the genomic sequence, with the exception of three introns in hri2+. For p573, site-directed mutagenesis was used to substitute a methionine codon for a lysine-253 codon in hri1+, and p596 encodes Hri1-ΔNp, with a deletion of residues 2 to 186. hri1+ and hri2+ cDNAs were also inserted between the NdeI and BamHI sites of S. pombe expression vectors pREP4 and pREP1 (45), a region that includes the nmt1+ promoter that is repressed in the presence of thiamine (44). The resulting plasmid, p627, is marked by ura4+ and encodes Hri1p, and p628 expressing Hri2p is marked by S. cerevisiae LEU2 suitable for complementation of leu1− in S. pombe. The human PKR cDNA was similarly cloned into pREP4, generating p603. The S. pombe gene, encoding eIF2α with its endogenous promoter, was inserted between the SacII and XhoI sites of S. pombe shuttle vector pSP1, which is marked with LEU2 (17). The resulting eIF2α-encoding plasmid was designated p601, and a mutant version, containing alanine substituted for Ser-51, was named p600.
Yeast strains and growth conditions.
S. pombe strains used in this study are derived from strain SP223 (h− ade6-216 leu1-32 ura4-D18) (66), including strains WY457 (hri1::ura4+), WY458 (hri2::leu1+), and WY459 (hri1::ura4+ hri2::leu1+). Confirmatory experiments for hri1+ were also carried out using a hri1::ura4+ (WY460) derivative of S. pombe ED665 (h− ade6-210 leu1-32 ura4-D18). S. pombe strains were grown in yeast extract plus supplements (YES) or Edinburgh minimum medium (EMM) (48), supplemented with 225 μg of adenine/ml, 225 μg of leucine/ml, and 225 μg of uracil/ml as required. Glucose was present at a concentration of 3% in the EMM and YES. Complete synthetic EMM was made by adding all amino acids at a concentration of 225 μg/ml into EMM (60). S. pombe strains were grown at 30°C on agar plates or in liquid culture with agitation.
Plasmids overexpressing Hri1p, Hri2p, or human PKR were introduced into S. pombe strain SP223 by selecting for uracil or leucine prototrophy in EMM supplemented with thiamine, an inhibitor of the nmt1+ promoter. As indicated, pSP1 encoding eIF2α was also introduced in strain SP223 and selected for by leucine prototrophy. All strains were grown to saturation in EMM supplemented with 5 μg of thiamine/ml and then streaked onto agar EMM in the presence or absence of thiamine. Agar plates were incubated at 30°C for 4 days and electronically imaged.
Deletions of hri1+ and hri2+ from the S. pombe strains were generated in one step by homologous recombination by transforming linear DNA containing ura4+ substituted for the entire coding region of hri1+ or leu1+ substituted for the entire coding region of hri2+ (48). Transformants were selected for by uracil or leucine prototrophy. The Δhri1 and Δhri2 knockouts were confirmed by PCR analysis and by Southern blot analysis using genomic DNA cleaved with restriction enzyme EcoRV combined with NdeI or HincII. Radiolabeled DNA probes included a 1.2-kb DNA fragment containing the 5′ noncoding portion of hri1+ or a 1.1-kb fragment including the 3′ noncoding portion of hri2+. Southern analysis was carried out using high stringency conditions, and the hybridized probes were visualized using X-ray film with an intensifying screen at −80°C.
To determine the impact of the loss of hri1+ and hri2+ on growth of S. pombe, the isogenic strains SP223, WY457, WY458, and WY459 were inoculated at A600 = 0.05 in liquid YES or EMM, as indicated, and grown at 30°C until saturation. At the specified time points, cell samples were removed from cultures and measured for density at A600. Heat shock was induced as follows: strains were grown to an A600 = 0.25 in liquid YES supplemented with all 20 amino acids and incubated at 48°C for 15 min. Addition of the amino acids to the YES medium insured minimal phosphorylation of eIF2α in nonstressed conditions. Following the heat shock, cultures were placed in an ice bath and then shifted to 30°C incubation as previously described (19). Cell growth was monitored by removing aliquots from the cultures at the indicated times and measuring density at A600. Arsenite exposure was carried out by culturing strains SP223, WY457, WY458, and WY459 to exponential phase in YES medium supplemented with all 20 amino acids, followed by the introduction of 200 μM NaAsO2, 400 μM NaAsO2, or no stress, as indicated. Alternatively, arsenate stress was analyzed by exposing this strain collection to 50 μM Na2HAsO4, 100 μM Na2HAsO4, or no stress. Cultures were monitored for growth by measuring absorbance at 600 nm. Strains were also inoculated in YES medium supplemented with 100 μM sodium arsenate at 30°C with agitation for 1 hour. Cells were then collected by centrifugation, rinsed, resuspended in the same volume of YES medium, and monitored for growth.
Bacterial expression of Hri1p and Hri2p and in vitro kinase assays.
E. coli strain BL21(DE3) (F− ompTrB− containing lysogen DE3), transformed with Hri1p expression plasmids p555, p573, or p596 or Hri2p expression plasmid p598, were grown at 37°C in Luria-Bertani medium supplemented with 100 μg of ampicillin/ml until mid-logarithmic phase, at which point 1 mM isopropyl-β-d-thiogalactoside was added to the culture and incubated for an additional 3 h. Cells were collected by centrifugation, washed in a solution of 20 mM Tris-HCl (pH 7.8) and 500 mM NaCl, and then resuspended in solution A (20 mM Tris-HCl [pH 7.8], 500 mM NaCl, 10% glycerol) and protease inhibitors (100 μM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 1 μM leupeptin, and 0.15 μM aprotinin) with 10 mM imidazole, followed by cell lysis using a French press. Lysates were clarified by centrifugation and applied onto a column containing nickel-chelation resin (Qiagen, Hilden, Germany). Polyhistidine-tagged Hri proteins were washed with solution A containing elevated concentrations of imidazole and eluted with solution A supplemented with 200 mM imidazole. Hri1p and Hri2p were visualized by electrophoresis using a sodium dodecyl sulfate (SDS)-12.5% polyacrylamide gel. Difficulties were initially encountered with Hri2p, which copurified with a 76,000-molecular-weight E. coli protein, presumed to be DnaK. Copurification with this chaperone has also been observed during expression of rabbit HRI. With extensive washing steps using increasing concentrations of imidazole, Hri2p was purified in a form distinct from the contaminating E. coli protein.
Kinase reactions were carried out with 50 ng of purified Hri1p or Hri2p, 10 μCi of [γ-32P]ATP (in a final concentration of 50 μM) and kinase buffer [20 mM Tris-HCl (pH 7.8), 50 mM NaCl, 10 mM Mg(OAc)2, 5 μM β-mercaptoethanol and protease inhibitors], and 0.75 μg of recombinant eIF2α or a mutant version of eIF2α with Ala substituted for Ser-51 (S51A), as indicated, in a 20-μl volume. Recombinant eIF2α substrate is a modified version from S. cerevisiae that was shown to be an effective substrate in in vitro assays using a variety of eIF2α kinases isolated from organisms ranging from yeasts to mammals (77, 88, 89). Alternatively, either 20 μg of lysates prepared from insect Sf9 cells expressing rabbit HRI or 50 ng of purified human PKR or yeast GCN2 were incubated in the same assay conditions. Samples were incubated at 30°C for 5 min, a time point that was found to be in the linear range of the assay. Reactions were arrested by the addition of 5 μl of 5× SDS-sample buffer, followed by heating at 95°C for 2 min. Radiolabeled proteins were separated by electrophoresis in a SDS-12.5% polyacrylamide gel, and the gels were fixed by Coomassie staining, dried, and subjected to autoradiography. The eIF2α band was excised from polyacrylamide gels, and the incorporation of 32P was quantified using liquid scintillation counting. Inhibition of eIF2α kinase activity was performed similarly, with the addition of increasing concentrations (0, 0.5, 2.5, 5, 10, and 20 μM) of hemin (Sigma) (10). Hemin was prepared as described previously (10) by dissolution in 1N KOH followed by neutralization with 200 mM Tris-HCl (pH 7.9) and 1 M HCl and subsequent addition of ethylene glycol.
Measurements of in vivo eIF2α phosphorylation by immunoblot analysis.
Using the nmt1+ promoter as described above, S. pombe strain SP223 overexpressing Hri1p, Hri2p, or human PKR was collected by centrifugation, washed with cold water, and resuspended in solution A supplemented with protease inhibitors. Cells were broken with acid-washed glass beads and clarified by centrifugation, and 40 μg of each protein sample was separated by electrophoresis in a SDS-12.5% polyacrylamide gel. Proteins were transferred to nitrocellulose filters, and the filters were blocked with TBS-T solution (20 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.1% Tween 20) and 4% nonfat milk powder (76). Filters were then incubated with affinity-purified antibody that specifically recognizes eIF2α phosphorylated at Ser-51 (Research Genetics) or antisera prepared against an eIF2α polypeptide that recognized either phosphorylated or nonphosphorylated eIF2α. Filters were washed with TBS-T, and the eIF2α-antibody complex was detected using horseradish peroxidase-labeled secondary antibody and chemifluorescent substrate. To assess the impact of heat shock, arsenite, or arsenate exposure on eIF2α phosphorylation, S. pombe strains SP223, WY457, WY458, and WY459 were grown in YES medium at 30°C and subjected to incubation at 48°C for 15 min or exposed to 200 μM sodium arsenite or 100 μM sodium arsenate for 1 hour, as indicated. Cell lysate preparation and immunoblot analysis of eIF2α phosphorylation was carried out as described above.
RESULTS
Identification of HRI-related protein kinases in S. pombe.
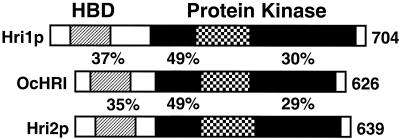
The rabbit HRI sequence (13) was used as a query to search for related protein kinases in S. pombe by using the BLAST program and the completed yeast genomic sequence (86). We identified two different putative serine/threonine protein kinases, whose genes we designated hri1+ and hri2+, with sequence similarities extending from the catalytic domain to the amino-terminal regulatory domain of rabbit HRI (Fig. 1). To obtain hri1+ and hri2+ cDNAs, we carried out RT-PCR using poly(A)+ RNA prepared from S. pombe strain SP223 and oligonucleotide primers complementary to the 5′ and 3′ portions of the predicted open reading frames. Sequence characterization of the isolated cDNAs indicated that Hri1p is 704 amino acid residues in length, with a predicted molecular weight of 85,000, and Hri2p is 639 residues long, with a size of 75,000 (Fig. 1 and 2). Alignments between the cDNA and genomic sequences indicated that hri2+ contained three introns, between nucleotides 116 and 202, 242 and 290, and 826 and 889, relative to the initiation codon. No introns were identified in hri1+.
FIG. 1.
Diagram of alignment of S. pombe Hri1p and Hri2p with rabbit HRI. Boxes represent sequences of the HRI-related enzymes, with the length of each eIF2α kinase indicated to the right. Percent identities are highlighted between Hri1p or Hri2p and the protein kinase region and the amino-terminal regulatory domain, including a heme binding domain (HBD) of rabbit (Oryctolagus cuniculus) HRI (OcHRI). The percent identities extending the length of the eIF2α kinases are as follows: Hri1p and Hri2p, 29%; Hri1p and OcHri, 27%; and Hri2p and OcHRI, 26%. The eIF2α kinases share an insert region of variable length and sequence, indicated by the checkered box, between subdomains IV and V of the protein kinase domain. In addition to the heme binding region in the amino terminus of rabbit HRI, a second binding site has been found in the kinase insert region. Precise boundaries and sequence requirements have not yet been defined for these heme binding regions of rabbit HRI.
FIG. 2.
Sequence comparison between rabbit HRI and S. pombe Hri1p and Hri2p. Sequence alignments were compiled in part by using the Vector NTI program. Boxes indicate identical residues between the aligned sequences. Gaps, indicated by dashes, were incorporated to maximize the alignment, and positions of aligned residues are listed on the right side of the alignment. Locations of the subdomains in the catalytic region are indicated above the alignment. Heme binding sites have been localized to rabbit HRI amino-terminal residues 1 to 154 and to the kinase insert region of residues 301 to 420 (58). Asterisks indicate residues in the amino-terminal regulatory region that were invariant in a multiple-sequence alignment that included the illustrated rabbit (Oryctolagus cuniculus) HRI (OcHRI) (13), S. pombe Hri1p and Hri2p, and HRI homologues from humans (83), mice (5), rats (46), chickens (14), zebra fish (accession no. AW127775), and frogs (Silurana tropicalis) (accession no. AW644355). GenBank accession numbers for S. pombe hri1+ and hri2+ are AF536223 and AF536224, respectively.
The greatest extent of sequence similarity between the rabbit and S. pombe protein kinases resides in the catalytic domains (Fig. 1 and 2). Significant sequence similarities also reside in the amino-terminal regulatory domain, which has been shown to be important for heme binding in rabbit HRI (58, 73) (Fig. 2). As illustrated in Fig. 2, in the alignment that extended between the amino termini of S. pombe Hri1p and Hri2p and HRI enzymes from mammals, chicken, frogs, and zebra fish, sixteen residues were identified to be invariant. During the course of our analysis of the S. pombe genome, we also found a third eIF2α kinase, corresponding to GCN2, encoded between adjacent cosmids SPBP18G and SPBC36B7, which were derived from chromosome II. The GCN2 has a predicted length of 1,576 residues and includes the histidyl-tRNA synthetase-regulatory domain shown to be important for activation of the S. cerevisiae eIF2α kinase in response to nutrient deprivation (23, 57, 81, 82, 89). Based on our genomic analysis, we conclude that there are three proteins in S. pombe related to the eIF2α kinase family.
Hri1p and Hri2p are heme-regulated eIF2α kinases with specificity to serine-51.
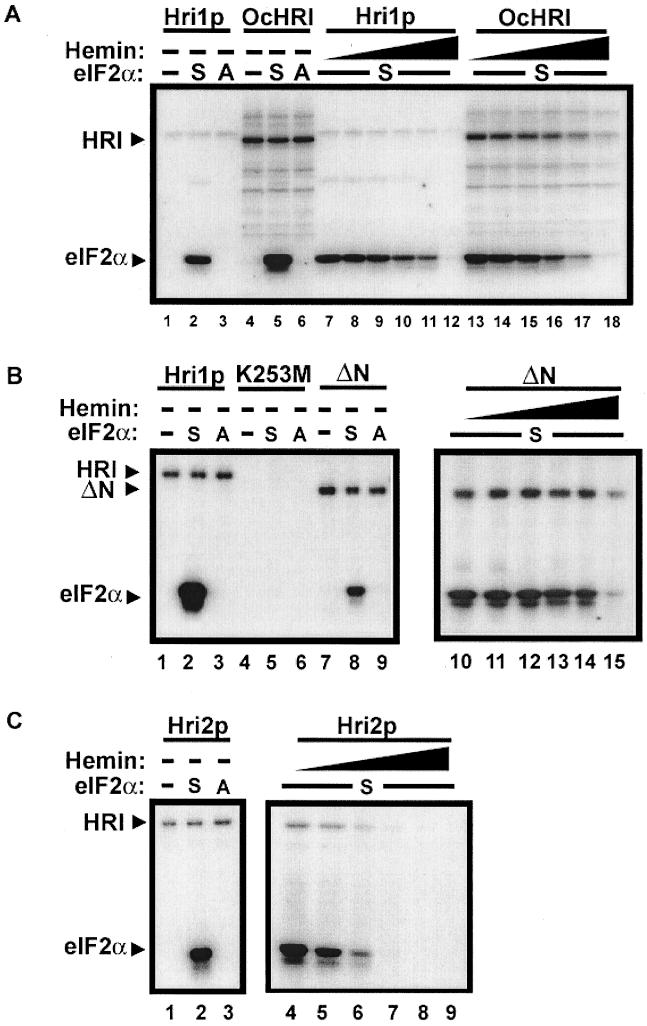
To address biochemically whether Hri1p and Hri2p are eIF2α kinases and whether their activity is inhibited by hemin, we expressed recombinant versions of these proteins by using the T7 promoter expression system in E. coli. Polyhistidine tags were incorporated at the amino termini of both HRI-related kinases, and these proteins were purified using nickel chelation resin (see Materials and Methods). Hri1p migrated in SDS-polyacrylamide gel electrophoresis (PAGE) with a molecular weight of 90,000, and Hri2p migrated with a molecular weight of 82,000, each about 10% higher than predicted by their cDNA sequences. Both proteins were recognized by an antibody specific to the polyhistidine tag (data not shown). Purified Hri1p and Hri2p were introduced into kinase reactions containing recombinant eIF2α and [γ-32P]ATP, and following SDS-PAGE, radiolabeled proteins were visualized by autoradiography (Fig. 3). Both Hri1p and Hri2p were found to phosphorylate eIF2α, and no phosphorylation was found in the absence of protein kinase or when mutant eIF2α (containing Ala for the phosphorylation site Ser-51) was used as the substrate in the reaction mixture. The specificity for Ser-51 in eIF2α was identical to that found in control reactions containing rabbit HRI (Fig. 3A). Furthermore, Hri1p and Hri2p were phosphorylated as reported previously for mammalian HRI. Substitution of methionine for Lys-253, a conserved residue in subdomain II of the kinase domain shown to be essential for rabbit HRI activity (13), blocked both Hri1p autophosphorylation and eIF2α kinase activity (Fig. 3B).
FIG. 3.
Hri1p and Hri2p phosphorylation of eIF2α is repressed by hemin. Shown are the results of kinase reactions with Hri1p and OcHRI (A), Hri1p or K253M or ΔN mutant versions of Hri1p (B), and Hri2p (C). Purified recombinant Hri1p or Hri2p or lysates prepared from insect Sf9 cells expressing OcHRI were incubated with recombinant eIF2α and [γ-32P]ATP in the presence of increasing concentrations of hemin, as described in Materials and Methods. Radiolabeled proteins were separated by SDS-PAGE and visualized by autoradiography, with phosphorylated HRI and eIF2α indicated by the arrowheads. −, no eIF2α or no hemin was added to the kinase reaction mixture; S, wild-type eIF2α was used as the substrate; A, mutant eIF2α with Ala substituted for Ser-51 was used in the reaction. Concentrations of 0 to 20 μM are indicated by the solid triangles. The radiolabeled eIF2α band was excised from the polyacrylamide gels, and the incorporation of 32P was quantified using liquid scintillation counting. Radioactivity incorporated into eIF2α at a zero concentration of hemin was defined as 100% of eIF2α phosphorylation.
We next addressed whether Hri1p and Hri2p were regulated by hemin. In the example of rabbit HRI, we found hemin inhibited eIF2α kinase activity with a Ki of 0.5 μM (Fig. 3A). Consistent with the noted sequence similarity, Hri1p and Hri2p were also inhibited by hemin with Ki concentrations of 2.5 μM and 1.5 μM, respectively (Fig. 3). N-terminal sequences of rabbit HRI were shown to be important for hemin regulation (11, 73), and we prepared a version of recombinant Hri1p from which residues 2 to 186 had been deleted. Hri1-ΔNp exhibited reduced eIF2α kinase activity compared with full-length kinase and was inhibited at only the higher hemin concentration of 20 μM (Fig. 3B). In control experiments, the activities of eIF2 kinases GCN2 and PKR, which are not physiologically regulated by heme, were also reduced by 20 μM hemin (data not shown). This suggests that high levels of hemin can have nonspecific inhibitory properties in the in vitro kinase reactions. We conclude that purified Hri1p and Hri2p specifically phosphorylate eIF2α at Ser-51 and that this activity can be inhibited by hemin.
Overexpression of eIF2α kinases in S. pombe inhibits cell growth by phosphorylation of serine-51 of eIF2α.
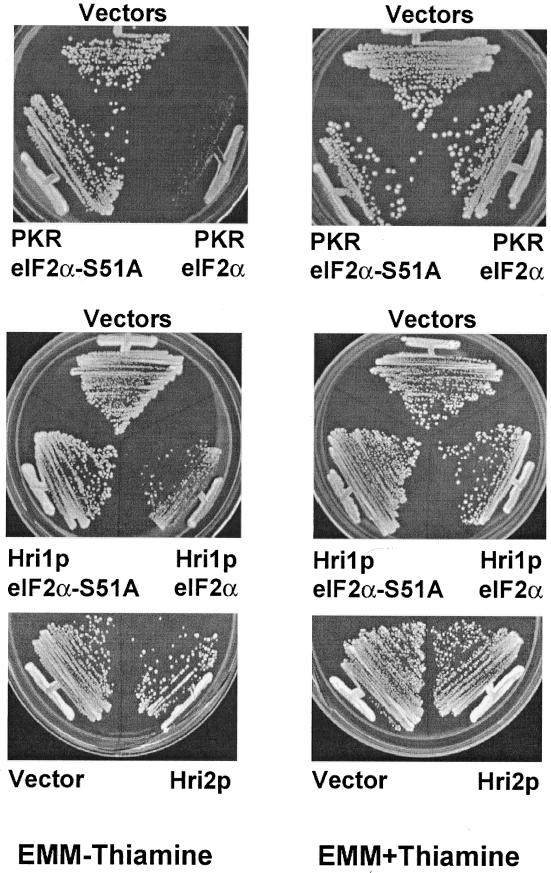
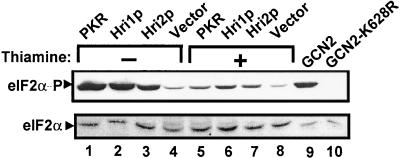
To determine whether phosphorylation of eIF2α can alter protein synthesis and growth of S. pombe cells, we expressed plasmid-encoded Hri1p and Hri2p in strain SP223 by using the thiamine-repressed promoter from nmt1+. As a control, we initially expressed human PKR that was previously shown to be an effective regulator of translation when expressed in S. cerevisiae (22, 59, 76, 88). Expression of PKR significantly reduced growth of S. pombe cells in minimal medium when expressed in the absence of thiamine (Fig. 4). We also found expression of Hri1p and, to a lesser extent, Hri2p, reduced growth specifically in the absence of thiamine. To address the importance of phosphorylation of eIF2α, we coexpressed the wild-type gene encoding the initiation factor subunit or the S51A mutant version. Consistent with the idea that phosphorylation of eIF2α specifically at Ser-51 leads to a reduction in translation initiation, we found that coexpression of eIF2α-S51A reversed the growth defect associated with PKR and Hri1p (Fig. 4). To directly determine whether elevated expression of Hri1p, Hri2p, or PKR leads to increased phosphorylation of eIF2α, we used antibody specific to eIF2α phosphorylated at Ser-51 in immunoblot assays. Expression of the eIF2α kinases in S. pombe elevated phosphorylation of eIF2α only when grown in the absence of thiamine, which are the growth conditions inducing expression of the eIF2α kinases (Fig. 5). The specificity of the antibody for phosphorylated eIF2α was confirmed by a similar immunoblot analysis using lysates prepared from S. cerevisiae expressing either wild-type or a kinase-deficient mutant, gcn2-K628R. The activity of the lone eIF2α kinase in S. cerevisiae, GCN2, was induced by growing these cells in the presence of sulfometuron methyl, an inhibitor of branched-chain amino acid biosynthesis (82). As expected, phosphorylation of Ser-51 of eIF2α was only detected in the presence of functional GCN2 (Fig. 5). Total levels of eIF2α were measured using polyclonal antisera that recognize both phosphorylated and nonphosphorylated forms of the initiation factor, and similar levels of eIF2α were found between the different S. pombe lysate preparations. Therefore, the induced levels of eIF2α phosphorylation are not due to changes in the total eIF2α protein levels.
FIG. 4.
Elevated expression of human PKR and Hri1p in S. pombe reduces growth. Using the nmt1+ promoter, which is inhibited by thiamine, the eIF2α kinase PKR, Hri1p, or Hri2p was expressed in S. pombe strain SP223 from a high-copy-number plasmid. Additionally, wild-type eIF2α, or a mutant variant containing Ala substituted for Ser-51, was coexpressed with PKR or Hri1p as described in Materials and Methods. S. pombe strains were streaked onto agar plates containing minimal medium supplemented with 5 μg of thiamine/ml (right) or without thiamine addition (left). Agar plates were incubated for 3 days at 30°C and electronically imaged. Cells expressing PKR (PKR/eIF2α) or Hri1p (Hri1p/eIF2α) had slower growth than control cells carrying the parent pREP4 and pSP1 plasmids (Vectors). Coexpression of eIF2α-S51A suppressed this growth defect.
FIG. 5.
Phosphorylation of eIF2α is induced in response to elevated expression of human PKR, Hri1p, or Hri2p. Equal amounts of lysate prepared from S. pombe strain SP223 expressing the indicated eIF2α kinase were characterized by immunoblot analysis using antiserum that detects total eIF2α protein (bottom panel) or antibody that specifically recognizes eIF2α phosphorylated at Ser-51 (eIF2α−P). Each eIF2α kinase was expressed from a high-copy-number plasmid using the thiamine-repressible nmt1+ promoter. For the vector lane, only the parent plasmid was introduced into the strain. Lysates analyzed in lanes 1, 2, 3, and 4 were prepared from cells cultured in EMM in the absence of thiamine, allowing for elevated expression of the eIF2α kinases. In lanes 5, 6, 7, and 8, thiamine was added to EMM, repressing expression of the indicated eIF2α kinase. For controls, the immunoblot analysis was also carried out using lysates prepared from S. cerevisiae expressing GCN2 or kinase-defective gcn2-K628R (lanes 9 and 10) that was grown in the presence of 1 μg of SM (an inhibitor of branched chain amino acid synthesis)/ml.
Hri1p and Hri2p function in S. pombe stress resistance to heat shock or arsenic.
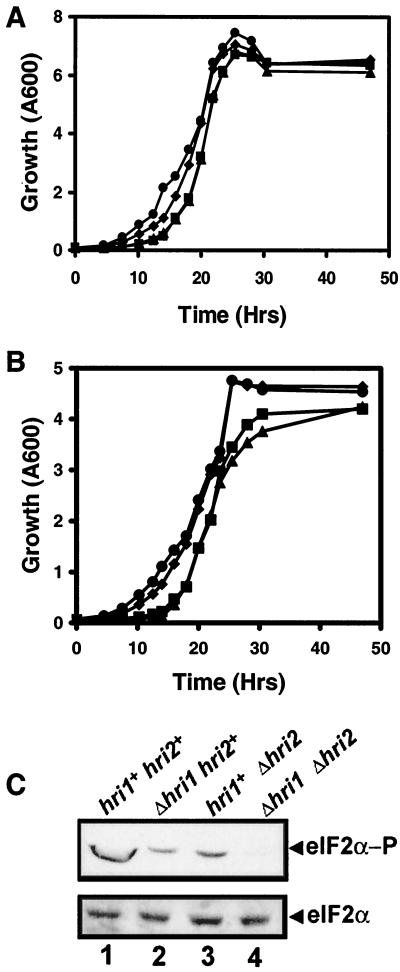
The importance of hri1+ and hri2+ in S. pombe stress resistance was addressed by deleting the two genes individually or in combination and measuring the growth properties of the mutant strains compared to the isogenic wild-type cells. The entire coding regions of hri1+ and hri2+ were replaced with the marker genes ura4+ and leu1+, respectively, by homologous recombination in haploid strain SP223. Gene deletions were screened by PCR analysis and subsequently confirmed by Southern analysis of genomic DNA cleaved by different restriction enzymes (data not shown). Experiments illustrated were confirmed with multiple independent deleted versions of SP223. Deletion of hri1+ and hri2+ individually or in combination did not impair cell viability in liquid or agar medium. Measurements of cell growth in rich medium, YES, or EMM indicated that deletion of hri2+ had no significant impact, while deletion of hri1+ in fact gave a modest enhancement of growth (Fig. 6A and B). When Δhri1 was combined with Δhri2, the growth rate of the mutant strain was identical to that of cells from which hri1+ alone was deleted. Furthermore, no differences were detected when glycerol was substituted for glucose in the rich medium grown under aerobic or anaerobic conditions (data not shown). Levels of eIF2α phosphorylation in the SP223-derived cells were measured by immunoblot analysis using the antibody specific to phosphorylated eIF2α. Deletion of either hri1+ or hri2+ partially diminished eIF2α phosphorylation levels in cells grown in YES compared to wild-type cells (Fig. 6C). In strain WY459 with combined Δhri1 Δhri2, the levels of eIF2α were significantly lower but detectable, indicative of a single remaining eIF2α kinase GCN2.
FIG. 6.
Deletion of hri1+ and hri2+ does not significantly alter growth in rich or minimal medium. S. pombe strains SP223 (▪, hri1+ hri2+), WY457 (♦, Δhri1 hri2+), WY458 (▴, hri1+ Δhri2), and WY459 (•, Δhri1 Δhri2) were grown in YES (A) or EMM (B). Aliquots were removed from each culture at the listed times and monitored for absorbance at 600 nm. (C) S. pombe SP223-derived strains with the indicated genotypes were grown to mid-exponential phase in YES medium at 30°C, and equal amounts of lysate prepared from each culture were analyzed by immunoblot using antibody that specifically recognizes eIF2α phosphorylated at Ser-51 (eIF2α−P) or antiserum that detects total eIF2α protein.
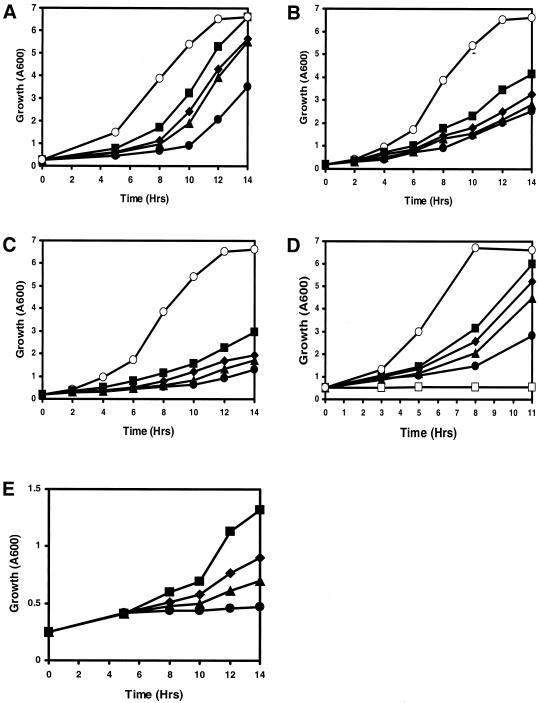
We next compared the growth rates of the wild-type and mutant S. pombe strains during different stress conditions. Addition of iron chelator, 3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine(ferrozine) (at concentrations between 100 and 500 μg/ml) to EMM equally reduced growth of the wild type and cells with hri1+ and hri2+ deleted (data not shown). Exposure to heat shock or oxidative stress induced by arsenite activates HRI in reticulocytes and in fetal liver nucleated progenitor cells; therefore, we analyzed the SP223-derived cells in YES medium subjected to these stress conditions. Following heat shock at 48°C, cell cultures were shifted to 30°C and monitored for growth. In response to heat stress, the wild-type strain SP223 (hri1+ hri2+) achieved exponential growth about 3 h later than nonstressed cells (Fig. 7A). Deletion of either hri1+ (WY457) or hri2+ (WY458) further reduced growth following heat shock compared to that of the isogenic wild-type strain. This growth delay was further exacerbated in the combined Δhri1 Δhri2 strain (WY459), which began to grow about 4 hours later than the identically heat-shocked wild-type strain (Fig. 7A).
FIG. 7.
hri1+ and hri2+ facilitate growth resistance of S. pombe to heat shock or arsenic exposure. S. pombe strains SP223 (▪, hri1+ hri2+), WY457 (♦, Δhri1 hri2+), WY458 (▴, hri1+ Δhri2), and WY459 (•,Δhri1 Δhri2), which were inoculated in YES medium supplemented with all 20 amino acids, were subjected to the indicated stress condition and monitored for growth by measuring absorbance at 600 nm. As controls, each of these strains was analyzed in parallel with the others in the absence of stress, with results similar to that observed for the wild-type strain SP223 (○, hri1+ hri2+). (A) The SP223-derived strains were subject to heat shock and then shifted to 30°C culture conditions as described in Materials and Methods. At the indicated times, cell samples were removed from the cultures and measured for absorbance at 600 nm. (B and C) Strains were grown in YES medium containing 200 μM (B) or 400 μM (C) sodium arsenite. (D) Strains were grown in YES medium containing 50 μM sodium arsenate. Additionally, each of these strains was analyzed in the presence of 100 μM sodium arsenate, with results similar to that observed for the wild-type strain SP223 (□, hri1+ hri2+). (E) S. pombe strains were cultured in YES medium supplemented with 100 μM sodium arsenate at 30°C for 1 hour, rinsed, and resuspended in the same volume of YES medium in the absence of the metalloid. Cultures were incubated at 30°C with agitation and monitored for growth by absorbance at 600 nm.
The impact of arsenic stress was addressed by culturing the SP223-derived strains in liquid YES medium supplemented with either arsenite or arsenate. We found that growth of the wild-type strain was delayed in response to exposure to 200 μM or 400 μM sodium arsenite (Fig. 7B and C). Deletion of the hri genes further retarded growth in the presence of arsenite, with the combined Δhri1 Δhri2 mutant cells showing the greatest sensitivity. Growth of the wild-type strain was delayed by about 2 h in response to exposure to 50 μM sodium arsenate (Fig. 7D). Arsenate sensitivity was further increased when either hri gene was deleted. Consistent with our previous stress conditions, the combined Δhri1 Δhri2 strain showed the greatest growth impact, with minimal growth compared to that of the isogenic wild-type strain similarly exposed to arsenate. At a higher concentration of sodium arsenate (100 μM), growth of the wild-type and hri-mutant versions of S. pombe were equally blocked (Fig. 7D). However, if strains were exposed to 100 μM sodium arsenate for 1 hour and shifted to YES medium in the absence of stress, the wild-type SP223 cells were able to resume growth (Fig. 7E). This resumption of growth was delayed in the cells with the hri genes deleted, with the order of severity being Δhri1 resuming growth faster than Δhri2 and the combined Δhri1 Δhri2 strain being completely impeded for growth.
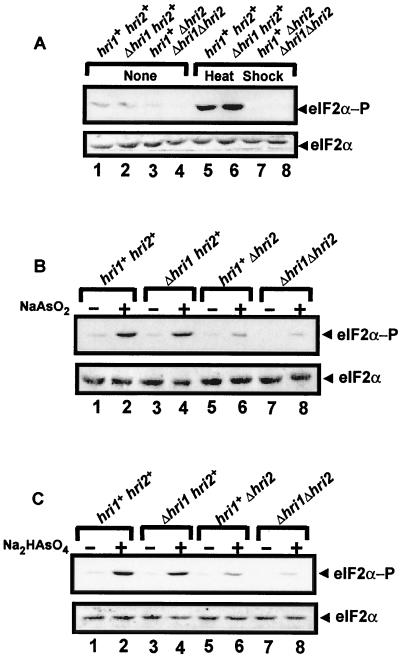
Phosphorylation of eIF2α by Hri1p and Hri2p is induced by heat shock or arsenic.
The diminished growth resistance of Δhri1 Δhri2 cells exposed to heat shock, arsenite, or arsenate suggests that these stress conditions modulate translational expression by phosphorylation of eIF2α. We compared the levels of eIF2α phosphorylation in wild-type SP223 and the Δhri1 and Δhri2 mutant derivatives. Phosphorylation of eIF2α in the wild-type cells (hri1+ hri2+) was significantly enhanced in response to heat shock (Fig. 8A). While Δhri1 had a minimal effect on eIF2α phosphorylation, deletion of hri2+ reduced the levels of eIF2α phosphorylation in response to heat exposure compared with that found in the combined Δhri1 Δhri2 strain. Phosphorylation of eIF2α was also enhanced in response to treatment with arsenite or arsenate (Fig. 8B and C). While deletion of hri1+ had a very modest impact on eIF2α phosphorylation in response to either arsenic compound, the levels of phosphorylation in the Δhri2 strain were significantly reduced, to less than 30% of that measured in the wild-type SP223 cells. By combining the hri1+ and hri2+ deletions in strain WY459, there was a further reduction of eIF2α phosphorylation in response to arsenite or arsenate exposure. These results indicate that phosphorylation of eIF2α by Hri-related proteins in S. pombe is induced in diverse environmental stress conditions. Furthermore, our results are consistent with the premise that the contributions of individual Hri1p and Hri2p activities differ during the course of the stress responses, with loss of Hri2p having a greater impact on growth and eIF2α phosphorylation levels in response to heat shock, arsenite, or arsenate exposure.
FIG. 8.
Loss of hri1+ and hri2+ impairs phosphorylation of eIF2α in response to stress conditions. The S. pombe SP223-derived strains with the indicated genotypes were grown to mid-exponential phase in YES medium supplemented with all 20 amino acids at 30°C and subjected to the presence or absence of heat shock (A), sodium arsenite (B), or sodium arsenate (C), as described in the Materials and Methods. Equal amounts of lysate prepared from each culture were analyzed by immunoblotting using antibody that specifically recognizes eIF2α phosphorylated at Ser-51 (eIF2α−P) or antiserum that detects total eIF2α protein (bottom panel).
DISCUSSION
Long-term exposure to arsenic compounds contributes to many disorders, including liver toxicity, peripheral neuropathies, cardiovascular diseases, and different cancers (1, 6, 39). Despite the toxicity of arsenic compounds, this metalloid is presently used in the treatment of protozoan infections and acute promyelocytic leukemia (3, 7, 64, 69). The mechanisms by which eukaryotic cells sense exposure to arsenic and their adaptive changes that are important for detoxifying this metalloid are not fully understood. In this study, we found that two eIF2α kinases related to mammalian HRI are important for the survival of S. pombe exposed to arsenite, arsenate, or heat shock. Deletion of both Hri1p and Hri2p from S. pombe strains reduced phosphorylation of eIF2α in response to exposure to these stress conditions and lowered growth tolerance compared to wild-type cells (Fig. 7 and 8). These results indicate that the HRI-related enzymes are important for sensing different stress conditions in S. pombe and implicate phosphorylation of eIF2α as a participant in the coordinate expression of related stress proteins.
Role of tandem HRI-related enzymes in S. pombe stress response.
Prior to this report, only single versions of each eIF2α kinase family member were reported for a given organism, and this study represents the first example of characterization of multiple copies of an eIF2α kinase family member in an eukaryotic organism. Consistent with their homology, which extends from the amino-terminal regulatory region to the kinase catalytic domains, Hri1p and Hri2p function jointly in response to exposure to different environmental stresses. Analysis of mutant strains from which either hri1+ or hri2+ was deleted indicated that Hri2p was the principal eIF2α kinase for response to addition of arsenite or arsenate to the medium. Loss of Hri1p showed only a modest impact on induced eIF2α phosphorylation in response to heat shock or arsenic exposure (Fig. 8). However, in each of these stress conditions, deletion of hri1+ did have an impact on growth, albeit less than that resulting from loss of hri2+ (Fig. 7). Furthermore, the combined loss of Hri1p and Hri2p led to the most severe growth sensitivity to these stress conditions. These results are consistent with the idea that Hri1p and Hri2p work in concert to facilitate heat and arsenic stress resistance, although Hri1p had a modest effect on the magnitude of eIF2 phosphorylation in our experimental conditions.
The basis for the enhanced contribution of Hri2p for eIF2α phosphorylation in response to heat shock or arsenic stress conditions may involve differences in the specific activity of this eIF2α kinase compared to that of Hri1p. Alternatively, the levels of Hri2p may be greater than those of Hri1p, directing S. pombe to rely more heavily on this eIF2α kinase for translational control during arsenite exposure or heat shock. We propose that phosphorylation of eIF2α is important for inducing the expression of stress-response proteins important for survival during environmental insults. In the well-characterized translational control pathway in S. cerevisiae, phosphorylation of even modest levels of eIF2α by GCN2 protein kinase can lead to a significant induction of GCN4 translation and transcription of its target genes. By comparison, higher levels of eIF2α phosphorylation reduce total protein synthesis. This observation may provide a rationale to explain why the apparent modest levels of eIF2α phosphorylation by Hri1p can contribute significantly to stress resistance.
Although S. pombe does not have a GCN4 homologue, other related transcription factors may facilitate its eIF2α kinase stress response. Many of the genes involved in arsenite stress are also induced by heat shock (6, 20, 68). Mechanisms of arsenite stress resistance in S. pombe include sequestering the metal by binding to phytochelatins, which are polymers derived from GSH by the action of phytochelatin synthase (16, 54). While phytochelatins are important for tolerance to toxic metals in S. pombe, phytochelatin synthase is not present in S. cerevisiae (15). Such differences between the detoxification pathways in these two fungi and their possible linkage with certain eIF2α kinases suggests one reason why HRI-related enzymes are present in only S. pombe. Arsenite also induces expression of metallothioneins, and metallothionein-deficient mice chronically exposed to arsenite experience more frequent and severe damage to liver and kidney tissues than control animals (40, 53). Further detoxification mechanisms include transporters than can evict metals from cells (41). These detoxification steps may be interfaced with a larger coordinate stress response that includes translational control by eIF2α phosphorylation. In the case of arsenate, toxicity stems from the ability of this arsenic compound to replace phosphate in phosphorolytic reactions. Arsenate can also be reduced to arsenite by a mechanism involving glutathione and the enzyme arsenate reductase (6). Therefore, similarities in eIF2α phosphorylation levels between S. pombe strains exposed to arsenite or arsenate may result in part from the use of this biotransformation pathway.
Regulation of the activity of HRI-related enzymes by heme, heat shock, and arsenic.
Rabbit HRI is a stable homodimer that can undergo autophosphorylation and catalyze eIF2α phosphorylation (4, 11, 12). Repression of HRI by heme involves two distinct types of binding sites (58). The first is a high-affinity site involving sequences in the amino terminus of HRI that stably binds heme. Heme binding at this site is proposed to be important for proper folding of HRI, and purified HRI associated with heme at the first site is autophosphorylated and has enhanced eIF2α kinase activity (4, 9, 11, 73). By comparison, the second site, incorporated into the insert region of the catalytic domain of HRI, can reversibly associate with heme and is thought to be responsible for repression by heme. We have also found that purified Hri1p and Hri2p are repressed by heme, although at higher concentrations than that measured for mammalian HRI (Fig. 3). In our initial in vivo characterization of iron regulation, using the chelator ferrozine, we found that addition of ferrozine at concentrations between 100 and 500 μg/ml retarded equally the growth of S. pombe SP223 and Δhri1 and Δhri2 mutant cells. This growth inhibition was suppressed by the addition of iron to the medium, indicating that the chelator reduced the intracellular level of iron. Furthermore, we did not see any induction of eIF2α phosphorylation in the S. pombe SP223 strain following incubation with ferrozine (data not shown). These results may indicate that iron metabolism per se is not linked to Hri1p and Hri2p functions, in contrast to the requirement for iron metabolism described for mammalian erythroid cells, with its important role in coordinating globin synthesis to iron availability. Alternatively, in the S. pombe cells exposed to ferrozine, there may be heme turnover issues that impact regulation of the eIF2α kinases in vivo.
Chaperones also copurify with rabbit HRI, and Hsc70 interaction with HRI is proposed to maintain the protein kinase in an inactive conformation, preventing its autophosphorylation even during heme deficiency (11, 34, 70, 87). Proteins damaged by heat shock or arsenite-induced oxidation may titrate Hsc70 from the HRI complex, facilitating autophosphorylation and increased eIF2α kinase activity. Additionally, Hsc70 is required for arsenite induction of mammalian HRI activity, emphasizing the role of this chaperone in HRI folding to an activatable conformation inducible by this metalloid (42, 71, 74).
A final explanation for the stress activation of Hri1p and Hri2p might include the potential negative impact of the arsenite and heat shock on heme levels (8, 20). In mammalian cells, a variety of stress-associated agents, including heat shock and exposure to arsenic compounds, induce the coordinate expression of proteins involved in antioxidant and cytoprotective functions. Among these genes is heme oxygenase, which catalyzes the rate-limiting step in heme catabolism that ultimately leads to the production of bilirubin and the antioxidant NQO [NAD(P)H:quinone oxidoreductase] (24, 31). NQO is important for the catalysis of the reduction of quinones, blocking their participation in oxidative stress and redox cycling. Mammalian genes such as γ-glutamylcysteine synthase, which is involved in the synthesis of glutathione, and glutathione S-transferase, which conjugates glutathione with reactive oxygen species, are reported to be induced by this heme oxygenase-mediated catabolic pathway (2, 35, 84). Reactive oxygen species are required for activation of HRI (42). Furthermore, carbon monoxide, a reported physiological regulator of rabbit HRI, is generated in the heme degradation reaction catalyzed by heme oxygenase (75). Interestingly, this stress-mediated coordinate gene expression involves the enhanced expression of ATF4, a gene subject to translational control in response to eIF2α phosphorylation in mammalian cells (27, 31). ATF4, heterodimerized with another bZIP protein, Nrf2, is thought to regulate transcriptional expression of the heme oxygenase gene by binding directly to regulatory sequences within its promoter.
Environmental stress, gene expression, and eIF2α kinases.
Recognition and remediation of environmental stresses in different eukaryotic organisms is thought to require different combinations of eIF2α kinases. Research to date suggests that the eIF2α kinase PKR is restricted to mammals, PEK/Perk functions throughout metazoic organisms, and GCN2 is distributed in both unicellular and multicellular eukaryotes. In this study, we found that HRI homologues facilitate stress responses in S. pombe in addition to the previously characterized vertebrate systems. That HRI is absent from the invertebrate organisms whose genomes have been analyzed, D. melanogaster and C. elegans, presumably is due to loss of this enzyme during their evolution or during their selection and development as model laboratory organisms. It is noteworthy that a reported eIF2α kinase, PfPK4, in the malarial parasite Plasmodium falciparum appears to be inhibited by elevated levels (i.e., 10 μM) of hemin (47). PfPK4 has a 230-residue N-terminal sequence flanking its kinase domain, with no similarity to HRI or other eIF2α kinase regulatory regions. The association of PfPK4 with the host erythrocyte membrane during the final stage of the parasite's development may suggest a role for this kinase in monitoring the host environment during invasion of erythrocytes. Future genome sequencing of other unicellular and metazoic eukaryotic organisms is needed to establish the prevalence of HRI.
Phosphorylation of eIF2α can induce gene-specific translation important for coordinate expression of genes required to remedy cellular stress. While GCN4 homologues are also found in many other fungi, including Neurospora crassa (43), Aspergillus niger (78), and Candida albicans (55), there is no GCN4 homologue in S. pombe or in higher eukaryotic organisms. In mammalian cells, translational expression of another bZIP protein, ATF4, has been reported to be induced by GCN2 and PEK/Perk in response to amino acid limitation and ER stress, respectively (27). Furthermore, the DNA binding activity of ATF4 was reported to be enhanced in response to arsenite treatment of rat pheochromocytoma PC12 cells (25). Such targeted induction of gene-specific translation may also occur in response to eIF2α phosphorylation by mammalian HRI, although this premise remains to be experimentally addressed. Therefore, stress pathways associated with eIF2α kinases are modular, with GCN2 protein kinase inducing distinct transcriptional regulators in different organisms. In S. pombe, at least six bZIP proteins have been characterized (18, 36, 50, 51, 67, 79, 85) and are potential regulatory targets for eIF2α phosphorylation by Hri1p and Hri2p. Interestingly, two proteins, Pap1p and Atf1p, are activated in response to a range of environmental stresses, including UV light, osmotic stress, heat shock, and exposure to the protein synthesis inhibitor anisomycin by a mechanism that involves a stress-activated mitogen-activated protein kinase pathway which includes the protein kinase Sty1p (18, 49, 85). The potential control of Pap1p or Atf1p or other bZIP proteins by the HRI-related proteins in S. pombe awaits further genetic and biochemical studies.
Acknowledgments
We thank Ernie Hannig, Charles Hoffman, and Patrick Romano for helpful discussions and sharing of plasmids and strains, Keith Remo for technical assistance, and the Biochemistry Biotechnology Facility at Indiana University for technical support.
This study was supported in part by National Institutes of Health grants R01GM49164 and R01GM643540 (R.C.W.) and R01DK53223 (J.-J. C.).
REFERENCES
- 1.Abernathy, C. O., Y. P. Liu, D. Longfellow, H. V. Aposhian, B. Beck, B. Fowler, R. Goyer, R. Menzer, T. Rossman, C. Thompson, and M. Waalkes. 1999. Arsenic: health effects, mechanisms of actions, and research issues. Environ. Health Perspect. 107:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam, J., D. Stewart, C. Touchard, S. Boinapally, A. M. Choi, and J. L. Cook. 1999. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 274:26071-26078. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, S. V., and M. P. Barrett. 2000. Anti-sleeping sickness drugs and cancer chemotherapy. Parasitol. Today 16:7-9. [DOI] [PubMed] [Google Scholar]
- 4.Bauer, B. N., M. Rafie-Kolpin, L. Lu, A. Han, and J. J. Chen. 2001. Multiple autophosphorylation is essential for the formation of the active and stable homodimer of heme-regulated eIF2α kinase. Biochemistry 40:11543-11551. [DOI] [PubMed] [Google Scholar]
- 5.Berlanga, J. J., S. Herrero, and C. de Haro. 1998. Characterization of the hemin-sensitive eukaryotic initiation factor 2α kinase from mouse nonerythroid cells. J. Biol. Chem. 273:32340-32346. [DOI] [PubMed] [Google Scholar]
- 6.Bernstam, L., and J. Nriagu. 2000. Molecular aspects of arsenic stress. J. Toxicol. Environ. Health Part B Crit. Rev. 3:293-322. [DOI] [PubMed] [Google Scholar]
- 7.Borst, P., and M. Ouellette. 1995. New mechanisms of drug resistance in parasitic protozoa. Annu. Rev. Microbiol. 49:427-460. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. L., and K. T. Kitchin. 1996. Arsenite, but not cadmium, induces ornithine decarboxylase and heme oxygenase activity in rat liver: relevance to arsenic carcinogenesis. Cancer Lett. 98:227-231. [PubMed] [Google Scholar]
- 9.Chefalo, P. J., J. Oh, M. Rafie-Kolpin, B. Kan, and J. J. Chen. 1998. Heme-regulated eIF-2α kinase purifies as a hemoprotein. Eur. J. Biochem. 258:820-830. [DOI] [PubMed] [Google Scholar]
- 10.Chefalo, P. J., J. M. Yang, K. V. Ramaiah, L. Gehrke, and J. J. Chen. 1994. Inhibition of protein synthesis in insect cells by baculovirus-expressed heme-regulated eIF-2 alpha kinase. J. Biol. Chem. 269:25788-25794. [PubMed] [Google Scholar]
- 11.Chen, J.-J. 2000. Heme-regulated eIF2α kinase, p. 529-546. In N. Sonenberg, J. W. B. Hershey, and M. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 12.Chen, J. J., and I. M. London. 1995. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem. Sci. 20:105-108. [DOI] [PubMed] [Google Scholar]
- 13.Chen, J. J., M. S. Throop, L. Gehrke, I. Kuo, J. K. Pal, M. Brodsky, and I. M. London. 1991. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc. Natl. Acad. Sci. USA 88:7729-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen, J. H., E. G. Coles, V. Robinson, A. Pasini, and D. G. Wilkinson. 2001. Screening from a subtracted embryonic chick hindbrain cDNA library: identification of genes expressed during hindbrain, midbrain and cranial neural crest development. Mech. Dev. 102:119-133. [DOI] [PubMed] [Google Scholar]
- 15.Clemens, S., E. J. Kim, D. Neumann, and J. I. Schroeder. 1999. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 18:3325-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobbett, C. S. 2000. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 123:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cottarel, G., D. Beach, and U. Deuschle. 1993. Two new multi-purpose multicopy Schizosaccharomyces pombe shuttle vectors, pSP1 and pSP2. Curr. Genet. 23:547-548. [DOI] [PubMed] [Google Scholar]
- 18.Degols, G., and P. Russell. 1997. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 17:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degols, G., K. Shiozaki, and P. Russell. 1996. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 16:2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Razo, L. M., B. Quintanilla-Vega, E. Brambila-Colombres, E. S. Calderon-Aranda, M. Manno, and A. Albores. 2001. Stress proteins induced by arsenic. Toxicol. Appl. Pharmacol. 177:132-148. [DOI] [PubMed] [Google Scholar]
- 21.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 22.Dever, T. E., J. J. Chen, G. N. Barber, A. M. Cigan, L. Feng, T. F. Donahue, I. M. London, M. G. Katze, and A. G. Hinnebusch. 1993. Mammalian eukaryotic initiation factor 2 alpha kinases functionally substitute for GCN2 protein kinase in the GCN4 translational control mechanism of yeast. Proc. Natl. Acad. Sci. USA 90:4616-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong, J., H. Qiu, M. Garcia-Barrio, J. Anderson, and A. G. Hinnebusch. 2000. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 6:269-279. [DOI] [PubMed] [Google Scholar]
- 24.Elbirt, K. K., and H. L. Bonkovsky. 1999. Heme oxygenase: recent advances in understanding its regulation and role. Proc. Assoc. Am. Physicians 111:438-447. [PubMed] [Google Scholar]
- 25.Fawcett, T. W., Q. Xu, and N. J. Holbrook. 1997. Potentiation of heat stress-induced hsp70 expression in vivo by aspirin. Cell Stress Chaperones 2:104-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han, A. P., C. Yu, L. Lu, Y. Fujiwara, C. Browne, G. Chin, M. Fleming, P. Leboulch, S. H. Orkin, and J. J. Chen. 2001. Heme-regulated eIF2α kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 20:6909-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding, H. P., I. I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6:1099-1108. [DOI] [PubMed] [Google Scholar]
- 28.Harding, H. P., H. Zeng, Y. Zhang, R. Jungries, P. Chung, H. Plesken, D. D. Sabatini, and D. Ron. 2001. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7:1153-1163. [DOI] [PubMed] [Google Scholar]
- 29.Harding, H. P., Y. Zhang, A. Bertolotti, H. Zeng, and D. Ron. 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5:897-904. [DOI] [PubMed] [Google Scholar]
- 30.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271-274. [DOI] [PubMed] [Google Scholar]
- 31.He, C. H., P. Gong, B. Hu, D. Stewart, M. E. Choi, A. M. Choi, and J. Alam. 2001. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 276:20858-20865. [DOI] [PubMed] [Google Scholar]
- 32.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-88. In N. Sonenberg, J. W. B. Hershey, and M. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 33.Hinnebusch, A. G. 1997. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J. Biol. Chem. 272:21661-21664. [DOI] [PubMed] [Google Scholar]
- 34.Hung, J. J., T. J. Cheng, M. D. Chang, K. D. Chen, H. L. Huang, and Y. K. Lai. 1998. Involvement of heat shock elements and basal transcription elements in the differential induction of the 70-kDa heat shock protein and its cognate by cadmium chloride in 9L rat brain tumor cells. J. Cell. Biochem. 71:21-35. [PubMed] [Google Scholar]
- 35.Ishii, T., K. Itoh, S. Takahashi, H. Sato, T. Yanagawa, Y. Katoh, S. Bannai, and M. Yamamoto. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 275:16023-16029. [DOI] [PubMed] [Google Scholar]
- 36.Kanoh, J., Y. Watanabe, M. Ohsugi, Y. Iino, and M. Yamamoto. 1996. Schizosaccharomyces pombe gad7+ encodes a phosphoprotein with a bZIP domain, which is required for proper G1 arrest and gene expression under nitrogen starvation. Genes Cells 1:391-408. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman, R. J. 2000. Double-stranded RNA-activated protein kinase PKR, p. 503-528. In N. Sonenberg, J. W. B. Hershey, and M. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 38.Kaufman, R. J., D. Scheuner, M. Schroder, X. Shen, K. Lee, C. Y. Liu, and S. M. Arnold. 2002. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3:411-421. [DOI] [PubMed] [Google Scholar]
- 39.Kitchin, K. T. 2001. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 172:249-261. [DOI] [PubMed] [Google Scholar]
- 40.Klaassen, C. D., J. Liu, and S. Choudhuri. 1999. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 39:267-294. [DOI] [PubMed] [Google Scholar]
- 41.Li, Z. S., Y. P. Lu, R. G. Zhen, M. Szczypka, D. J. Thiele, and P. A. Rea. 1997. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. USA 94:42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu, L., A.-P. Han, and J.-J. Chen. 2001. Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 21:7971-7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo, Z., M. Freitag, and M. S. Sachs. 1995. Translational regulation in response to changes in amino acid availability in Neurospora crassa. Mol. Cell. Biol. 15:5235-5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maundrell, K. 1990. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem. 265:10857-10864. [PubMed] [Google Scholar]
- 45.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127-130. [DOI] [PubMed] [Google Scholar]
- 46.Mellor, H., K. M. Flowers, S. R. Kimball, and L. S. Jefferson. 1994. Cloning and characterization of cDNA encoding rat hemin-sensitive initiation factor-2 alpha (eIF-2 alpha) kinase. Evidence for multitissue expression. J. Biol. Chem. 269:10201-10204. [PubMed] [Google Scholar]
- 47.Mohrle, J. J., Y. Zhao, B. Wernli, R. M. Franklin, and B. Kappes. 1997. Molecular cloning, characterization and localization of PfPK4, an eIF-2α kinase-related enzyme from the malarial parasite Plasmodium falciparum. Biochem. J. 328:677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen, A. N., A. Lee, W. Place, and K. Shiozaki. 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11:1169-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nojima, H., S. H. Leem, H. Araki, A. Sakai, N. Nakashima, Y. Kanaoka, and Y. Ono. 1994. Hac1: a novel yeast bZIP protein binding to the CRE motif is a multicopy suppressor for cdc10 mutant of Schizosaccharomyces pombe. Nucleic Acids Res. 22:5279-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohmiya, R., C. Kato, H. Yamada, H. Aiba, and T. Mizuno. 1999. Isolation of multicopy suppressors of the calcium sensitivity of a mutant lacking the bZIP transcription factor Atf1 in fission yeast. Mol. Gen. Genet. 261:297-306. [DOI] [PubMed] [Google Scholar]
- 52.Olsen, D. S., B. Jordan, D. Chen, R. C. Wek, and D. R. Cavener. 1998. Isolation of the gene encoding the Drosophila melanogaster homolog of the S. cerevisiae GCN2 eIF-2α kinase. Genetics 149:1495-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park, J. D., Y. Liu, and C. D. Klaassen. 2001. Protective effect of metallothionein against the toxicity of cadmium and other metals (1). Toxicology 163:93-100. [DOI] [PubMed] [Google Scholar]
- 54.Perego, P., and S. B. Howell. 1997. Molecular mechanisms controlling sensitivity to toxic metal ions in yeast. Toxicol. Appl. Pharmacol. 147:312-318. [DOI] [PubMed] [Google Scholar]
- 55.Pereira, S. A., and G. P. Livi. 1995. A GCN-like response in Candida albicans. Cell Biol. Int. 19:65-69. [DOI] [PubMed] [Google Scholar]
- 56.Proud, C. G. 1995. PKR: a new name and new roles. Trends Biochem. Sci. 20:241-246. [DOI] [PubMed] [Google Scholar]
- 57.Qiu, H., J. Dong, C. Hu, C. S. Francklyn, and A. G. Hinnebusch. 2001. The tRNA-binding moiety in GCN2 contains a dimerization domain that interacts with the kinase domain and is required for tRNA binding and kinase activation. EMBO J. 20:1425-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rafie-Kolpin, M., P. J. Chefalo, Z. Hussain, J. Hahn, S. Uma, R. L. Matts, and J. J. Chen. 2000. Two heme-binding domains of heme-regulated eukaryotic initiation factor-2alpha kinase. N terminus and kinase insertion. J. Biol. Chem. 275:5171-5178. [DOI] [PubMed] [Google Scholar]
- 59.Romano, P. R., S. R. Green, G. N. Barber, M. B. Mathews, and A. G. Hinnebusch. 1995. Structural requirements for double-stranded RNA binding, dimerization, and activation of the human eIF-2α kinase DAI in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 61.Santoyo, J., J. Alcalde, R. Mendez, D. Pulido, and C. de Haro. 1997. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2α (eIF-2α) kinase from Drosophila melanogaster. Homology to yeast GCN2 protein kinase. J. Biol. Chem. 272:12544-12550. [DOI] [PubMed] [Google Scholar]
- 62.Schmitt, M. E., T. A. Brown, and B. L. Trumpower. 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18:3091-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi, Y., K. M. Vattem, R. Sood, J. An, J. Liang, L. Stramm, and R. C. Wek. 1998. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18:7499-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soignet, S. L., P. Maslak, Z. G. Wang, S. Jhanwar, E. Calleja, L. J. Dardashti, D. Corso, A. DeBlasio, J. Gabrilove, D. A. Scheinberg, P. P. Pandolfi, and R. P. Warrell, Jr. 1998. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 339:1341-1348. [DOI] [PubMed] [Google Scholar]
- 65.Sood, R., A. C. Porter, K. Ma, L. A. Quilliam, and R. C. Wek. 2000. Pancreatic eukaryotic initiation factor-2α kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346:281-293. [PMC free article] [PubMed] [Google Scholar]
- 66.Steiner, N. C., K. M. Hahnenberger, and L. Clarke. 1993. Centromeres of the fission yeast Schizosaccharomyces pombe are highly variable genetic loci. Mol. Cell. Biol. 13:4578-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda, T., T. Toda, K. Kominami, A. Kohnosu, M. Yanagida, and N. Jones. 1995. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 14:6193-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamas, M. J., K. Luyten, F. C. Sutherland, A. Hernandez, J. Albertyn, H. Valadi, H. Li, B. A. Prior, S. G. Kilian, J. Ramos, L. Gustafsson, J. M. Thevelein, and S. Hohmann. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31:1087-1104. [DOI] [PubMed] [Google Scholar]
- 69.Tamas, M. J., and R. Wysocki. 2001. Mechanisms involved in metalloid transport and tolerance acquisition. Curr. Genet. 40:2-12. [DOI] [PubMed] [Google Scholar]
- 70.Thulasiraman, V., Z. Xu, S. Uma, Y. Gu, J. J. Chen, and R. L. Matts. 1998. Evidence that Hsc70 negatively modulates the activation of the heme-regulated eIF-2α kinase in rabbit reticulocyte lysate. Eur. J. Biochem. 255:552-562. [DOI] [PubMed] [Google Scholar]
- 71.Thulasiraman, V., B. G. Yun, S. Uma, Y. Gu, B. T. Scroggins, and R. L. Matts. 2002. Differential inhibition of Hsc70 activities by two Hsc70-binding peptides. Biochemistry 41:3742-3753. [DOI] [PubMed] [Google Scholar]
- 72.Uma, S., D. J. Barret, and R. L. Matts. 1998. Changes in the expression of the heme-regulated eIF-2 alpha kinase and heat shock proteins in rabbit reticulocytes maturing during recovery from anemia. Exp. Cell Res. 238:273-282. [DOI] [PubMed] [Google Scholar]
- 73.Uma, S., R. L. Matts, Y. Guo, S. White, and J. J. Chen. 2000. The N-terminal region of the heme-regulated eIF2α kinase is an autonomous heme binding domain. Eur. J. Biochem. 267:498-506. [DOI] [PubMed] [Google Scholar]
- 74.Uma, S., V. Thulasiraman, and R. L. Matts. 1999. Dual role for Hsc70 in the biogenesis and regulation of the heme-regulated kinase of the alpha subunit of eukaryotic translation initiation factor 2. Mol. Cell. Biol. 19:5861-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uma, S., B. G. Yun, and R. L. Matts. 2001. The heme-regulated eukaryotic initiation factor 2α kinase. A potential regulatory target for control of protein synthesis by diffusible gases. J. Biol. Chem. 276:14875-14883. [DOI] [PubMed] [Google Scholar]
- 76.Vattem, K. M., K. A. Staschke, and R. C. Wek. 2001. Mechanism of activation of the double-stranded-RNA-dependent protein kinase, PKR: role of dimerization and cellular localization in the stimulation of PKR phosphorylation of eukaryotic initiation factor-2 (eIF2). Eur. J. Biochem. 268:3674-3684. [DOI] [PubMed] [Google Scholar]
- 77.Vattem, K. M., K. A. Staschke, S. Zhu, and R. C. Wek. 2001. Inhibitory sequences in the N-terminus of the double-stranded-RNA-dependent protein kinase, PKR, are important for regulating phosphorylation of eukaryotic initiation factor 2α (eIF2α). Eur. J. Biochem. 268:1143-1153. [DOI] [PubMed] [Google Scholar]
- 78.Wanke, C., S. Eckert, G. Albrecht, W. van Hartingsveldt, P. J. Punt, C. A. van den Hondel, and G. H. Braus. 1997. The Aspergillus niger GCN4 homologue, cpcA, is transcriptionally regulated and encodes an unusual leucine zipper. Mol. Microbiol. 23:23-33. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe, Y., and M. Yamamoto. 1996. Schizosaccharomyces pombe pcr1+ encodes a CREB/ATF protein involved in regulation of gene expression for sexual development. Mol. Cell. Biol. 16:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wek, R. C. 1994. eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem. Sci. 19:491-496. [DOI] [PubMed] [Google Scholar]
- 81.Wek, R. C., B. M. Jackson, and A. G. Hinnebusch. 1989. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc. Natl. Acad. Sci. USA 86:4579-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wek, S. A., S. Zhu, and R. C. Wek. 1995. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 15:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiemann, S., B. Weil, R. Wellenreuther, J. Gassenhuber, S. Glassl, W. Ansorge, M. Bocher, H. Blocker, S. Bauersachs, H. Blum, J. Lauber, A. Dusterhoft, A. Beyer, K. Kohrer, N. Strack, H. W. Mewes, B. Ottenwalder, B. Obermaier, J. Tampe, D. Heubner, R. Wambutt, B. Korn, M. Klein, and A. Poustka. 2001. Toward a catalog of human genes and proteins: sequencing and analysis of 500 novel complete protein coding human cDNAs. Genome Res. 11:422-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wild, A. C., H. R. Moinova, and R. T. Mulcahy. 1999. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 274:33627-33636. [DOI] [PubMed] [Google Scholar]
- 85.Wilkinson, M. G., M. Samuels, T. Takeda, W. M. Toone, J. C. Shieh, T. Toda, J. B. Millar, and N. Jones. 1996. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10:2289-2301. [DOI] [PubMed] [Google Scholar]
- 86.Wood, V., R. Gwilliam, M. A. Rajandream, M. Lyne, R. Lyne, A. Stewart, J. Sgouros, N. Peat, J. Hayles, S. Baker, D. Basham, S. Bowman, K. Brooks, D. Brown, S. Brown, T. Chillingworth, C. Churcher, M. Collins, R. Connor, A. Cronin, P. Davis, T. Feltwell, A. Fraser, S. Gentles, A. Goble, N. Hamlin, D. Harris, J. Hidalgo, G. Hodgson, S. Holroyd, T. Hornsby, S. Howarth, E. J. Huckle, S. Hunt, K. Jagels, K. James, L. Jones, M. Jones, S. Leather, S. McDonald, J. McLean, P. Mooney, S. Moule, K. Mungall, L. Murphy, D. Niblett, C. Odell, K. Oliver, S. O'Neil, D. Pearson, M. A. Quail, E. Rabbinowitsch, K. Rutherford, S. Rutter, D. Saunders, K. Seeger, S. Sharp, J. Skelton, M. Simmonds, R. Squares, S. Squares, K. Stevens, K. Taylor, R. G. Taylor, A. Tivey, S. Walsh, T. Warren, S. Whitehead, J. Woodward, G. Volckaert, R. Aert, J. Robben, B. Grymonprez, I. Weltjens, E. Vanstreels, M. Rieger, M. Schafer, S. Muller-Auer, C. Gabel, M. Fuchs, C. Fritzc, E. Holzer, D. Moestl, H. Hilbert, K. Borzym, I. Langer, A. Beck, H. Lehrach, R. Reinhardt, T. M. Pohl, P. Eger, W. Zimmermann, H. Wedler, R. Wambutt, B. Purnelle, A. Goffeau, E. Cadieu, S. Dreano, S. Gloux, V. Lelaure, et al. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871-880. [DOI] [PubMed] [Google Scholar]
- 87.Xu, Z., J. K. Pal, V. Thulasiraman, H. P. Hahn, J. J. Chen, and R. L. Matts. 1997. The role of the 90-kDa heat-shock protein and its associated cohorts in stabilizing the heme-regulated eIF-2α kinase in reticulocyte lysates during heat stress. Eur. J. Biochem. 246:461-470. [DOI] [PubMed] [Google Scholar]
- 88.Zhu, S., P. R. Romano, and R. C. Wek. 1997. Ribosome targeting of PKR is mediated by two double-stranded RNA-binding domains and facilitates in vivo phosphorylation of eukaryotic initiation factor-2. J. Biol. Chem. 272:14434-14441. [DOI] [PubMed] [Google Scholar]
- 89.Zhu, S., A. Y. Sobolev, and R. C. Wek. 1996. Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2. J. Biol. Chem. 271:24989-24994. [DOI] [PubMed] [Google Scholar]