Abstract
The single-cell trichomes in wild-type Arabidopsis are either unbranched or have two to five branches. Using transgenic Arabidopsis plants expressing a green fluorescent protein–microtubule-associated protein4 fusion protein, which decorates the microtubular cytoskeleton, we observed that during trichome branching, microtubules reorient with respect to the longitudinal growth axis. Considering branching to be a localized microtubule-dependent growth reorientation event, we investigated the effects of microtubule-interacting drugs on branch induction in trichomes. In unbranched trichomes of the mutant stichel, a change in growth directionality, closely simulating branch initiation, could be elicited by a short treatment with paclitaxel, a microtubule-stabilizing drug, but not with microtubule-disrupting drugs. The growth reorientation appeared to be linked to increased microtubule stabilization and to aster formation in the treated trichomes. Taxol-induced microtubule stabilization also led to the initiation of new branch points in the zwichel mutant of Arabidopsis, which is defective in a kinesin-like microtubule motor protein and possesses trichomes that are less branched. Our observations suggest that trichome cell branching in Arabidopsis might be mediated by transiently stabilized microtubular structures, which may form a component of a multiprotein complex required to reorient freshly polymerizing microtubules into new growth directions.
INTRODUCTION
The shape of a cell is largely determined by its major growth axis. In general, a rounded cell morphology reflects an isotropic mode of growth, whereas an asymmetric cell shape is generated by anisotropic growth, that is, more growth in one direction than in others. Cells can also grow in different directions by initiating branches at specific locations. The initiation of a branch in a unidirectionally growing cell may thus be traced to a localized and transient alteration in the direction of cell growth, after which the cell reverts to its original mode of growth but now extends itself in two (or more) different directions. Studies performed using diverse organisms such as Schizosaccharomyces pombe (Sawin and Nurse, 1998) and Physcomitrella patens (Doonan et al., 1988) as well as cell types such as mammalian cancer cell lines (Sabry et al., 1991), nerve cells (Williamson et al., 1996), and root hair cells in plants (Bibikova et al., 1999) have firmly established the role of the microtubular component of the cytoskeleton in these processes. It is accepted that microtubule reorientation can lead to a change in growth orientation (Williamson, 1991; Joshi, 1998). The spatial orientation of microtubules is generated by their interactions with various molecules and complexes such as microtubule-organizing centers (MTOCs), which both nucleate and affect the positioning of microtubules in the cytoplasm (Marc, 1997; Vaughn and Harper, 1998). MTOCs may originate at sites other than their place of action and be transported to their specific locations with the aid of microtubule motor proteins such as dynein and kinesins (Asada and Collings, 1997). Alternatively, MTOCs may also self-organize into ordered arrays in the presence of certain motor proteins (Nedelec et al., 1997).
In most plant cells, however, the origin, identity, and precise locations of MTOCs have remained relatively elusive because specific organelles such as centrosomes are not found in higher plants (Lambert, 1995; Vaughn and Harper, 1998). On the other hand, studies over the last 10 to 15 years have provided strong evidence linking changes in the orientation of plant cell growth to gross alterations in cortical microtubule arrays (Cyr, 1994; Cyr and Palevitz, 1995). A complete switch from anisotropic to isotropic patterns of growth has been observed under the influence of plant growth regulators, drugs that interact with microtubules, and diverse chemicals (Shibaoka, 1994; Baskin and Wilson, 1997; Kropf et al., 1998). The microtubule reorientation noted during the process of, or leading to, branching in various animal cells has not, however, been observed in plants. This discrepancy arises because most single plant cells, except for the trichomes in plants such as Arabidopsis, do not branch naturally and are therefore not amenable for such studies. Instead, most plant cells reorient themselves, either by directional bending through extension growth or by initiating fresh cell divisions in the direction that requires new growth.
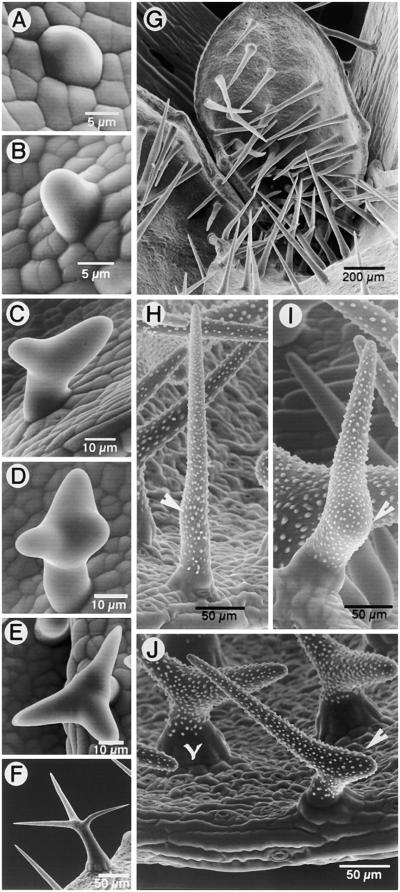
Arabidopsis trichomes thus provide an interesting cell type for studying the process of single-cell branching in plants. Trichomes in Arabidopsis are aerial, epidermal, unicellular structures whose development involves a sequence of morphogenetic events to convert an epidermal protrusion into an anisotropically growing tubular form (Huelskamp et al., 1994; Marks, 1997). The tubular cell may remain unbranched, split into two branches, or branch further to produce a three- to five-branched trichome (Figure 1). The decision to branch appears to be made early during trichome development, and the divergent orientations of branch initials on the main trichome stalk clearly indicate underlying alterations in the direction of growth. Later events during trichome cell morphogenesis include the processes of extension growth and wall thickening, which ultimately produce the mature, characteristically stellate form of the trichome (Figure 1; Szymanski et al., 1998).
Figure 1.
Stages during Development of Branched and Unbranched Trichome Cells in Arabidopsis.
(A) A trichome initial appears as a bulge on the leaf epidermis.
(B) A tubular trichome initial extends perpendicular from the leaf epidermis. It may remain unbranched, as in (G), or initiate various degrees of branching, as in (C), (D), and (H) to (J).
(C) A tubular trichome cell shows an apical split to produce a Y-shaped two-branched trichome. Further growth may result in a trichome with equally extending or unequal branches, as in (J).
(D) Three branches are formed by an initial apical split, producing the proximal and distal branches (in relation to the longitudinal axis of the leaf) followed by a subapical branch point formation on the distal branch.
(E) Typical stellate morphology of branched trichomes on wild-type Arabidopsis leaves.
(F) A mature trichome with fully extended branches and papillate decorations.
(G) Trichomes of the stichel mutant are straight, unbranched, and spike-like. Similar unbranched trichomes are found on inflorescence stems in wild-type Arabidopsis and with various frequencies on the different radical and cauline leaves.
(H) A trichome of the zwichel mutant (zwi9311-11) with a subtle bulge on its surface (arrowhead), indicating an incipient branch point.
(I) A zwichel trichome showing a prominent bulge (arrowhead), indicating its arrest at a slightly more advanced stage of branch initiation than that in (G).
(J) Nearly 59.6% of the trichomes on zwi9311-11 have two branches (Luo and Oppenheimer, 1999), with a lopsided branch growth (arrowhead) or almost equally extending branches (Y-shaped).
We have recently shown that the actin cytoskeleton plays a dominant role during extension growth of trichome branches and serves to elaborate and maintain an already established branching pattern (Mathur et al., 1999). Early trichome development was shown to rely more on microtubular cytoskeleton–linked processes because microtubule-interacting drugs changed growth directionality completely, from the normal anisotropic trichome cell growth to isotropic growing forms. These drugs also reduced or totally abolished branching of the trichome cell (Mathur et al., 1999). Having laid the conceptual framework for cytoskeletal involvement during trichome morphogenesis in our previous study, we further utilized the trichome system to investigate in more detail the behavior and role of the microtubular cytoskeleton during the process of trichome cell branching in Arabidopsis.
To study the microtubule cytoskeleton during trichome morphogenesis, we have used transgenic Arabidopsis plants expressing a green fluorescent protein (GFP)–microtubule-associated protein4 (MAP4) fusion protein, which has been shown to decorate microtubule arrays in plant cells (Marc et al., 1998). Our observations on branching trichomes and drug-induced growth alterations in growth direction indicate that microtubule reorientation precedes branch initiation. We found that a transient drug-induced stabilization of microtubules in the developing trichomes of two branching mutants, stichel and zwichel, leads to the production of new or additional growth foci that resemble branch initiation points. These observations suggest that transiently occurring, stable subpopulations of microtubules may provide the necessary nucleating sites needed for microtubule reorientation and consequent branching of trichome cells.
RESULTS
Localized Growth Reorientation Is the First Indication of Trichome Branching
Trichomes on Arabidopsis leaves range from totally unbranched to having as many as five branches. Although the relative percentages of particular trichome types on a given leaf can be established, no precise spatial coordinates can be assigned to them. Before embarking on an investigation of the role of microtubular cytoskeleton in branching trichome cells, we felt it was vital to observe the earliest stages of branching. We used scanning electron microscopy (SEM) to establish these early stages in trichome development. Figure 1 defines the phenotypic context in which branch initiation was used in this study. All trichomes, irrespective of their ultimate shape, initiate as small bulges on the leaf epidermis (Figure 1A). Continued perpendicular growth (Figure 1B) results in an unbranched trichome (as in Figure 1G). A two-branched trichome arises from a split at the tip, or a lop-sided V-shape may result from one branch arising subapically (Figure 1C). An additional subapical bulge on the distal (with respect to the longitudinal leaf axis) branch produces a three-branched trichome initial (Figures 1D and 1E). Additional branching may also occur before trichome maturation to produce four- or five-branched trichomes. However, once branch initiation has occurred, the final stellate shape of the trichome cell is achieved mainly by extension and elaboration of the branches (Figures 1E and 1F). Figures 1B to 1D show that the early stages of branching appear as subtle bulges only.
Because wild-type Arabidopsis displays a random mixture of trichome types with respect to branching, we used two trichome mutants to ensure experimental clarity. The mutant stichel possesses a majority of unbranched trichomes (98.2% ± 0.76%) on the early vegetative leaves (Figure 1G) (Luo and Oppenheimer, 1999). In late-developing and cauline leaves, the proportion of trichomes displaying bulges (similar to those seen in Figure 1H) or exhibiting clearly bifurcated branches may increase up to 2.3%. Trichomes of the mutant zwichel are either unbranched or exhibit a range of phenotypes resembling stages in branch initiation (Figures 1H to 1J). These include trichomes with a subtle bulge on the main stem (Figures 1H and 1I), trichomes with an underdeveloped branch and one elongated branch (Figure 1J), trichomes with two nearly equal branches (Figure 1J), and a few swollen trichome cells with underdeveloped branching. Plants of the strong zwichel allele that we used (zwi9311-11) have ∼40.4% unbranched and 59.6% two-branched trichomes (Luo and Oppenheimer, 1999). Trichomes with three branches were not seen in our SEM observations.
The SEM study thus allowed us to view the branch initials as subtle, localized bulges on the trichome cell whose subsequent extension confirmed the growth reorientation event that must have taken place. Observations on zwichel further clarified our view of the stages through which a branch initial may proceed before its visible manifestation as a fully extended branch. Throughout this study, the formation of a new bulge or a clear growth in a direction different from that of the parent cell was taken to indicate growth reorientation and development of the branch initials.
Microtubule Reorientation Takes Place during Trichome Branch Formation
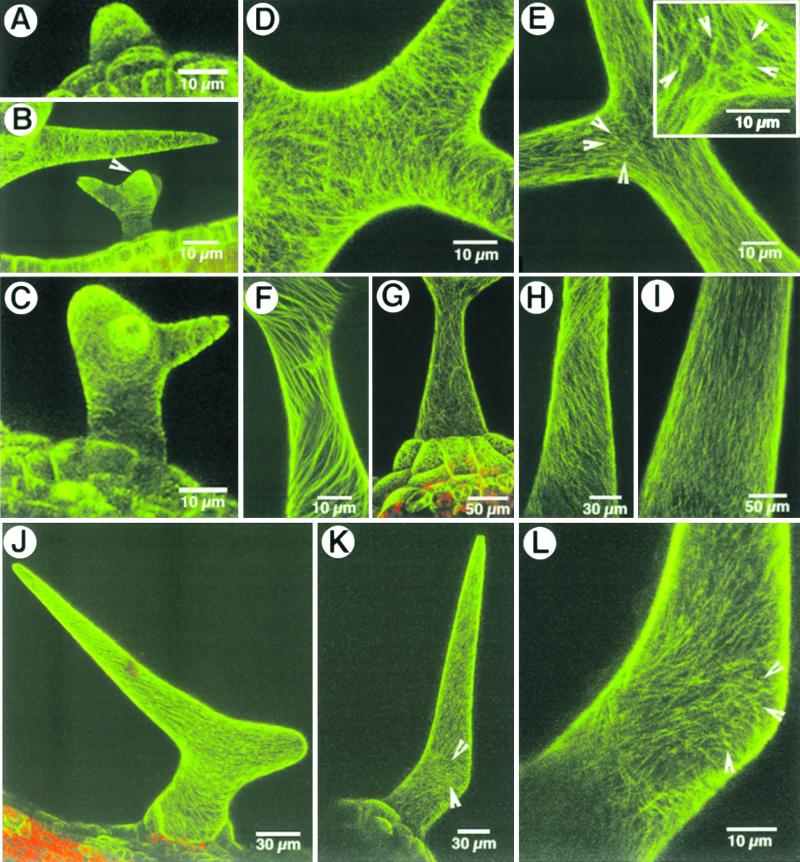
Arabidopsis plants carrying the GFP–MAP4 transgene were phenotypically normal, were fertile, and displayed the usual branching and distribution of trichomes on the leaf and inflorescence stem epidermis. Noninvasive visualization of the microtubule arrays in both unbranched and branched trichomes allowed us to directly correlate the external trichome morphology with its microtubular cytoskeleton. Figure 2 shows the microtubular arrays in branched and unbranched trichomes. Visualization of the microtubular arrays was difficult in the earliest stage of trichome development because of the transparency of the developing cells and their proximity to underlying cells of the leaf. During the tubular growth stage of wild-type trichomes, the cortical microtubules remained predominantly transversely arranged (Figure 2A). As the trichome cell developed further, the stalk and extending branch portions retained a transverse cortical microtubule array (e.g., in the trichome in the lower part of Figure 2B and in the stalk in Figure 2C), but subcortical microtubules in some wild-type trichomes began to show a dichotomy toward their apical ends that coincided with the appearance of new growth foci denoting the branch initials (Figures 2B and 2C). Subsequently, a clear reorientation of the cortical microtubule arrays at the branch junction resulted in numerous microtubules crisscrossing each other (Figure 2D).
Figure 2.
Microtubular Arrays in Trichome Cells of Arabidopsis.
(A) to (C) Early stages of trichome development show transverse cortical microtubule arrays in the tubular stage (A), at the branching stage (B) (the trichome cell in the lower part of figure), and in a more developed trichome with an extended stalk and one branch (C). The arrowhead in (B) indicates a branch initial. The upper part of (B) shows a microtubule mesh composed of transverse and oblique microtubules. The predominant orientation remains transverse with respect to the axis of extension growth.
(D) Cortical microtubules reorient at the branch junction and produce numerous cross-points at which they appear to traverse each other.
(E) Subcortical microtubules form a crisscross pattern at the branch junction and appear to radiate from small, more fluorescent knots of microtubules (arrowheads and box). The knots appear to be limited to the vicinity of the branch junction.
(F) A change from transverse to oblique orientation is observed as microtubules mature. The obliquely oriented microtubules appear as clockwise and counterclockwise spirals that cross each other.
(G) A mature trichome with basal accessory cells displaying a fine meshwork of oblique and crisscrossing microtubules.
(H) and (I) Unbranched wild-type and stichel mutant trichomes display a transverse to oblique reorientation of microtubule spirals (H) before assuming a predominantly longitudinal orientation (I) in the mature trichome.
(J) A two-branched zwichel trichome maintains transverse to oblique cortical microtubule arrays, like the wild-type trichomes.
(K) and (L) A subtle bulge in a zwichel trichome (K) shows numerous branched/reorienting microtubules (arrowheads) deviating from the predominant oblique cortical microtubule array observed elsewhere in the trichome cell. In a magnified view of the bulge shown in (K), the deviating microtubules appear to be linked to brightly fluorescent knots (arrowheads) (L).
Observations of subcortical microtubule arrays specifically at the trichome branch junction revealed the presence of branched microtubules that differed from the more longitudinally oriented microtubules found in extended portions such as the trichome stalk and portions of the branches (Figure 2E). These microtubules appeared to branch out from brightly fluorescent knots (between 0.3 and 0.8 μm thick and 0.05 to 0.3 μm in diameter), which could be clearly distinguished from the relatively straight microtubules leading to and away from them (e.g., in the lower part of Figure 2E representing a portion of the trichome stalk). Although such bright fluorescent spots could have been created by microtubules crossing each other, we did not observe similar knots in the trichome stalk or extended branch arms (e.g., Figure 2B, where the upper part of the figure shows an extended trichome branch). As the trichomes extended to their final stellate form, the predominantly transverse cortical microtubule array progressively organized into oblique spirals (Figure 2F). The clockwise and counterclockwise spirals crossed each other, resulting in a fine cortical meshwork of obliquely running microtubule spirals in the mature trichome cell (Figure 2G). Unbranched wild-type as well as stichel trichomes also displayed such oblique spiral microtubule arrays during their extension growth (Figure 2H), whereas the cortical microtubules in mature, unbranched trichomes attained a net longitudinal orientation (Figure 2I). The dense microtubule knots observed in branching trichomes were conspicuously absent in unbranched trichomes. Cortical microtubule arrays in zwichel trichomes displayed a pattern of microtubule orientation similar to that of the wild type, changing in a tip-directed wave from initially transverse to progressively oblique arrays (Figures 2J and 2K). Branched and reorienting microtubules and the fluorescent knots, possibly denoting microtubule aggregates, were clearly observed in the small bulges denoting incipient branch points that formed in zwichel trichomes (e.g., Figures 2K and 2L).
We concluded that cortical microtubule reorientation progresses from transverse to oblique arrays as trichomes mature and approaches a near-longitudinal configuration in unbranched trichomes. Microtubule reorientation is extremely conspicuous at the branch initiation points. In a majority of trichomes, the branch junction displayed some dense microtubule centers.
Microtubules at the branch point are more stable than elsewhere in the trichome cell. In an attempt to unravel the nature of the dense and more fluorescent microtubule knots, we treated seedlings by submerging them in 10 μM oryzalin, the microtubule-depolymerizing drug. Microtubules linked to the bright knots were among the last to disappear from the trichomes (data not shown). The disappearance of the microtubules and the dense knots took from 10 to 30 min among different trichomes on the same leaf. Microtubule arrays disappeared very early (within 5 to 10 min) in the youngest trichomes, whereas their disappearance was delayed in the more mature ones. The observed differences in time for microtubule depolymerization may merely reflect the state of maturity of a trichome cell and the permeability of its cell wall to oryzalin.
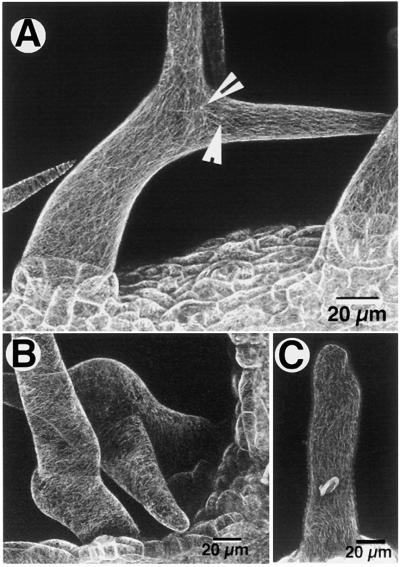
Oryzalin-treated seedlings were thoroughly washed with five changes of liquid Murashige and Skoog medium and were allowed to recover on plates of solid Murashige and Skoog medium for 1 hr. Subsequent confocal microscopic observations of treated seedlings showed a slow recovery of cortical microtubules, extending over 6 to 24 hr. Most mature trichome cells showed complete recovery of cortical microtubule arrays within 6 to 12 hr after treatment, with no discernable change in their overall morphology (Figure 3A). Despite the technically hindered visualization of reconstituting microtubule knots near the branch points, a result of laser-induced injury to the trichome cells on repeated confocal observation, we found that similar knots had reappeared near branch points in recovered and developing trichomes (Figure 3A, arrows). Trichomes at an early stage of development at the time of the oryzalin treatment also reconstituted their cortical microtubule arrays but, in addition, displayed morphological changes (Figure 3B). Certain trichomes recovered only partially over the 24-hr period; they had broken, longitudinally oriented microtubules and a generally enlarged cell shape (Figure 3C).
Figure 3.
Reconstitution of Cortical Microtubule Arrays on Drug-Free Murashige and Skoog Medium after Their Depolymerization by 10 μM Oryzalin.
(A) Cortical microtubules in mature trichomes that had depolymerized during the 30-min oryzalin treatment reappeared after 6 to 12 hr with no discernable change in microtubule distribution or trichome morphology. Note the reappearance of the bright knots (arrowheads).
(B) Trichomes that had been exposed to oryzalin at an early stage of their development also reconstituted their cortical microtubule arrays, but their morphology changed to that of a generally bloated cell.
(C) A trichome in which the cortical microtubules were reconstituted after the drug treatment but were unable to reorganize their normal orientation and also displayed a general shape change.
On the basis of the late disappearance of the fluorescent knots of microtubules, we speculated that these knots might represent more stable microtubules. This conjecture prompted us to investigate whether microtubules in trichomes could be stabilized by exogenous drug treatments. Because the microtubule knots appeared in places in which microtubule reorientation was clearly visible (e.g., branch junction), we asked whether microtubule stabilization could also lead to microtubule reorientation. Considering microtubule reorientation a prerequisite for growth reorientation (branch formation), we further reasoned that a visible manifestation of microtubule reorientation would be a change in the growth directionality of the trichome cell and, by extrapolation, a change in the formation of a branch point. Taking cues from our previous study (Mathur et al., 1999), we used paclitaxel (taxol), a microtubule-stabilizing drug, and oryzalin and propyzamide, which disrupt microtubule assembly (Morejohn, 1991), for control treatments. Latrunculin B, an actin-interacting drug, was used to demonstrate the specificity of the microtubule-mediated processes.
Because of the mixed nature and unpredictability of trichome types on the wild-type leaf, we performed our experiments with the mutant stichel, in which the majority of trichomes are unbranched (Huelskamp et al., 1994; Luo and Oppenheimer, 1999).
Drug Treatments Produce Shape Changes in stichel Trichomes
Continuous culture of stichel seedlings for as long as 6 days on medium containing 1 μM paclitaxel, oryzalin, or propyzamide resulted in radially swollen trichome cells (Figure 4A). Confocal microscopy showed that prolonged treatment with 1 μM paclitaxel resulted in a rearrangement of the normally transverse microtubular arrays into polygonal lattice work composed of thick, sinuous, longitudinally oriented bundles of microtubules (Figure 4B). In trichomes that had already matured by the time of treatment and therefore did not exhibit morphological changes, whorls of longitudinally bundled microtubules were seen (Figure 4C). An optical section through the head of a hammer-shaped trichome cell that had been obtained after prolonged paclitaxel treatment revealed the presence of numerous aster-like microtubule arrays (Figure 4D). Although treatment with oryzalin and propyzamide also resulted in an external phenotype similar to that obtained by paclitaxel treatment (Figure 4A), the cortical microtubule arrays completely disappeared in the oryzalin- or propyzamide-treated trichomes (data not shown). Moreover, the response elicited by 1 μM propyzamide treatment was much weaker than that obtained with oryzalin at the same concentration. Thus, all subsequent experiments used only paclitaxel and oryzalin.
Figure 4.
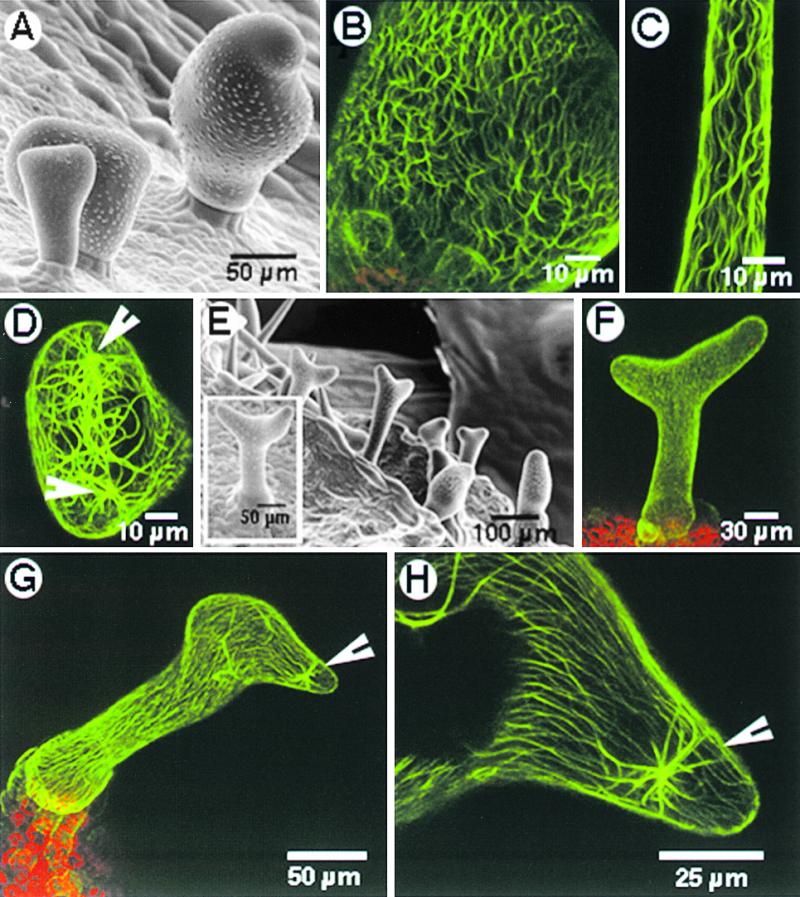
Microtubule-Interacting Drugs Induce Changes in Morphology as well as in the Microtubular Cytoskeleton in stichel Trichomes.
(A) Cells swell radially on treatment with microtubule-disrupting drugs such as oryzalin, propyzamide, and the microtubule-stabilizing drug paclitaxel. The degree of swelling appears to be linked to the stage of the particular trichome cell at the time of treatment.
(B) A bloated trichome obtained after continuous maintenance on 1 μM paclitaxel shows polygonal arrays formed by thick, longitudinally bundled microtubules.
(C) Trichomes that had already matured at the time of paclitaxel treatment did not exhibit changes in external morphology but displayed spirals of longitudinally bundled microtubules instead of the transverse cortical arrays observed in untreated wild-type trichomes (as in Figure 2E).
(D) An optical section providing a top view through a “hammerhead-like” trichome obtained by prolonged treatment with 1 μM paclitaxel shows thick microtubule bundles apparently radiating from nucleating points (arrowheads).
(E) A short 2-hr treatment with 20 μM paclitaxel reoriented the growth of the unbranched trichomes of stichel. Trichomes exhibited different degrees of branching, with the angle between the two branches varying from shallow to deep (box).
(F) A stichel trichome that branched after a 2-hr treatment with 20 μM paclitaxel displayed the normal transverse cortical microtubule after recovery on Murashige and Skoog basal medium.
(G) A stichel trichome exhibited growth reorientation and contained thick, persistent bundles of microtubules in the vicinity of the growth reorientation point (arrowhead) 4 days after the treatment.
(H) A magnified view of part of the trichome shown in (G) revealed an aster-like microtubule configuration near the point of growth reorientation (arrowhead).
We found that prolonged and continuous drug treatments resulted in clear changes in the morphology of stichel trichomes and, in general, converted the normal anisotropic growth pattern toward isotropic cell growth. Although stabilized microtubule populations were observed in paclitaxel-treated trichomes, the shape changes (Figures 4A and 4B) were too gross for these radially swollen trichomes to qualify as branching trichomes. On the other hand, short treatments for 30, 60, or 120 min with low concentrations (1 μM) of paclitaxel, oryzalin, or propyzamide did not produce any visible trichome phenotypes or noticeable changes in the microtubular cytoskeleton. This prompted us to use short (120-min) treatments with a high concentration (20 μM) of paclitaxel and oryzalin.
Short treatments of stichel seedlings with 20 μM oryzalin removed all cortical microtubules in the trichomes. Upon recovery, after 3 to 5 days on Murashige and Skoog basal medium plates, the seedlings displayed a few trichomes with a slight general swelling (data not shown) that clearly had not advanced to the gross shape changes observed after continuous culture on 1 μM oryzalin (similar to Figure 4A). These trichomes displayed a diffuse transverse cortical microtubule array, indicating that they had indeed recovered from the 2-hr microtubule-disrupting oryzalin treatment (data not shown).
A 120-min treatment with 20 μM paclitaxel resulted in a variety of trichome phenotypes, depending on the stage of a leaf and the developmental stage of the trichomes on that leaf at the time of treatment. A representative leaf of the stichel mutant observed 3 days after the drug treatment showed the following trichome morphologies: apparently unaffected trichomes, trichome cells with various degrees of changes in growth orientation, and totally ballooned trichomes. These findings were taken to reflect the effect of drug treatment on trichomes of different developmental stages—that is, already matured, transitioning toward maturity, at a midstage of development, and at an early stage of development. Thus, stichel trichomes that had already matured showed a slight, general bulging around their midsections but no bifurcation of their tips. Trichomes treated at a slightly earlier developmental stage showed a bloated hammerhead tip or a pronounced subapical swelling, during which midstage trichomes changed mostly into Y-shaped structures (14% ± 2.1%; Figure 4E), with the angle of the groove between the two arms varying from shallow to deep (see the box in Figure 4E). Trichomes located toward the leaf petiole or on leaves that developed later grew normally without any bulges or tip bifurcations.
Observation of the microtubule arrays after 10 hr of paclitaxel treatment revealed a thickening of bundles and the formation of certain brightly fluorescent microtubule asters. However, ∼48 hr after the paclitaxel treatment, most trichomes had rearranged their cortical microtubules into the normal transverse to oblique arrays (Figure 4F). Many of these paclitaxel-treated stichel trichomes now resembled branched zwichel trichomes (cf. Figures 2J and 4F) in both their external morphology and microtubular cytoskeleton. In a few trichomes, however, the thickened microtubule bundles persisted longer and could be seen even after growth reorientation had taken place (Figures 4G and 4H). In such trichomes, dense microtubule bundles/asters could be seen near the points of growth reorientation (Figure 4H).
We conclude that even a short treatment of stichel trichomes with paclitaxel could initiate stable microtubule populations in the trichome cell. Further, taxol-treated stichel trichomes showed a clear reorientation of microtubules and a concomitant growth reorientation similar to that observed during branch formation in trichomes.
Microtubule Stabilization Produces New Branch Initials in zwichel Trichomes
After observing growth reorientation in unbranched stichel trichomes, we investigated whether a similar effect might be elicited in another trichome branching mutant, zwichel. A strong allele of zwichel (zwi-9311-11) displays only unbranched and two-branched trichomes (Luo and Oppenheimer, 1999). The trichome branching defect in zwichel mutants has been attributed to the malfunctioning of a kinesin-like calmodulin binding protein (KCBP; Oppenheimer et al., 1997).
Zwichel seedlings were treated as were the stichel seedlings with 20 μM paclitaxel or oryzalin for 120 min, washed well, and allowed to recover on Murashige and Skoog basal medium. Control seedlings did not show any change in trichome morphology and branch initiation points. In contrast, zwichel seedlings treated with oryzalin showed radially swollen trichomes, similar to stichel trichomes after prolonged drug treatment (cf. Figures 4A and 5A). Microtubule arrays in these swollen trichomes had resumed their transverse orientation, although they were very diffuse and difficult to visualize in comparison with the control trichomes (data not shown).
Figure 5.
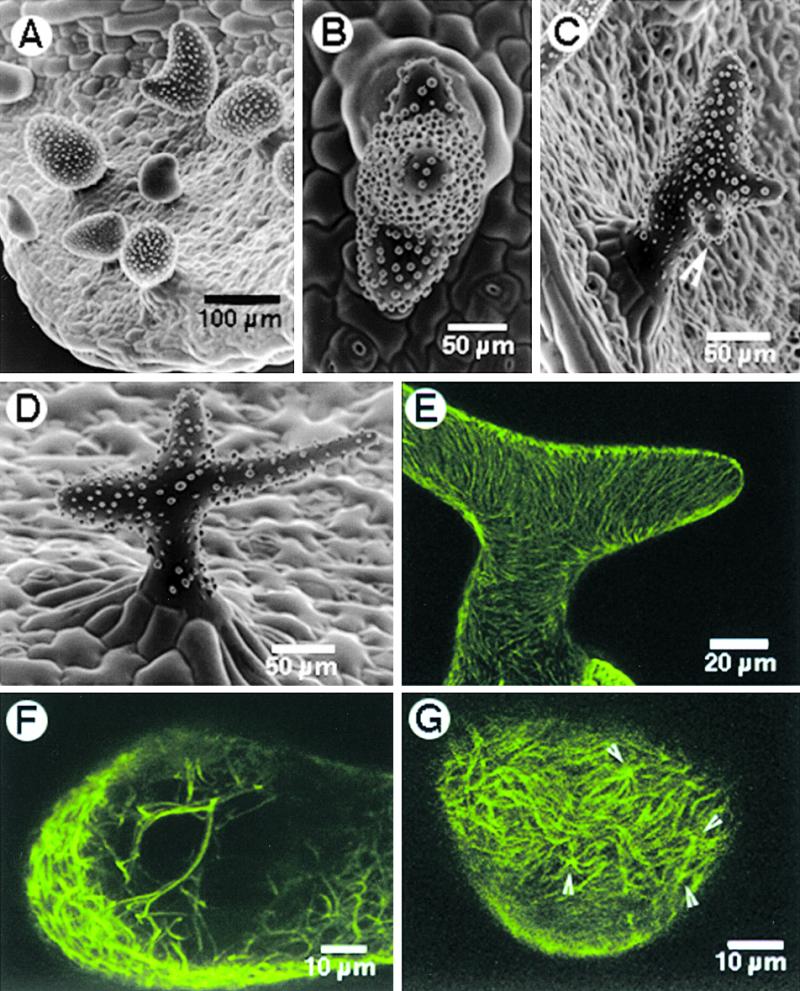
Effect of Oryzalin and Paclitaxel Treatment on zwichel 9311-11 Trichomes.
(A) Treatment with 20 μM oryzalin for 2 hr resulted in radially swollen trichomes.
(B) SEM view from the top of a single trichome, showing three branch initials. The result was obtained after treating seedlings for 2 hr with 20 μM paclitaxel, followed by washing and recovery on Murashige and Skoog basal medium.
(C) A single zwichel trichome with an additional incipient branch (arrowhead). Note that the positioning of such de novo–induced branching was random.
(D) Branches extended in some of the three-branched trichomes obtained after treatment with 20 μM paclitaxel.
(E) A trichome cell with bundled cortical microtubules at 10 hr after treatment with 20 μM paclitaxel.
(F) An optical section through a trichome 10 hr after paclitaxel treatment, revealing numerous thick, subcortical, cross-linked microtubule bundles.
(G) Examination of the cortical microtubule array on a subtle bulge arising on an unbranched zwichel trichome, nearly 24 hr after the paclitaxel treatment, revealed the presence of numerous microtubule asters (arrowheads).
Paclitaxel treatment resulted in the induction of new growth points on zwichel trichomes (Figures 5B and 5C); in a few cases, these branches extended even farther apart to produce a clear multibranched trichome (Figure 5D). Observations of the microtubule arrays 10 hr after the paclitaxel treatment showed that the cortical microtubules had thickened visibly (cf. Figure 5E with Figures 2J and 2K). Optical sectioning of a trichome around the same time revealed the presence of thick, cross-linked microtubule bundles (Figure 5F). Optical sectioning of other zwichel trichomes that showed subtle bulges on their surfaces 24 hr after the 120-min paclitaxel treatment revealed the presence of clear microtubule asters in the bulges (Figure 5G).
We conclude that taxol-induced microtubule stabilization and aster formation (Figure 5G) in zwichel trichomes were somehow able to compensate for the effects of reduced KCBP activity, which is thought to be responsible for the trichome branching phenotype of the zwichel mutant.
The Actin Cytoskeleton Is Not Involved in Trichome Branch Initiation
Wild-type Arabidopsis and stichel plants expressing the GFP–talin fusion protein (Kost et al., 1998; Mathur et al., 1999) were investigated after continuous paclitaxel treatment (Figures 6A and 6B). In each case, the trichome cells exhibited the cellular phenotype associated with the paclitaxel treatment (Figures 6A to 6C). In general, the F-actin strands remained elongated and followed the contours of the bloated cells (Figures 6A and 6B). In some stichel trichomes, however, the F-actin strands appeared to be thicker than those observed in the control (untreated) plants (Figure 6C).
Figure 6.
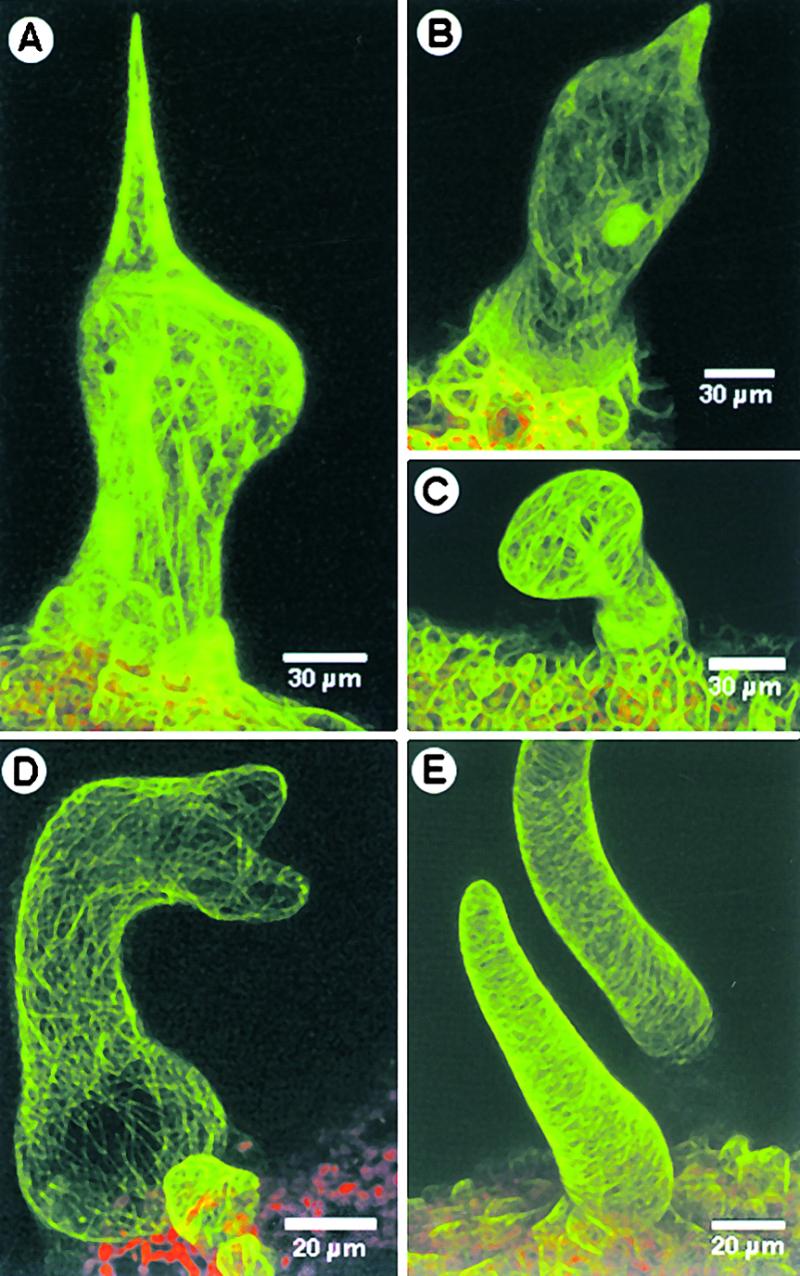
Effects of Paclitaxel Treatment on the Actin Cytoskeleton and of Latrunculin B on the Microtubular Cytoskeleton in Trichomes.
(A) A radially swollen wild-type trichome obtained after treatment with paclitaxel exhibits intact and elongated F-actin strands.
(B) A trichome of stichel with a subapical bulge, obtained after prolonged paclitaxel treatment, shows elongated F-actin strands following the cell periphery.
(C) A stichel trichome with a swollen apex displayed thick continuous actin bundles.
(D) A wild-type trichome became distorted, with unextended branches. The transverse cortical microtubule array appears undisturbed.
(E) Stichel trichomes treated with latrunculin B are short, stubby, and wavy. Note that the cortical microtubule array appears undisturbed.
We also treated wild-type Arabidopsis and stichel seedlings expressing the GFP–MAP4 fusion protein with latrunculin B (1 μM) to disrupt the actin cytoskeleton and obtained distorted trichomes as reported previously (Mathur et al., 1999). The distorted wild-type trichomes maintained their normal cortical microtubule arrays (Figure 6D). Trichomes of stichel appeared stunted, and they exhibited a blunt tip instead of the spike shape associated with the mutant trichomes but displayed the normal transverse microtubule arrays (Figure 6E). No additional branch initiation was seen.
DISCUSSION
The formation of a branch in a single cell indicates two major underlying processes: the decision to reorient the growth direction at a particular point in the cell body and mobilization of the cell machinery to implement this decision. The decision to branch and reorient cell growth in a new direction may be guided by extrinsic cues, as in neuronal development (Tanaka and Sabry, 1995), or may be under strict, intrinsic genetic control. The availability of numerous trichome branching mutants in Arabidopsis indicates that the phenomenon of single-cell branching in this plant is under genetic control (Huelskamp et al., 1994, 1999; Folkers et al., 1997; Krishnakumar and Oppenheimer, 1999; Luo and Oppenheimer, 1999). However, despite the availability of numerous branching mutants, to date only a single gene involved in trichome cell branching has been cloned. The cloning of the ZWICHEL gene, which encodes KCBP (Oppenheimer et al., 1997; Reddy et al., 1997), has opened new areas of investigation on the involvement and interplay of cytoskeletal elements in trichome cell branching. To our knowledge, this study is the first to investigate directly the role of the microtubular cytoskeleton in trichome branching.
Branching can be broadly defined as directional growth that has become reoriented in relation to the parent axis of a growing cell. Our adherence to this broad definition in our observations of different trichomes (Figure 1) in the wild type and the mutants stichel and zwichel led us to consider the occurrence of even subtle bulges on the trichome cell surface to be visible manifestations of branch point initiation. Although stichel trichomes were used as a source for unbranched trichomes, zwichel trichomes revealed much more information about branch initiation. Observation of a range of zwichel trichome types that exhibited subtle variations in the degree of localized wall expansion suggested that although not all branch initials may develop into fully extended branches, they nevertheless denote a growth reorientation with regard to the axis of the parent cell. In consideration of this point, we investigated the arrangement of microtubule arrays at well-defined branch points in wild-type trichomes and also in subtle bulges of zwichel trichomes. These microtubule images were then compared with the cortical microtubule arrays seen in unbranched wild-type and stichel trichomes as well as the microtubule arrays in regions of the trichome stalk and extended branches. We found that during trichome cell development, cortical microtubules can reorient from initial transverse arrays into slightly oblique to nearly longitudinal arrays. At the branch junction especially, microtubules reorient in relation to the longitudinal growth axis of the parent cell, form bifurcations, and cross each other. Portions of extending branches again display predominantly transverse cortical microtubule arrays.
Because microtubule reorientation apparently occurred in each situation in which growth directionality was altered, we decided to investigate whether microtubule reorientation was the cause or the effect of growth reorientation. Our previous study had shown that treatment with microtubule drugs resulted in changes in trichome morphology, ostensibly because of microtubular rearrangement (Mathur et al., 1999). We therefore treated seedlings of stichel, a mutant in which the majority of trichomes are unbranched, with microtubule-affecting drugs. Continuous treatments with low concentrations of drugs (both stabilizing and depolymerizing) resulted in similar gross alterations in trichome cell morphology and established that microtubule reorientation was indeed the cause for, rather than the result of, alterations in the direction of growth.
Stable Microtubules Form an Integral Part of the Microtubular Cytoskeleton in Trichomes
Irrespective of the number of branches present, small, strongly fluorescent microtubular foci, which were more stable to challenge by the microtubule-depolymerizing drug oryzalin, were observed in the vicinity of the branch points but not elsewhere in branching trichomes. Studies in both animal and plant systems have suggested that stable microtubules form an integral component of MTOCs (Kimble and Kuriyama, 1992; Vaughn and Harper, 1998). In plants, the absence of organelles such as the centrosome has led to the belief that MTOCs originate on the nuclear envelope and are transported to specific intracellular locations by microtubule motor proteins (Asada and Collings, 1997; Baluska et al., 1997). Alternatively, spontaneous and de novo assembly of such MTOCs may occur in the cell cortex by enhanced microtubule stability (Cyr and Palevitz, 1995; Marc, 1997; Nedelec et al., 1997). Whatever their site of origin, MTOCs function to organize, configure, and reorient microtubules into precise, defined arrays and thus facilitate innumerable microtubule-mediated processes (Cyr and Palevitz, 1995; Vaughn and Harper, 1998). Microtubule reorientation as observed during branch initiation in trichomes may thus involve the presence of stable MTOCs at the cell cortex. Further, if indeed such centers form an integral functional component of the scheme leading to branch initiation, their stability would be a major necessity for branching, and impairments in their functioning would be expected to lead to branching disorders.
Although evidence to confirm the status of the microtubule knots that we observed as MTOCs is lacking at present, the observation that such spots were more stable than other microtubules in the trichome cell led our investigations in two directions. The first involved testing our hypothesis that if stability were a major factor for microtubule reorientation leading to branch initiation, artificial creation of similarly stabilized microtubules during trichome development could lead to the initiation of fresh growth points on a trichome cell. This was tested by using paclitaxel to stabilize microtubules in developing trichomes and looking for microtubule and growth reorientation after the treatment.
Paclitaxel-Induced Microtubule Stabilization Leads to a Change in Growth Directionality in stichel and zwichel Trichomes
Paclitaxel (taxol) is a well-known microtubule-stabilizing drug (Schiff et al., 1979; De Brabander et al., 1981). In addition to decreasing the critical tubulin concentration for microtubule assembly, taxol induces the formation of ectopic microtubule asters in cells, often without any relation to the usual MTOCs (Verde et al., 1991; Hyman and Karsenti, 1998). Although prolonged treatment with low taxol concentrations did not produce branch initiation, general swelling of the trichome cells was obtained, indicating a shift in growth reorientation. Microtubules were found to be tightly bundled into parallel/polygonal arrays after these taxol treatments. On the basis of this observation, we reasoned that a short treatment of the seedlings with a high concentration of taxol might produce a transiently stable microtubule population. Provided that such stabilized but randomly distributed microtubules could act as seeds for freshly polymerizing microtubules, we expected to see both microtubule and growth reorientation in the trichome cells. Because trichome cells at different stages of development would be affected in any given treatment, a variety of cell morphologies ranging from unaffected to strongly affected trichomes would be expected from such an experiment. Subsequent observations on stichel seedlings treated with taxol for a short time supported the above assumptions.
The second point of investigation utilized a strong allele of the zwichel mutant of Arabidopsis in which trichomes either remain unbranched or develop an incipient to well-developed branch (Figure 1; Luo and Oppenheimer, 1999). Various scenarios have been suggested to account for the zwi phenotype, generally involving a defective microtubule motor function wherein the KCBP encoded by ZWI may have a direct role in the transport of a MTOC from its site of origin to its site of action (Oppenheimer, 1998). An alternative explanation comes from the observation that different kinesin-like proteins may trigger microtubule self-assembly by influencing microtubule stability at the cell cortex, either alone or as part of a MTOC (Marc, 1997; Nedelec et al., 1997). Considering our observations on paclitaxel-stabilized stichel trichomes and in view of the previous two senarios described for the zwichel phenotype, we investigated the effect of microtubule stabilization on zwichel trichomes. Transient stabilization of microtubules led to both microtubule and growth reorientation in zwichel trichomes; moreover, extra growth foci were also seen (Figure 5).
Although the observations on paclitaxel-treated stichel/zwichel trichomes point to a role for stable microtubules during trichome cell branching, they also provide a hint about the nature of the STI/ZWI gene products. Given the genetic evidence, Krishnakumar and Oppenheimer (1999) have hypothesized the presence of a multiprotein complex required for trichome branch initiation. Except for ZWI, the different components of the complex are not well defined at the molecular level. Nevertheless, the proposed complex may play an integral role in the assembly and direction of cytoskeletal components such as cortical microtubules, and a partial or complete instability of such a complex could impair the branching process. Moreover, because many different genes are likely to be involved, we expect that the degree of branching defect would be determined by several factors, such as the nature and hierarchical position of a particular gene, its degree of redundancy, and the severity of the mutation in that gene. Accordingly, the expected trichome phenotype for a mutant showing a complete or partial inability to organize or stabilize such a complex for branch initiation would be an unbranched (similar to stichel) or a sparsely branched (similar to zwichel) trichome. Cortical microtubules in both stichel and zwichel mutants are similar to those in the wild type except that both mutants are impaired in their branching ability. However, the strong alleles of both mutants used in our study produced growth reorientation and branching when treated with paclitaxel. In our experiments, the induced stabilization of microtubules by transient exogenous application of taxol may have directly or indirectly provided the necessary stability needed for the proposed multiprotein complex to function and initiate microtubule (and subsequently growth) reorientation. We thus speculate that both STICHEL and ZWICHEL could play a stabilizing role for an organizing complex required for branch initiation. Recent genetic evidence for ZWICHEL also supports such a conjecture.
At least seven different alleles are available for the zwi mutation, their phenotypic variations apparently depending on the allelic strength (Huelskamp et al., 1994; Krishnakumar and Oppenheimer, 1999). The variations in branch abnormalities in these different alleles may thus reflect the degree of functionality of the KCBP in the organizing complex. Furthermore, the idea of a multiprotein complex being required for trichome branching has gained additional support with the isolation of extragenic suppressors of the zwi-3 mutation (Krishnakumar and Oppenheimer, 1999).
Microtubule-Disrupting Drugs and Latrunculin Do Not Promote Branching
Prolonged culture of stichel seedlings on medium containing oryzalin produces a phenotype similar to that obtained with paclitaxel. However, contrary to the observations with paclitaxel, even a short treatment with 20 μM oryzalin completely destroys the cortical microtubule array. Branch initiation was not observed after either long or short treatment with oryzalin, even though the treated stichel trichomes recovered their cortical microtubule arrays. This points to a clear role for microtubule stabilization rather than their reconstitution after depolymerization. However, this observation also indicates the vital role of proper microtubule dynamics and cortical microtubule arrays in generating the shape of the trichome cell.
The role of actin microfilaments in maintaining the trichome pattern has been previously established (Mathur et al., 1999). However, our observations of normal microtubule arrays in latrunculin-treated trichomes, together with the absence of any indications of trichome branching in stichel, suggest that branch initiation per se may not involve the actin cytoskeleton. The presence of a modified but intact F-actin cytoskeleton in stichel trichomes treated with paclitaxel further supports this notion. Interactions between actin microfilaments and microtubules in deciding the precise location of a branch initiation point, however, cannot be ruled out entirely.
In conclusion, our investigations on the microtubular cytoskeleton in Arabidopsis trichomes presented here show that microtubule reorientation is the key to a change in growth directionality that leads to branch initiation. A possible link between microtubule stability and reorientation was discovered by observing relatively stable microtubule foci in trichome cells. We subsequently demonstrated with the aid of paclitaxel that the direction of trichome cell growth could indeed be altered in two Arabidopsis mutants, stichel and zwichel, by stabilizing their microtubule arrays. These results suggest a possible stabilizing role of STICHEL and ZWICHEL gene products as part of an organizing complex required for trichome cell branching. Although the precise mechanism by which microtubule stability is translated into a growth reorientation is unclear at this stage, our results provide evidence for a general role of the microtubular cytoskeleton in the spatial patterning of a trichome cell as well as the more specific process of branching in a higher plant cell.
METHODS
Generation of Transgenic Arabidopsis Plants Carrying a Cauliflower Mosaic Virus 35S-GFP-MAP4 Transgene
A GFP–MAP4 construct was kindly provided by J. Olmsted (University of Rochester, NY; Olson et al., 1995). The GFP was replaced by a plant-optimized version (Kost et al., 1998), and the construct was introduced into a binary plant transformation vector (pCAMBIA3000) kindly provided by R. Jefferson (CSIRO, Canberra, Australia). Agrobacterium tumefaciens–mediated transformation of Arabidopsis thaliana ecotype Landsberg erecta was performed as described by Valvekens et al. (1988), and 10 independent, transgenic lines were generated.
Other Plant Materials
Transgenic Arabidopsis plants (Landsberg erecta ecotype) carrying a 35S-GFP-talin transgene have been previously described (Kost et al., 1998). The 35S-GFP-talin and 35S-GFP-MAP4 transgenes were introduced into the Arabidopsis trichome branching mutant stichel NI1 (Landsberg erecta background), kindly provided by M. Huelskamp (Lehrstuhl für Entwicklungsgenetik, Tübingen, Germany), and zwi9311-11 (RLD background), kindly provided by D. Oppenheimer (University of Alabama, Tuscaloosa), by genetic crosses or transforming with the 35S-GFP-MAP4 construct directly. Homozygous lines (T3 generation) were used for all experiments.
Inhibitor Treatments
Microtubule-interacting drugs paclitaxel (1 to 20 M; Calbiochem, La Jolla, CA), propyzamide (1 μM; Sumitomo Chemical Co., Osaka, Japan), and oryzalin (1 to 10 μM; Crescent Chemical Co., Singapore Division), and the actin inhibitor latrunculin B (1 and 10 μM; Calbiochem) were used. Twenty seedlings (5 to 7 days old) with an emerging pair of primary leaves were placed in a 5-cm plastic dish containing 2 mL of liquid Murashige and Skoog medium (Murashige and Skoog, 1962) to which the inhibitors had been added directly. Depending on the experiment, seedlings were either treated for short periods (30 to 180 min) with high concentrations of inhibitor (20 μM paclitaxel, 10 μM oryzalin, 30 μM propyzamide, and 10 μM latrunculin) or maintained on a low concentration of inhibitor (1 μM in each case) for as long as 6 days. After the short treatment, seedlings were thoroughly washed for 2 hr with five changes of liquid Murashige and Skoog basal medium before being transferred to phytagel Murashige and Skoog basal medium plates. For the longer treatments, seedlings were placed on a 1-cm-wide Whatman filter paper strip that dipped into 2 mL of Murashige and Skoog liquid medium containing the inhibitor. Plants were kept upright in a tissue culture room and observed at 24-hr intervals. For each experiment, seedlings placed in liquid Murashige and Skoog medium containing 1.0 to 10 μL of DMSO or ethanol (the solvents used for the inhibitors) were used as controls. All experiments were repeated at least five times.
Microscopy and Image Processing
In any given experiment, one-half of the plants were randomly chosen, placed on a double-sided sticky tape, frozen in liquid nitrogen, and scanned at 30-Pa pressure at 30 kV with a scanning electron microscope (model JEOL-JSM 5310-LV; JEOL, Tokyo, Japan; Ahlstrand, 1996). The remaining plants were observed with a Kr/Ar laser scanning confocal microscope (model MRC-1024; Bio-Rad, Microscopy Division, Herts, UK). Images were stored as PICT files and processed with the Adobe Photoshop 3.0 program (Adobe Systems Inc., Mountain View, CA).
Acknowledgments
We thank Dr. Joanne Olmsted for the GFP–MAP4 gene, Dr. Martin Huelskamp for providing seeds of stichel, Dr. David Oppenheimer for seeds of zwichel, Sumitomo Chemicals (Japan) for the gift of propyzamide, Dr. Benedikt Kost for discussions and critical comments on the manuscript, Yang-Sun Chan for help with scanning electron microscopy, and Wei-Ping Tang and EeLing Ng for general technical assistance. N.-H.C. was supported by Department of Energy Grant No. 94-ER20143.
References
- Ahlstrand, G. (1996). Low temperature low voltage scanning microscopy (LTLVSEM) of uncoated frozen biological materials: A simple alternative. In Proceedings of Microscopy and Microanalysis, G. Bailey, J. Corbett, R. Dimlich, J. Michael, and N. Zaluzec, eds (San Francisco, CA: San Francisco Press), pp. 9–18.
- Asada, T., and Collings, D. (1997). Molecular motors in higher plants. Trends Plant Sci. 2, 29–37. [Google Scholar]
- Baluska, F., Volkmann, D., and Barlow, P.W. (1997). Nuclear components with microtubule-organizing properties in multicellular eukaryotes: Functional and evolutionary considerations. Int. Rev. Cytol. 175, 91–135. [DOI] [PubMed] [Google Scholar]
- Baskin, T.I., and Wilson, J.E. (1997). Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 113, 439–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova, T.N., Blancaflor, E.B., and Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17, 657–665. [DOI] [PubMed] [Google Scholar]
- Cyr, R.J. (1994). Microtubules in plant morphogenesis: Role of the cortical array. Annu. Rev. Cell Biol. 10, 153–180. [DOI] [PubMed] [Google Scholar]
- Cyr, R.J., and Palevitz, B.A. (1995). Organization of cortical microtubules in plant cells. Curr. Opin. Cell Biol. 7, 65–71. [DOI] [PubMed] [Google Scholar]
- De Brabander, M., Geuens, G., Nuydens, R., Willebrords, R., and De May, J. (1981). Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of centrosomes and kinetochores. Proc. Natl. Acad. Sci. USA 78, 5608–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan, J.H., Cove, D.J., and Lloyd, C.W. (1988). Microtubules and microfilaments in tip growth: Evidence that microtubules impose polarity on protonemal growth in Physcomitrella patens. J. Cell Sci. 89, 533–540. [Google Scholar]
- Folkers, U., Berger, J., and Huelskamp, M. (1997). Cell morphogenesis of trichomes in Arabidopsis: Differential control of primary and secondary branching by branch initiation regulators and cell growth. Development 124, 3779–3786. [DOI] [PubMed] [Google Scholar]
- Huelskamp, M., Misera, S., and Jurgens, G. (1994). Genetic dissection of trichome cell development in Arabidopsis. Cell 76, 555–566. [DOI] [PubMed] [Google Scholar]
- Huelskamp, M., Schnittger, A., and Folkers, U. (1999). Pattern formation and cell differentiation: Trichomes in Arabidopsis as a genetic model system. Int. Rev. Cytol. 186, 147–178. [DOI] [PubMed] [Google Scholar]
- Hyman, A., and Karsenti, E. (1998). The role of nucleation in patterning microtubule networks. J. Cell Sci. 111, 2077–2083. [DOI] [PubMed] [Google Scholar]
- Joshi, H.C. (1998). Microtubule dynamics in living cells. Curr. Opin. Cell Biol. 10, 35–44. [DOI] [PubMed] [Google Scholar]
- Kimble, M., and Kuriyama, R. (1992). Functional components of microtubule-organizing centres. Int. Rev. Cytol. 136, 1–50. [DOI] [PubMed] [Google Scholar]
- Kost, B., Spielhofer, P., and Chua, N.-H. (1998). A GFP–mouse talin fusion protein labels plant actin filaments in vivo and visualizes actin cytoskeleton in growing pollen tubes. Plant J. 16, 393–402. [DOI] [PubMed] [Google Scholar]
- Krishnakumar, S., and Oppenheimer, D.G. (1999). Extragenic suppressors of the Arabidopsis zwi-3 mutation identify new genes that function in trichome branch formation and pollen tube growth. Development 126, 3079–3088. [DOI] [PubMed] [Google Scholar]
- Kropf, D.L., Bisgrove, S.R., and Hable, W.E. (1998). Cytoskeletal control of polar growth in plant cells. Curr. Opin. Cell Biol. 10, 117–122. [DOI] [PubMed] [Google Scholar]
- Lambert, A.-M. (1995). Microtubule organizing centres in higher plants: Evolving concepts. Bot. Acta 108, 535–537. [Google Scholar]
- Luo, D., and Oppenheimer, D.G. (1999). Genetic control of trichome branch number in Arabidopsis: The roles of the FURCA loci. Development 126, 5547–5557. [DOI] [PubMed] [Google Scholar]
- Marc, J. (1997). Microtubule organizing centres in plants. Trends Plant Sci. 2, 223–230. [Google Scholar]
- Marc, J., Granger, C.L., Brincat, J., Fisher, D.D., Kao, T., McCubbin, A.G., and Cyr, R. (1998). A GFP–MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 10, 1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks, M.D. (1997). Molecular genetic analysis of trichome development in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 137–163. [DOI] [PubMed] [Google Scholar]
- Mathur, J., Spielhofer, P., Kost, B., and Chua, N.-H. (1999). The actin cytoskeleton is required to elaborate and maintain spatial patterning during trichome cell morphogenesis in Arabidopsis thaliana. Development 126, 5559–5568. [DOI] [PubMed] [Google Scholar]
- Morejohn, L.C. (1991). The molecular pharmacology of plant tubulin and microtubules. In The Cytoskeletal Basis of Plant Growth and Form, C. Lloyd, ed (London: Academic Press), pp. 29–44.
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nedelec, F.J., Surrey, T., Maggs, A.C., and Leibler, S. (1997). Self-organization of microtubules and motors. Nature 389, 305–308. [DOI] [PubMed] [Google Scholar]
- Olson, K.R., McIntosh, J.R., and Olmsted, J.B. (1995). Analysis of MAP4 function in living cells using green fluorescent protein (GFP) chimeras. J. Cell Biol. 130, 636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer, D.G. (1998). Genetics of plant cell shape. Curr. Opin. Plant Biol. 1, 520–524. [DOI] [PubMed] [Google Scholar]
- Oppenheimer, D.G., Pollock, M.A., Vacik, J., Szymanski, D.B., Ericson, B., Feldmann, K., and Marks, M.D. (1997). Essential role of a kinesin-like protein in Arabidopsis trichome morphogenesis. Proc. Natl. Acad. Sci. USA 94, 6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A.S.N., Safadi, F., Narasimhulu, S.B., Golovkin, M., and Hu, X. (1997). A novel plant calmodulin-binding protein with a kinesin heavy chain motor domain. J. Biol. Chem. 271, 7052–7060. [DOI] [PubMed] [Google Scholar]
- Sabry, J.H., O'Connor, T.P., Evans, L., Toroian-Raymond, A., Kirschner, M.W., and Bentley, D. (1991). Microtubule behaviour during the guidance of pioneer neuron growth cones in situ. J. Cell Biol. 115, 1381–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., and Nurse, P. (1998). Regulation of cell polarity by microtubules in fission yeast. J. Cell Biol. 142, 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff, P.B., Fant, J., and Horwitz, S.B. (1979). Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–667. [DOI] [PubMed] [Google Scholar]
- Shibaoka, H. (1994). Plant hormone–induced changes in the orientation of cortical microtubules: Alterations in the cross-linking between microtubules and the plasma membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 527–544. [Google Scholar]
- Szymanski, D.B., Ross, J.A., Pollock, S.M., and Marks, M.D. (1998). Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Tanaka, E., and Sabry, J. (1995). Making the connection: Cytoskeletal rearrangements during growth cone guidance. Cell 83, 171–176. [DOI] [PubMed] [Google Scholar]
- Valvekens, D., Van Montagu, M., and Van Lijsebettens, M. (1988). Agrobacterium tumefaciens–mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 85, 5536–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn, K.C., and Harper, J.D.I. (1998). Microtubule-organizing centres and nucleating sites in land plants. Int. Rev. Cytol. 181, 75–149. [DOI] [PubMed] [Google Scholar]
- Verde, F., Berrez, J.-M., Antony, C., and Karsenti, E. (1991). Taxol-induced microtubule asters in mitotic extracts of Xenopus eggs: Requirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 112, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, R.E. (1991). Orientation of cortical microtubules in interphase plant cells. Int. Rev. Cytol. 129, 135–208. [Google Scholar]
- Williamson, T., Gordon-Weeks, P.R., Schachner, M., and Taylor, J. (1996). Microtubule reorganization is obligatory for growth cone turning. Proc. Natl. Acad. Sci. USA 93, 15221–15226. [DOI] [PMC free article] [PubMed] [Google Scholar]