Abstract
Among members of the bHLHZip family of transcriptional regulators, MondoA and Mlx have the unique property of cytoplasmic localization. We have proposed that MondoA-Mlx heterodimers accumulate in the nucleus in response to extracellular cues. Our previous work implicated heterodimerization between MondoA and Mlx and a conserved domain in the N terminus of MondoA as important determinants of MondoA-Mlx subcellular localization. MondoA and Mlx share sequence similarity in their bHLHZip domains and C termini. Here we show that for both MondoA and Mlx, this C-terminal domain has cytoplasmic localization activity that is required by the protein monomers to accumulate in the cytoplasm. This C-terminal domain is also a novel dimerization interface that functions independently of the leucine zipper to mediate heterotypic interactions between MondoA and Mlx. Dimerization between MondoA and Mlx inactivates the cytoplasmic localization activity of their C termini and is necessary for the heterocomplex to accumulate in the nucleus. MondoA-Mlx heterodimers, while poised for nuclear entry, are retained in the cytoplasm by conserved domains in the N terminus of MondoA. Mondo conserved regions (MCRs) II and III contribute to cytoplasmic localization of MondoA-Mlx by functioning as a CRM1-dependent nuclear export signal and as a novel binding site for 14-3-3 family members, respectively. We propose that the nuclear accumulation of MondoA and Mlx is a two-step process. First, heterodimerization abolishes the cytoplasmic localization activity of their C termini. Second, an extracellular signal(s) must overcome the cytoplasmic localization function imparted by CRM1 and 14-3-3 binding to the N terminus of MondoA.
The subcellular localization of transcription factors often contributes to the regulation of gene expression (30, 51, 52). Many transcription factors are sequestered in the cytoplasm away from their targets and accumulate in the nucleus only in response to extracellular cues. For example, NF-κB and NF-AT associate with proteins that mask their nuclear localization signals (NLSs); MIZ1 and SREBP are retained in the cytoplasm via associations with microtubules and the endoplasmic reticulum, respectively; and many proteins, including the Forkhead transcription factors and histone deacetylase 4 (HDAC-4) and -5, are actively exported from the nucleus (7, 12, 13, 31, 40, 47, 55, 61). In addition, certain transcription regulators function as heterodimers and in several cases, as with the STATs and Smads, dimerization is required for nuclear entry (36, 38). In each of the given examples, extracellular signals have been identified that counteract these control mechanisms, resulting in the nuclear accumulation and subsequent activity of the transcription factor. Our group has recently identified two novel members of the basic region helix-loop-helix leucine zipper (bHLHZip) family of transcription factors, MondoA and Mlx (8, 9). MondoA and Mlx heterodimerize and localize to the cytoplasm in all cell types tested, but they activate transcription when targeted to the nucleus (8). We have proposed that MondoA-Mlx heterodimers function as activators of transcription and accumulate in the nucleus in response to extracellular signals.
MondoA and Mlx are related in structure and function to members of the Myc/Max/Mad network of bHLHZip transcriptional regulators (20). Max functions as the center of this transcription factor network and has no intrinsic transcriptional activity. Max is capable of forming homodimers weakly, but it preferentially forms heterodimers with members of the Myc family, the Mad family, Mnt/Rox, or Mga via their respective bHLHZip domains (1, 3, 10, 26-28, 42, 46, 60). Myc-Max and Mad-Max heterodimers are thought to reciprocally regulate the expression of CACGTG-dependent target genes required for cell growth and proliferation by functioning as transcriptional activators and transcriptional repressors, respectively (18, 20, 23).
Mlx, Max-like protein x, originally identified as a dimerization partner for Mad1, shares many similarities with Max (9). Of known bHLHZip proteins, Mlx is most similar at the sequence level to Max and, like Max, Mlx forms homodimers poorly but preferentially heterodimerizes with Mad1. Mad1-Mlx heterodimers also bind the CACGTG subclass of E-box elements and repress transcription from synthetic promoters containing single or multiple copies of this element. Like Mad1-Max transcriptional repression, Mad1-Mlx transcriptional repression requires interaction with the mSin3A/HDAC corepressor complex. Surprisingly, Mlx has a more limited dimerization spectrum than Max, interacting with only Mad1 and Mad4 of the Mad family and weakly with Mnt/Rox, but with none of the Myc family proteins (9, 41). The similarities to Max suggested that Mlx also functions as the center of a transcriptional network. Supporting this hypothesis, our group has described interactions between Mlx and a novel family of transcriptional activators, the Mondo family (8).
Just as Mlx appears to be an analog of Max, MondoA appears to share characteristics with Myc. MondoA does not form homodimers but readily heterodimerizes with Mlx to bind and activate transcription from the Myc-Max consensus CACGTG E-box elements. Like Myc family members, MondoA has an autonomous transcriptional activation domain in its amino terminus (8). Furthermore, Myc family members have a conserved domain termed Myc Box II that is required for its function as a transforming oncogene. MondoA and MondoB/WBSCR14/ChREBP, a MondoA paralog, both have sequence elements that are similar to Myc Box II (unpublished observation), although the significance of this conserved domain to their function has not been examined. Together these data suggest that MondoA and Myc may regulate similar target genes and, therefore, similar cellular processes. In support of this hypothesis, we have recently demonstrated synthetic lethal interactions between Drosophila melanogaster Myc and Mondo (unpublished data).
In marked contrast to the typical nuclear localization of Myc-Max, MondoA-Mlx heterodimers localize to the cytoplasm in all cell types tested (reference 8 and unpublished observation). Regulation of MondoA-Mlx subcellular localization is not well understood. In response to the nuclear export inhibitor leptomycin B (LMB), MondoA-Mlx heterodimers accumulate in the nucleus, suggesting that that the heterodimer is exported from the nucleus by CRM1. Furthermore, a MondoA mutant lacking its N-terminal 322 amino acids localizes to the cytoplasm; however, when coexpressed with Mlx, both proteins localize to the nucleus (8). Therefore, the N terminus of MondoA, heterodimerization with Mlx, and active nuclear export contribute to the cytoplasmic localization of MondoA-Mlx.
Here we investigate the domains that control the subcellular localization of MondoA and Mlx. Following their bHLHZip domains, the C termini of MondoA and Mlx are similar; however, no function has been assigned to this domain. We show that the conserved C terminus of MondoA and Mlx is a novel protein-protein interaction domain that mediates heterotypic interactions between MondoA and Mlx. Furthermore, we show that this C-terminal domain of both MondoA and Mlx is required for the cytoplasmic localization of the protein monomers. Heterodimerization of MondoA and Mlx, via either the leucine zipper or the C-terminal domain, inactivates the cytoplasmic localization activity at their C termini and is necessary, but not sufficient, for nuclear accumulation of the heterodimer. Our previous data showed that a region encompassing Mondo conserved regions (MCRs) I to V in the N terminus of MondoA contains a potent autonomous cytoplasmic localization domain that contributes to the subcellular localization of the heterodimer. We show here that MCRII is a CRM1-dependent nuclear export signal (NES) and that MCRIII is a novel binding site for 14-3-3 proteins. The NES activity of MCRII and 14-3-3 binding both contribute to the cytoplasmic localization activity of the N terminus of MondoA.
MATERIALS AND METHODS
Subcloning.
FLAGMlx, ΔLZMlx, MondoA, MondoA(322-919), and GalMondoA(125-321) expression constructs have been described previously (8, 9). PCR was used to make deletions of MondoA and Mlx and to fuse different regions of MondoA to the Gal DNA binding domain (DBD) in pFA-CMV (Stratagene) or to the LexA DBD in pBTM116-URA3 (6). PCR was used to fuse 14-3-3β to the VP16 activation domain in pVP16 (24). Anne Brunet provided FLAG14-3-3ζ. Michael B. Yaffe provided expression constructs for multiple glutathione S-transferase (GST)-14-3-3 isoforms, including GST-14-3-3β used to make VP16-14-3-3β. Point mutations were generated using the QuikChange mutagenesis kit (Stratagene). All constructs were verified by sequencing.
Cell culture and transfections.
NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% bovine calf serum (HyClone). For indirect immunofluorescence, cells were plated at 0.7 × 105 cells per well in 24-well plates and transfected the following day with 1 μg of expression vector per well using Lipofectamine Plus (Invitrogen). For cell photography, cells were plated on glass coverslips in 6-well plates and transfected with 2 μg of expression vector.
293T cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% bovine calf serum (HyClone). For immunoprecipitations, 4 × 105 cells were plated on 60-mm dishes and transfected using calcium phosphate the following day (2). Luciferase assays were performed as previously described (8).
Immunoprecipitations and Western blotting.
The day following transfection, 293T cytoplasmic lysates were made in JLB buffer (50 mM Tris-HCl [pH 8.0], 10% glycerol, 150 mM NaCl, 0.5% Triton X-100) containing 0.23 μM aprotinin, 2 μM leupeptin, 1.43 μM pepstatin, and 0.5 mM phenylmethylsulfonyl fluoride (21). Total protein concentration was determined by the Bradford assay, and 1 mg of total protein was used for each immunoprecipitation. Immunoprecipitations were rocked at 4°C for 1 h and washed four times in JLB buffer. Immunoprecipitates were analyzed by Western blotting with antibodies against Mlx (9), MondoA (8), the V5 epitope (Invitrogen), or 14-3-3 (Santa Cruz). Protein A-horseradish peroxidase conjugate (Bio-Rad), horseradish peroxidase-linked anti-mouse immunoglobulin G (Amersham), and ECL Western blotting detection reagents (Amersham) were used.
Immunofluorescence and localization quantitation.
Transfected NIH 3T3 cells were fixed in 1× phosphate-buffered saline (PBS) containing 3.7% formaldehyde for 15 min, washed with 1× PBS, and blocked with PBT (1× PBS, 0.1% Triton X-100, 1% bovine serum albumin, 0.1% sodium azide) for 30 min. The staining procedure was performed at room temperature. Primary antibodies, anti-FLAG M2 (Sigma) or anti-MondoA, were used at 1:1,000 and 1:500 dilutions, respectively. Secondary antibodies anti-mouse Alexa 488 and anti-rabbit Alexa 594 (Molecular Probes) were used at 1:500. Antibody dilutions were made in PBT. Cells were incubated with the primary antibodies for 1 h. Following two washes with PBT and blocking for 30 min in PBT, the cells were incubated with the secondary antibodies for 30 min. Cells were washed and blocked again in PBT. Nuclei were stained with 5 μg of Hoechst 33342 (Molecular Probes)/ml. For quantification, cells were treated with SlowFade Light AntiFade (Molecular Probes) prior to viewing. For photography, coverslips were mounted on slides with ProLong Antifade (Molecular Probes).
To quantify subcellular localization, independent transfections were carried out at least twice and at least 50 cells were counted for each transfection. To eliminate bias in scoring, the transfections were coded and scored blindly. In MondoA and FLAGMlx cotransfections, only cells expressing both MondoA and FLAGMlx were scored. The subcellular localization of MondoA or FLAGMlx was scored into five categories: nuclear only, nuclear greater than cytoplasmic, nuclear equivalent to cytoplasmic, cytoplasmic greater than nuclear, and cytoplasmic only. For presentation of the data, the five categories were condensed into three. The categories nuclear only and nuclear greater than cytoplasmic were combined into a “predominantly nuclear” category. Cytoplasmic greater than nuclear and cytoplasmic only were combined into a “predominantly cytoplasmic” category. Data are presented as histograms showing the percentage of total cells in each staining category. Error is expressed as the standard error of the mean.
Yeast two-hybrid assays.
LexA-MondoA(1-300) was transformed into the Saccharomyces cerevisiae strain DY5735 and was used to screen a mouse embryo day 9.5 and 10.5 cDNA library as described previously (4, 6, 53). Sequencing of VP16 fusions revealed partial clones of the following 14-3-3 isoforms: β, amino acids 1 to 164; ɛ, amino acids 78 to 255; and ζ, amino acids 1 to 139. To assay different protein-protein interactions, pVP16 and pBTM116-URA3 were used to make the VP16 activation domain and LexA DBD fusion constructs, respectively (6, 24). DY5735 was transformed with two plasmids, and interaction between the two fusion proteins was measured by a filter assay for β-galactosidase (2).
RESULTS
The conserved domains of MondoA and Mlx regulate cytoplasmic localization.
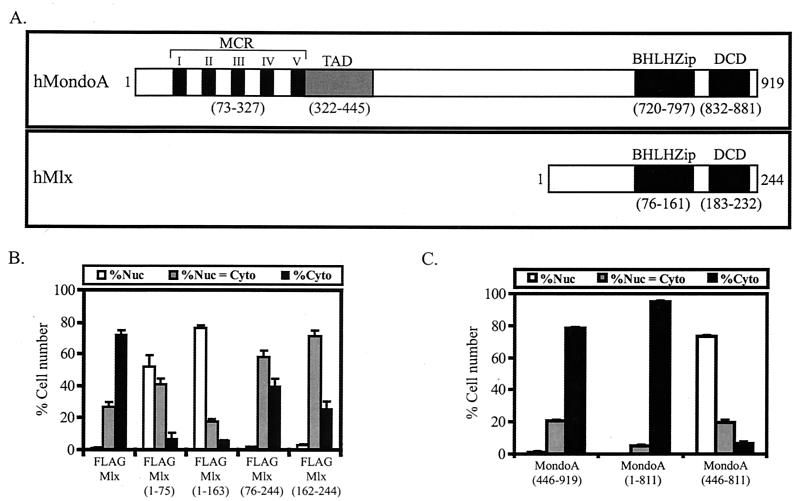
Mlx and MondoA localize predominantly to the cytoplasm (8). To determine the regions in each protein required for cytoplasmic localization, a series of deletion constructs were made based upon their domain structures (Fig. 1A). Different MondoA and FLAG epitope-tagged Mlx constructs (Fig. 1B and C) were transiently expressed in NIH 3T3 cells. MondoA and Mlx were detected by indirect immunofluorescence using anti-MondoA and anti-FLAG antibodies, respectively, and their subcellular localization was quantified. Mlx proteins lacking the C terminus, Mlx(1-163), or the bHLHZip domain and the C terminus, Mlx(1-75), localized to the nucleus (Fig. 1B). Thus, the C terminus of Mlx is required for cytoplasmic localization, whereas the N terminus has sequences that direct Mlx to the nucleus.
FIG. 1.
The conserved C termini of MondoA and Mlx are required for cytoplasmic localization. (A) Domain diagrams of human MondoA and human Mlx. MCR, Mondo conserved region; TAD, transcriptional activation domain; bHLHZip, basic helix-loop-helix leucine zipper domain; DCD, dimerization and cytoplasmic localization domain. (B and C) The indicated FLAGMlx (B) and MondoA (C) constructs were expressed in NIH 3T3 cells and their subcellular localization was quantified. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic.
The N terminus of MondoA has a cytoplasmic localization domain that encompasses the MCRs (8); however, MondoA(446-919), which lacks the MCRs, was cytoplasmic (Fig. 1C), indicating that additional sequences control MondoA subcellular localization. MondoA(446-811) lacks both the conserved N-terminal and C-terminal domains and localized to the nucleus, whereas MondoA(1-811) lacks the conserved C-terminal domain and localized to the cytoplasm. Therefore, the presence of either the N-terminal MCRs or the conserved C terminus of MondoA is sufficient for the cytoplasmic localization of MondoA. Furthermore, the nuclear accumulation of MondoA(446-811) suggests that this central region of MondoA contains an NLS. As the C termini of MondoA and Mlx both contribute to their subcellular localization and are similar in sequence, they may function via a common mechanism.
The C termini of MondoA and Mlx are novel dimerization domains.
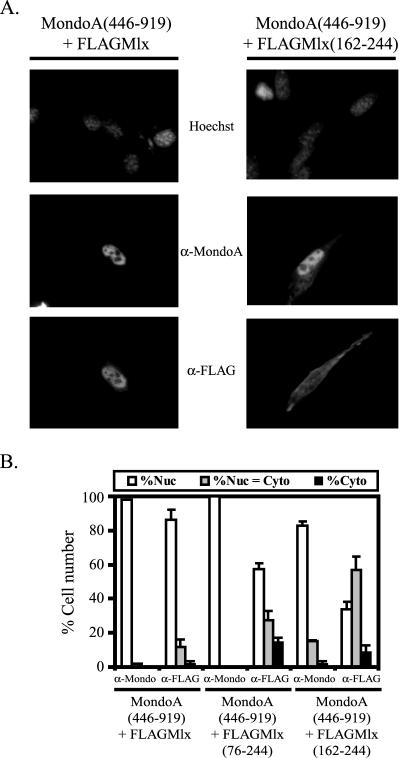
When MondoA(446-919) and Mlx are expressed separately, they localize to the cytoplasm (Fig. 1B and C); however, when they are expressed together, both localize to the nucleus (Fig. 2A and B). We hypothesized that dimerization between MondoA and Mlx might inactivate the cytoplasmic localization function at the C terminus of MondoA and Mlx and, therefore, dimerization might be required for the nuclear localization of MondoA-Mlx. To test this hypothesis, we determined the regions of Mlx necessary to “relocalize” MondoA(446-919) from the cytoplasm to the nucleus. When expressed alone, Mlx(76-244) or Mlx(162-244) localized almost equivalently between the cytoplasm and the nucleus (Fig. 1B); however, both proteins relocalized MondoA(446-919) to the nucleus (Fig. 2B). Mlx(76-244) has the leucine zipper domain, supporting the idea that dimerization is important for nuclear localization of MondoA(446-919). However, Mlx(162-244) lacks the leucine zipper domain and does not contain other known dimerization motifs. Together these data suggest that dimerization is required for the nuclear accumulation of MondoA-Mlx heterodimers and that the C termini of MondoA and Mlx may mediate interaction between these two proteins.
FIG. 2.
The C terminus of Mlx is sufficient for nuclear localization of MondoA(446-919). (A) Indirect immunofluorescence to detect localization of MondoA(446-919) when coexpressed with either FLAGMlx or FLAGMlx(162-244) in NIH 3T3 cells. (B) MondoA(446-919) was coexpressed with the indicated FLAGMlx deletion constructs in NIH 3T3 cells and the subcellular localization of the individual proteins was quantified. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic.
To test whether the C termini of MondoA and Mlx function as novel dimerization domains, we used a yeast two-hybrid assay to test for interaction (Fig. 3A). The C terminus of Mlx was fused to the DBD of LexA, and the C terminus of MondoA was fused to VP16. LexA-Mlx(162-244) interacted with VP16-MondoA(798-919), demonstrating that the C termini of Mlx and MondoA heterodimerize (Fig. 3A). Furthermore, the converse experiment testing LexA-MondoA(798-919) and VP16-Mlx(162-244) also showed an interaction. No interaction was detected between LexA-Mlx(162-244) and VP16-Mlx(162-244) or between LexA-MondoA(798-919) and VP16-MondoA(798-919), indicating that the C termini do not homodimerize.
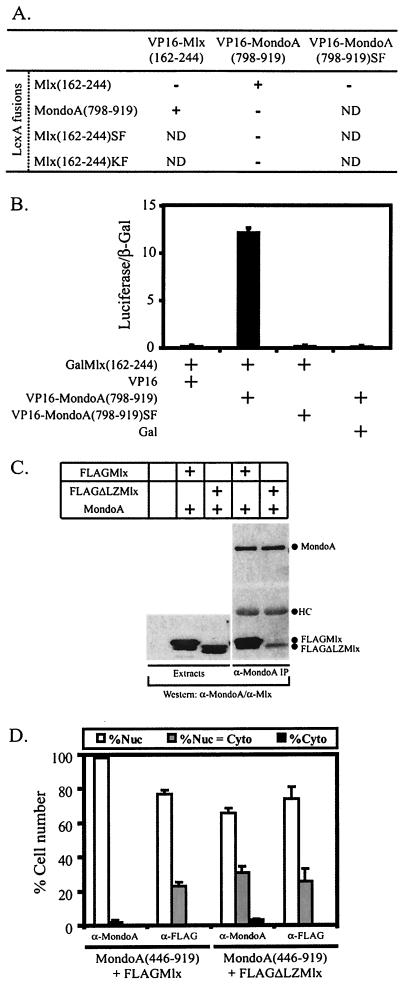
FIG. 3.
The C termini of MondoA and Mlx function as a novel heterodimerization domain. (A) A yeast two-hybrid assay was used to test for interaction between the indicated LexA and VP16 fusion proteins. β-Galactosidase activity was scored as follows: −, none; +, strong. SF, S198A/F199A for Mlx and S847A/F848A for MondoA; KF, K184A/F185A; ND, not determined. (B) HEK293 cells were transfected with the indicated Gal and VP16 fusion proteins and a Gal-dependent luciferase reporter gene. Luciferase and β-galactosidaseactivities were measured 24 h following transfection. Normalized luciferase activity is reported along with the standard error of the mean. (C) 293T cells were transiently transfected with the expression constructs indicated, followed by anti-MondoA and anti-Mlx Western blotting of MondoA immunoprecipitates. (D) MondoA(446-919) was coexpressed with either FLAGMlx or FLAGΔLZMlx in NIH 3T3 cells and the subcellular localization of the individual proteins was quantified. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic.
Three additional experiments demonstrate that the C termini of MondoA and Mlx heterodimerize in mammalian cells. First, in a mammalian two-hybrid assay, a fusion between the C terminus of Mlx and the DBD of Gal4 (Gal), GalMlx(162-244), interacted with VP16-MondoA(798-919) (Fig. 3B). In addition, VP16-Mlx(162-244) also interacted with GalMondoA(798-919) (data not shown). No interaction was observed between GalMlx(162-244) and VP16 alone or between Gal alone and VP16-MondoA(798-919), demonstrating that the interaction between the C termini of MondoA and Mlx was specific (Fig. 3B). Second, we tested whether a mutant Mlx protein lacking a leucine zipper, FLAGΔLZMlx (9), interacted with MondoA by using a coprecipitation assay (Fig. 3C). Lysates from 293T cells transiently expressing MondoA and either FLAGMlx or FLAGΔLZMlx were immunoprecipitated with an antibody against MondoA followed by Western blotting to detect associated proteins. Both FLAGMlx and FLAGΔLZMlx interacted with MondoA, suggesting that MondoA and Mlx can dimerize outside of their known dimerization interface, the leucine zipper. Finally, dimerization appears to be required for Mlx to relocalize MondoA(446-919) to the nucleus; therefore, we tested the activity of ΔLZMlx in this assay. Alone, ΔLZMlx was distributed throughout the cell (data not shown) but, identical to wild-type Mlx, it relocalized MondoA(446-919) to the nucleus (Fig. 3D), again suggesting that dimerization does not depend entirely on the leucine zipper. Taken together these four experiments provide compelling evidence that the C termini of MondoA and Mlx function as autonomous heterodimerization domains.
Dimerization between MondoA and Mlx regulates subcellular localization.
The C termini of Mlx and MondoA are required for the cytoplasmic localization of each protein (Fig. 1B and C). In addition, the C termini of MondoA and Mlx heterodimerize, and heterodimerization is required for nuclear accumulation of MondoA and Mlx. Consequently, we hypothesized that these two functions might be coupled: heterodimerization between MondoA and Mlx may inactivate their C-terminal cytoplasmic localization activity. Based on this hypothesis, we predicted that if MondoA and Mlx were unable to dimerize, then they should localize to the cytoplasm.
To test this model, we first identified residues important for MondoA and Mlx heterodimerization. Alignment of the C termini of the MondoA and Mlx family proteins revealed that lysine 184, phenylalanine 185, serine 198, and phenylalanine 199 (numbering relative to Mlx) are conserved between family members (data not shown). These residues were mutated, in pairs, to alanine, i.e., K184A/F185A and S198A/F199A, in LexA-Mlx(162-244) and tested for interaction with the C terminus of MondoA by using two-hybrid assays. Both LexA-Mlx(162-244)SF and LexA-Mlx(162-244)KF were expressed in yeast (data not shown), but they failed to interact with VP16-MondoA(798-919) (Fig. 3A). Similarly, a MondoA mutant, VP16-MondoA(798-919)S847A/F848A, with mutations equivalent to S198A/F199A in Mlx, did not interact with LexA-Mlx(162-244) in either the yeast or mammalian two-hybrid assay (Fig. 3A and B). Therefore, these conserved residues in the C terminus of MondoA and Mlx are important determinants of heterodimerization.
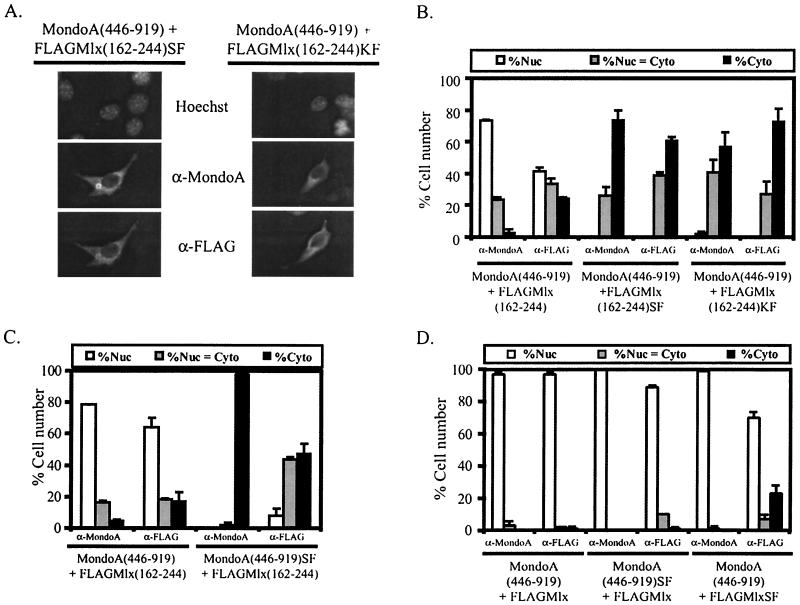
To test whether mutants that cannot heterodimerize retain cytoplasmic localization activity, we determined the subcellular localization of MondoA(446-919) in combination with the two Mlx mutants: MlxS198A/F199A and MlxK184A/F185A. To eliminate the influence of the leucine zipper, we introduced these mutations into Mlx(162-244). As before, Mlx(162-244) localized MondoA(446-919) to the nucleus; however, both Mlx mutants lacked this activity, and MondoA(446-919) localized to the cytoplasm (Fig. 4A and B). To confirm that dimerization is required to cancel cytoplasmic localization activity, we performed the reciprocal experiment and tested whether Mlx(162-244) localized MondoA(446-919)S847A/F848A to the nucleus (Fig. 4C). MondoA(446-919)S847A/F848A cannot heterodimerize with Mlx(162-244) and, as expected, remained in the cytoplasm when coexpressed with Mlx. Therefore, mutants of either MondoA or Mlx that cannot heterodimerize retain cytoplasmic localization activity. These data suggest that cytoplasmic localization activity conferred by the conserved C termini of MondoA and Mlx can be overcome when these domains heterodimerize. Based upon this link between dimerization and regulation of cytoplasmic localization, we call the region of C-terminal conservation in MondoA and Mlx the dimerization and cytoplasmic localization domain (DCD).
FIG. 4.
Heterodimerization of C-terminal conserved regions regulates MondoA-Mlx localization. (A) Photomicrograph showing the localization of MondoA(446-919) when coexpressed with either FLAGMlx(162-244)SF or FLAGMlx(162-244)KF. SF, S198A/F199A; KF, K184A/F185A. (B) MondoA(446-919) was coexpressed with the indicated FLAGMlx(162-244) protein in NIH 3T3 cells and the subcellular localization of the individual proteins was quantified. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic. (C) FLAGMlx(162-244) was coexpressed with either wild-type MondoA(446-919) or MondoA(446-919)SF in NIH 3T3 cells and the subcellular localization of the individual proteins was quantified. SF, S847A/F848A. (D) The indicated MondoA and Mlx constructs were coexpressed in NIH 3T3 cells and the cytoplasmic localization of the individual proteins was quantified.
The DCD of Mlx is sufficient to localize MondoA(446-919) to the nucleus; however, Mlx constructs encoding both the leucine zipper domain and the DCD had stronger nuclear localization activity than the DCD alone (Fig. 2 and data not shown), suggesting that dimerization through both the leucine zipper domain and DCD participates in inactivating the cytoplasmic localization function of the DCD. To test this, we introduced mutations into the C terminus of MondoA(446-919), S847A/F848A, and into the C terminus of full-length Mlx, S198A/F199A. Each of these mutant constructs retains its leucine zipper and should be capable of dimerization via this domain. Both combinations, MondoA(446-919)SF plus Mlx and MondoA(446-919) plus MlxSF, resulted in the nuclear localization of MondoA and Mlx (Fig. 4D). Therefore, even though dimerization via the DCDs of MondoA(446-919) and Mlx was abolished via mutation, dimerization via the leucine zipper was sufficient to overcome cytoplasmic localization activity.
MCRII and MCRIII contribute to the cytoplasmic localization function at the N terminus of MondoA.
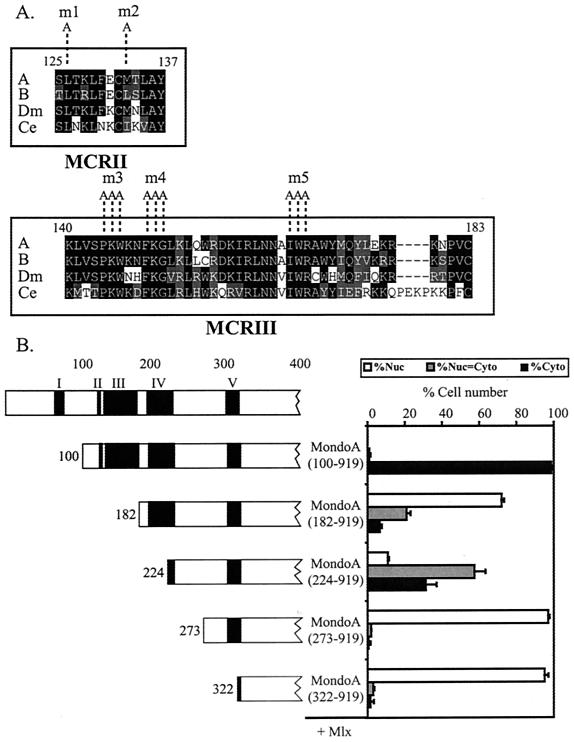
The N terminus of MondoA is highly conserved, with five blocks of homology identifiable between MondoA: a MondoA paralog, MondoB/WBSCR14/ChREBP (8, 16, 17, 58), and Mondo orthologs from Caenorhabditis elegans and D. melanogaster (Fig. 5). The blocks of homology are apparently unique to the Mondo family; therefore, we have termed them the Mondo conserved regions (MCRs) I to V (Fig. 5A). Our group previously reported that MondoA(322-919) lacks all five MCR domains and localized exclusively to the nucleus when expressed with Mlx, demonstrating that a region containing the MCRs was required for the cytoplasmic localization of MondoA-Mlx heterodimers (Fig. 5B) (8).
FIG. 5.
MCRII and -III function as a cytoplasmic localization domain. (A) Alignments of MCRII and MCRIII within the N terminus of Mondo. Sequences were aligned from the following reference sequences: A, human MondoA; B, human MondoB/WBSCR14/ChREBP; Ce, C. elegans Mondo; Dm, D. melanogaster Mondo. The GenBank accession numbers for human MondoA, human MondoB/WBSCR14/ChREBP, C. elegans Mondo, and D. melanogaster Mondo are AAG34121, NP_067430, AAL50027, and AAF53988, respectively. Protein blocks were identified with the Blocks and MEME algorithms. Boundaries of each MCR are labeled with amino acid numbers from MondoA. Identical amino acids are boxed in black. Similar amino acids are boxed in gray. The point mutations m1 through m5 are indicated. (B) A schematic diagram of the amino terminus of each MondoA deletion mutant. FLAGMlx was coexpressed with the indicated MondoA deletion mutants in NIH 3T3 cells and the subcellular localization of the individual proteins was quantified. Localization data for the MondoA deletion proteins are shown. The localization of MondoA and Mlx was quantified only in cells where both proteins were expressed. MondoA mutants localized to the cytoplasm when expressed alone (data not shown), indicating that their nuclear localization required coexpression of Mlx. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic.
To better define this cytoplasmic localization activity and to ascertain the contribution of the MCR domains, additional MondoA proteins with N-terminal deletions were coexpressed with Mlx (Fig. 5B). MondoA(273-919) and MondoA(182-919), which contain MCRV and MCRIV to -V, respectively, localized to the nucleus, suggesting that these domains do not contribute to cytoplasmic localization. By contrast, MondoA(100-919), which contains MCRII to -V, localized almost exclusively to the cytoplasm, suggesting that the majority of the cytoplasmic localization activity at the N terminus of MondoA is contained within MCRII and MCRIII. As MondoA(100-919) was completely cytoplasmic, it was not possible to test the effect of MCRI in this assay. However, fusions between MCRI and the green fluorescent protein gave a diffuse staining pattern throughout the cell similar to green fluorescent protein alone, suggesting that MCRI does not encode an autonomous cytoplasmic localization activity (data not shown).
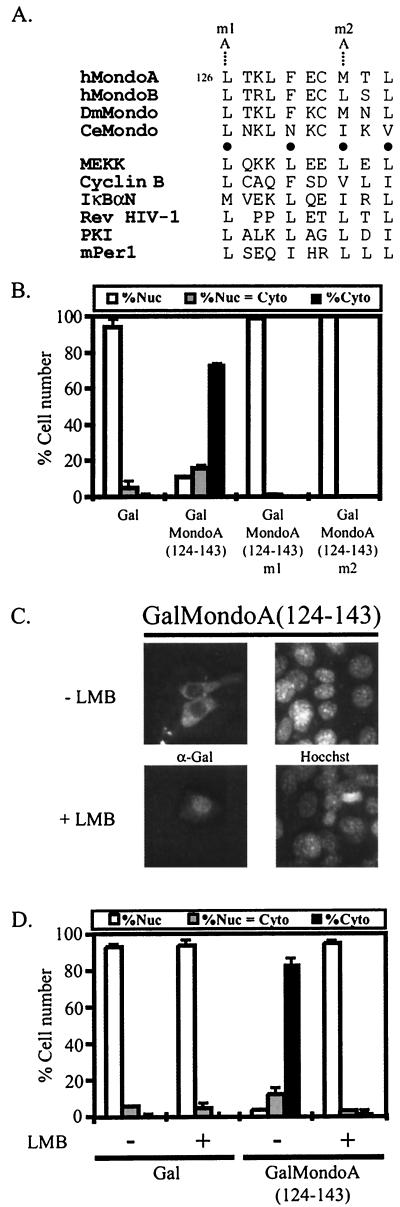
To determine how the N terminus of MondoA contributes to cytoplasmic localization, we investigated the activity of MCRII and -III in isolation. Inspection of the amino acid sequence of MCRII showed a region of hydrophobic residues with conserved spacing that matched the consensus NES recognized by the nuclear export receptor CRM1 (Fig. 6A) (25). To test if MCRII functions as an NES, MondoA(124-143) was fused to the DBD of Gal4. Gal has a weak NLS and, as expected, localized almost exclusively to the nucleus. Consistent with the hypothesis that MCRII can function as an NES, GalMondoA(124-143) localized primarily to the cytoplasm (Fig. 6B). To further characterize the NES activity of MCRII, we determined whether the hydrophobic residues were required for cytoplasmic localization. Two mutations, L126A (m1) and M133A (m2) (Fig. 5A), were introduced into GalMondoA(124-143). The two mutant proteins localized to the nucleus, demonstrating that these residues are required for cytoplasmic localization and implying that MCRII is a CRM1-dependent NES (Fig. 6B). To test this further, we determined the localization of GalMondoA(124-143) following treatment with the CRM1-specific nuclear export inhibitor LMB (33, 34, 56). Treatment with LMB resulted in the nuclear accumulation of GalMondoA(124-143) (Fig. 6C and D), suggesting that MCRII functions as a CRM1-dependent NES. Given the importance of the hydrophobic residues and the effect of LMB treatment on localization, we conclude that MCRII functions as a CRM1-dependent NES.
FIG.6.
MCRII is a CRM1-dependent NES. (A) MCRII export signals from Mondo family members aligned with known export signals from the indicated proteins. Black circles denote the conserved hydrophobic residues believed to be required for NES function. The NES in MCRII fits a φ-x3-φ-x2-φ-x-φ hydrophobic residue spacing consensus, where φ is a hydrophobic amino acid and x is any amino acid. Point mutations m1 and m2 are indicated. (B) Gal alone, GalMondoA(124-143), or one of the GalMondoA(124-143) mutants was expressed in NIH 3T3 cells and the subcellular localization of the individual proteins was quantified. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic. (C) Photomicrographs showing representative NIH 3T3 cells expressing GalMondoA(124-143) either without (−) or following (+) LMB treatment. (D) Gal and GalMondoA(124-143) were expressed in NIH 3T3 cells, and the subcellular localization of the individual proteins was quantified either without (−) or following (+) LMB treatment.
To identify proteins that interact with the N terminus of MondoA and might contribute to its cytoplasmic localization function, we carried out a yeast two-hybrid screen using LexA-MondoA(1-300) as bait. This region of MondoA includes MCRI to -IV. Approximately 4.3 × 106 primary transformants from a random-primed VP16 fusion cDNA library made from day 9.5 and 10.5 mouse embryos were screened. From 50 positive interacting clones, 6 encoded different 14-3-3 isoforms. Because 14-3-3 is known to regulate the cytoplasmic localization of many proteins, including Cdc25C, HDAC-4, HDAC-5, and FKHRL1 (14, 21, 35, 37, 54, 59), we thought it might also contribute to the cytoplasmic localization activity of the N terminus of MondoA.
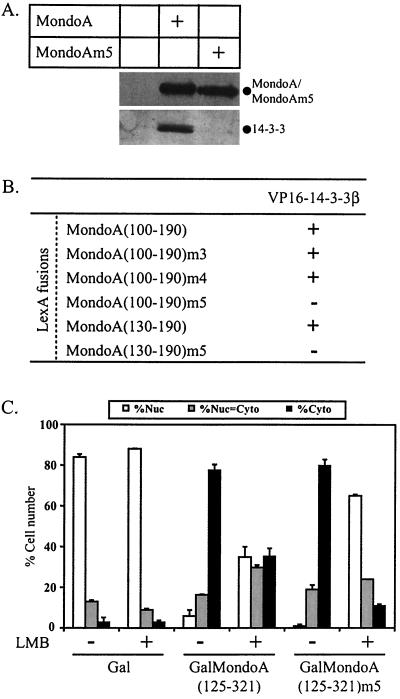
To validate the interaction between MondoA and 14-3-3 and to ascertain a possible role for 14-3-3 in the regulation of MondoA cytoplasmic localization, we determined whether MondoA interacts with 14-3-3 in vivo. 293T cells were transiently transfected with an expression construct for a V5 epitope-tagged version of MondoA, and Western blotting was performed to detect endogenous 14-3-3 in the anti-V5 immunoprecipitates. Both MondoA and 14-3-3 were apparent in the immunoprecipitate (Fig. 7A), indicating that MondoA and 14-3-3 interact in vivo.
FIG. 7.
MCRIII of MondoA interacts with 14-3-3. (A) MondoA or MondoAm5 including a V5 epitope tag were transiently expressed in 293T cells. Anti-V5 immunoprecipitations were performed, followed by anti-V5 and anti-14-3-3 Western blotting. (B) A yeast two-hybrid assay was used to test for interaction between VP16-14-3-3β and the indicated MondoA fusions to LexA. All LexA fusions failed to interact with VP16 alone and VP16-14-3-3β also failed to interact with LexA alone (data not shown). β-Galactosidase activity was scored as follows: −, none; +, strong. (C) Gal, GalMondoA(125-321), and GalMondoA(125-321)m5 were expressed in NIH 3T3 cells, and the subcellular localization of the individual proteins was quantified without (−) or following (+) LMB treatment. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic.
We next determined whether 14-3-3 binding mapped to a specific MCR. Yeast two-hybrid assays narrowed the binding site for 14-3-3 to amino acids 130 to 190 of MondoA (Fig. 7B). This region encompasses MCRIII but does not contain a recognizable consensus binding site for 14-3-3 (19, 57). To better characterize this novel 14-3-3 binding site, a series of point mutations were made within conserved residues of MCRIII. Three triple point mutations were introduced into LexA-MondoA(100-190) or LexA-MondoA(130-190), and these proteins were tested for interaction with VP16-14-3-3β. The mutations were P144A/K145A/W146A (m3), F149A/K150A/G151A (m4), and I166A/W167A/R168A (Fig. 5A). LexA-MondoA(100-190)m3 and LexA-MondoA(100-190)m4 interacted with VP16-14-3-3β, but LexA-MondoA(100-190)m5 or LexA-MondoA(130-190)m5 did not (Fig. 7B). Furthermore, the m5 mutations in full-length MondoA also abolished the interaction with endogenous 14-3-3 in vivo (Fig. 7A). MondoAm5 still interacted with Mlx (data not shown), suggesting that the point mutations do not grossly alter the overall fold or stability of the protein.
To test whether 14-3-3 binding contributes to the cytoplasmic localization activity of MondoA, we introduced the m5 point mutations into a Gal fusion that contains MCRII, -III, and -IV, GalMondoA(125-321), and determined the effect on subcellular localization. Surprisingly, like GalMondoA(125-321), GalMondoA(125-321)m5 localized primarily to the cytoplasm. However, the nuclear export inhibitor LMB had a more pronounced effect on GalMondoA(125-321)m5 than on the wild type (Fig. 7C), suggesting that loss of 14-3-3 binding resulted in either a reduction in nuclear export activity or an increase in nuclear localization activity. Together, these data suggest that MCRIII interacts with 14-3-3 and this interaction contributes to the cytoplasmic localization of MondoA.
Both CRM1 and 14-3-3 contribute to the N-terminal cytoplasmic localization activity of MondoA.
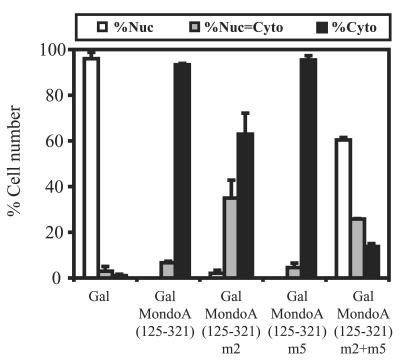
To determine whether there is functional interplay between MCRII and MCRIII, we introduced the mutations that eliminate the NES activity of MCRII or eliminate 14-3-3 binding to MCRIII, individually and in combination, into a larger construct, GalMondoA(125-321), which contains MCRII, -III, and -IV.
GalMondoA(125-321) localized to the cytoplasm, confirming that MCRII and MCRIII have cytoplasmic localization activity (8). By contrast, the MCRII mutant, GalMondoA(125-321)m2, showed an increase in equivalent cytoplasmic and nuclear staining, suggesting that impairment of the MCRII NES affects localization of this larger construct. However, the majority of the cells still showed a primarily cytoplasmic localization and there were very few cells that showed a completely nuclear staining pattern (Fig. 8). The MCRIII mutant, GalMondoA(125-321)m5, localized primarily to the cytoplasm, identical to GalMondoA(125-321). Therefore, elimination of either the NES activity of MCRII or 14-3-3 binding to MCRIII has a relatively minor effect on the N-terminal cytoplasmic localization activity of MondoA.
FIG. 8.
Nuclear export and 14-3-3 binding both contribute to cytoplasmic localization activity at the N terminus of MondoA. Gal, wild-type GalMondoA(125-321), and the indicated GalMondoA(125-321) mutants were expressed in NIH 3T3 cells, and the subcellular localization of the individual proteins was quantified. Nuc, predominantly nuclear; Nuc = Cyto, nuclear equivalent to cytoplasmic; Cyto, predominantly cytoplasmic.
A more dramatic effect was seen when MCRII and MCRIII were mutated in combination. GalMondoA(125-321)m2-GalMondoA(125-321)m5 localized to the nucleus in 60% of the cells, suggesting a significant reduction in the cytoplasmic localization function of the fusion protein. For comparison, mutation of MCRII or MCRIII alone resulted in only 2% or 0% cells, respectively, with a nuclear staining pattern. Gal alone localized to the nucleus in over 95% of the cells, suggesting that while the effect of the double mutation in MCRII and MCRIII is dramatic, it is incomplete and other sequences must also contribute to the cytoplasmic localization activity of this conserved domain. However, the pronounced effect observed in the double mutant demonstrates that MCRII and MCRIII both contribute to the cytoplasmic localization activity in the N terminus of MondoA.
DISCUSSION
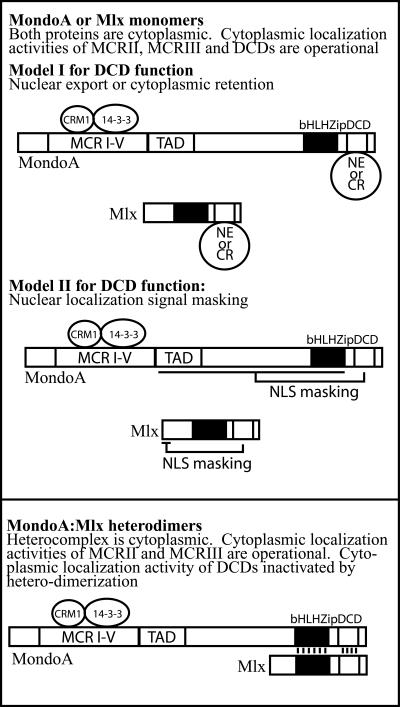
Conserved domains at the N and C termini of MondoA and Mlx control their subcellular localization. The DCD located at the C terminus of both proteins is required for the cytoplasmic localization of the protein monomers, whereas the N terminus of MondoA is required for the cytoplasmic localization of the MondoA-Mlx heterodimer. Our data demonstrate that the DCD has a dual function: cytoplasmic localization activity, and serving as a heterodimerization interface. Furthermore, as heterodimerization abolishes the cytoplasmic localization activity of the DCD, these two activities appear to be linked. The N terminus of MondoA encodes a potent, and dominant, cytoplasmic localization activity that depends in part on the binding of CRM1 and 14-3-3. We propose that nuclear accumulation of the MondoA-Mlx heterodimer is a multistep process. First, heterodimerization of MondoA and Mlx via an extended domain comprised of both the leucine zipper and the DCD renders the heterodimer competent for both DNA binding and nuclear entry. However, the heterodimer is retained in the cytoplasm through the function of the cytoplasmic localization activity at the N terminus of MondoA (Fig. 9). Thus, heterodimerization is necessary but not sufficient for nuclear localization. We propose, therefore, that the second step required for the nuclear localization of the MondoA-Mlx heterodimer is the inactivation of the N-terminal cytoplasmic localization activity of MondoA, which is presumably induced by a signaling event.
FIG. 9.
Multiple domains contribute to the subcellular localization of MondoA-Mlx heterodimers. See text for details. NE, nuclear export receptor; CR, cytoplasmic retention factor; TAD, transcription activation domain; NLS, nuclear localization signal; MCR, Mondo conserved region; DCD, dimerization and cytoplasmic localization domain; bHLHZip, basic region helix-loop-helix leucine zipper domain.
Though unknown, clues to determining the signal required to induce the nuclear entry of the MondoA-Mlx heterodimer come from recent reports examining the biological role of MondoB/WBSCR14, a MondoA paralog. MondoB/WBSCR14 was implicated as the carbohydrate response element-binding protein (ChREBP), suggesting it plays a role in regulating glucose homeostasis or energy metabolism (32, 58). As such, MondoA may have similar physiological roles. In support of this, MondoB/WBSCR14/ChREBP and MondoA are highly expressed in liver and skeletal muscle, respectively (8, 58; unpublished observation), the tissues that carry out most of the glucose metabolism in mammals (48). Furthermore, in response to treatment of rat primary hepatocytes with glucose, overexpressed MondoB/WBSCR14/ChREBP accumulated in the nucleus (32). In addition, Myc positively regulates a number of genes involved in energy metabolism (44, 50). The similarity between Myc and MondoA suggests that MondoA may also regulate genes involved in glucose metabolic pathways. In support of this, MondoA-Mlx can activate the Myc target, lactate dehydrogenase A, to levels similar to those seen with Myc-Max (data not shown). Together, these data suggest MondoA-Mlx may regulate genes involved in energy metabolism and that insulin, glucose, or a dietary metabolite may trigger the nuclear accumulation and the nuclear activity of the heterodimer.
How the DCD regulates cytoplasmic localization is not known; however, its conservation in both MondoA and Mlx suggests that it functions via a mechanism common to both proteins. It seems most likely that the DCD functions either by acting as an NES or by interacting with cytoplasmic retention proteins. A third possibility is that the DCDs of MondoA and Mlx mask their respective NLSs by an intramolecular mechanism (Fig. 9). The DCDs of MondoA and Mlx each contain a region with properly spaced hydrophobic residues that may function as a CRM1-dependent NES (11). However, fusions between the DCD of MondoA or Mlx to Gal localized to the nucleus (data not shown), suggesting that the DCD does not function autonomously. A context-dependent function of the subcellular localization function of the DCD is not without precedent. For example, the NES of HDAC-5 functions in a protein context-dependent manner (40).
In addition to functioning in regulating cytoplasmic localization, the DCD also functions as a novel heterodimerization interface. We have identified point mutations that abolish dimerization; however, the S198A/F199A mutations in Mlx had no effect on the cytoplasmic localization of full-length Mlx (data not shown), suggesting that these two functions do not overlap. We did not detect homotypic interactions between the DCDs, suggesting that these domains only mediate heterotypic interactions (Fig. 3A and B). The DCDs can heterodimerize independently of the leucine zipper; however, in conjunction with the leucine zipper, the DCD may provide an extended dimerization surface that may increase both the affinity and specificity of MondoA-Mlx heterodimerization and subsequent DNA binding. Furthermore, dimerization via either the leucine zipper or the DCDs of MondoA and Mlx is sufficient to abolish cytoplasmic localization activity; however, in isolation each domain is slightly less effective than when in combination (Fig. 2B and 4D). As such, it is likely that the extended leucine zipper-DCD dimerization interface is also required to completely inactivate the C-terminal cytoplasmic localization activity of MondoA and Mlx.
Searches of the sequence databases indicate that no other members of the bHLHZip family or other proteins have strong sequence homology to the DCD. Whereas the DCD is apparently unique to members of the Mondo and Mlx families, it is conceptually similar to the PAS domain found in a number of proteins of the BHLH class of transcription factors. The PAS domain can function in homotypic interactions, mediating interactions between transcription factors, or it can function in heterotypic interactions, mediating association with cellular chaperones (for a review, see reference 22). The fact that the subcellular localization of the aryl hydrocarbon receptor, a PAS-containing transcription factor, is also highly regulated extends this analogy between the DCD and PAS domain (5, 29, 39, 45).
Our data also suggest that both MondoA and Mlx contain regions important for nuclear localization. A MondoA mutant lacking both N- and C-terminal cytoplasmic localization activities, MondoA(446-811), localized to the nucleus (Fig. 1C), suggesting active nuclear import. MondoA(446-811) itself does not appear to contain a recognizable NLS and, therefore, may interact with a protein that has an NLS. Similarly, the predominantly nuclear localization of Mlx(1-75) (Fig. 1B) suggested that the N terminus of Mlx functions as an NLS; however, it could not direct a heterologous protein to the nucleus (data not shown). It is possible that the small size of Mlx(1-75), approximately 8 kDa, allows it to enter the nucleus by diffusion and it is sequestered there by functioning as a nuclear retention signal. We suggest that the combined actions of nuclear import and nuclear retention, mediated by the central region of MondoA and the N terminus of Mlx, respectively, allow for nuclear accumulation of the heterodimer.
When fused to Gal, MCRII is sufficient to target the fusion protein to the cytoplasm, suggesting an NES function. The well-characterized CRM1-dependent NES comprises hydrophobic residues, the spacing of which is important for function. Studies of the NES of the human T-cell leukemia virus type 1 Rex protein demonstrated that the spacing of hydrophobic residues in 3-3-1, 3-2-1, or 2-2-1 configurations allowed NES function, whereas spacing hydrophobic residues in a 2-3-1 configuration is unfavorable (11). The MCRII NES comprises Leu126, Phe130, Met133, and Leu135 in a 3-2-1 configuration. Mutation of Leu126 or Met133 completely inactivated the cytoplasmic localization activity of MCRII. L129 is also conserved; however, as an NES it would display an unfavorable 2-3-1 configuration. Finally, we found that LMB completely inactivated the cytoplasmic localization activity of MCRII. Together, these data strongly suggest that MCRII functions as a CRM1-dependent NES.
14-3-3 binds MCRIII in the context of full-length MondoA and in isolation. Many binding partners for 14-3-3 have been identified, the majority of which depend on phosphorylation for the interaction. There appear to be two consensus 14-3-3 binding sites (19, 57); however, neither site is present in MCRIII. Therefore, 14-3-3 binding may be direct through a noncanonical binding site or indirect via another protein that bridges 14-3-3 to MondoA. We have not been able to detect an interaction between endogenous MondoA and 14-3-3, likely because the levels of MondoA are quite low in the cell type utilized here. Furthermore, our experiments strongly suggest the interaction between MondoA and 14-3-3 is regulated and may be difficult to detect for this reason. We are currently developing additional antibodies that recognize MondoA with the hope of overcoming both of these limitations. By site-directed mutagenesis we have identified three amino acids, I166, W167, and R168, that are required for interaction with 14-3-3. We observed that a Gal fusion encoding MCRII to -IV which could not bind 14-3-3 was more sensitive to LMB than the wild type, suggesting a role for 14-3-3 in controlling cytoplasmic localization of MondoA. We cannot rule out the formal possibility that the mutations that eliminate 14-3-3 binding influence subcellular localization indirectly by altering the binding of other, yet to be identified, proteins or by altering the structure and/or activity of the other MCR domains. Given the conservative nature of these mutations and the fact that the m5 mutant is expressed, is stable, and can heterodimerize with Mlx, we do not favor these two alternative possibilities.
Mutations that eliminate CRM1 binding to MCRII or 14-3-3 binding to MCRIII do not dramatically affect subcellular localization. By contrast, the mutations that affect both domains led to a dramatic loss of cytoplasmic localization activity imparted by MCRII and MCRIII and resulted in the predominantly nuclear localization of GalMondoA(125-321). Because MCRII functions as an autonomous NES and because MCRIII in isolation showed no cytoplasmic localization function (data not shown), we suggest that MCRII and MCRIII do not have redundant functions.
The relative rates of nuclear import and export of a given protein contribute to its subcellular localization at steady state. 14-3-3 proteins are abundant and can contribute to both of these processes. Work from several labs suggests that 14-3-3 proteins drive cytoplasmic localization of their ligands indirectly by masking intrinsic NLSs or unmasking intrinsic NESs that lie close to their binding sites (15, 35, 40, 43, 59). In addition, 14-3-3 proteins are also capable of promoting nuclear accumulation by blocking access to CRM1-dependent nuclear export sequences (49). In the context of a fusion protein that contains both MCRII and MCRIII, mutations that eliminate 14-3-3 binding have little effect on the cytoplasmic localization, likely due to a dominant effect of CRM1 binding to MCRII. However, these same mutations make the fusion protein more susceptible to LMB. One model that could explain these data is that binding of 14-3-3 to MCRIII negatively regulates the NES activity of MCRII indirectly by blocking the activity of a nearby, but at present unidentified, NLS. We suggest that loss of 14-3-3 binding to MCRIII exposes the NLS and accounts for the increased sensitivity to LMB observed with the fusion protein that cannot bind 14-3-3. By contrast, loss of CRM1 binding alone is not sufficient to lead to the nuclear accumulation of the MCRII-III fusion protein when 14-3-3 is bound because the putative NLS is blocked.
Members of the Mondo and Mlx families possess several conserved and novel domains that regulate the subcellular localization of the heterodimer. Our data suggest that cells tightly regulate nuclear entry of the MondoA-Mlx heterodimers, implying that they regulate a process or processes critical for normal cellular function. Discovering the signal that induces the nuclear entry and the transcriptional activity of the MondoA-Mlx heterodimer is a critical step in unraveling its complex regulation and ultimately its downstream targets. Our detailed dissection of the sequences that control MondoA and Mlx subcellular localization provide the framework to vigorously test the mechanisms of signal-induced nuclear accumulation of the MondoA-Mlx heterodimer.
Acknowledgments
We thank Leena Bhoite and David Stillman for reagents, Anne Brunet and Mike Yaffe for 14-3-3 plasmids, Minoru Yoshida for leptomycin B, and David Virshup, Barbara Graves, and members of the Ayer lab for comments on the manuscript.
DNA sequencing and oligonucleotide synthesis were supported by Cancer Center Support Grant 2P30 CA42014. This work was supported by NIH grant GM55668 and funds from the Huntsman Cancer Foundation. D.E.A. is a Scholar of The Leukemia and Lymphoma Society.
REFERENCES
- 1.Amati, B., S. Dalton, M. W. Brooks, T. D. Littlewood, G. I. Evan, and H. Land. 1992. Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature 359:423-426. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1995. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 3.Ayer, D. E., and R. N. Eisenman. 1993. A switch from Myc:Max to Mad:Max heterocomplexes accompanies monocyte/macrophage differentiation. Genes Dev. 7:2110-2119. [DOI] [PubMed] [Google Scholar]
- 4.Ayer, D. E., Q. A. Lawrence, and R. N. Eisenman. 1995. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell 80:767-776. [DOI] [PubMed] [Google Scholar]
- 5.Berg, P., and I. Pongratz. 2001. Differential usage of nuclear export sequences regulates intracellular localization of the dioxin (aryl hydrocarbon) receptor. J. Biol. Chem. 276:43231-43238. [DOI] [PubMed] [Google Scholar]
- 6.Bhoite, L. T., Y. Yu, and D. J. Stillman. 2001. The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 15:2457-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billin, A. N., A. L. Eilers, K. L. Coulter, J. S. Logan, and D. E. Ayer. 2000. MondoA, a novel basic helix-loop-helix-leucine zipper transcriptional activator that constitutes a positive branch of a Max-like network. Mol. Cell. Biol. 20:8845-8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billin, A. N., A. L. Eilers, C. Queva, and D. E. Ayer. 1999. Mlx, a novel max-like BHLHZip protein that interacts with the max network of transcription factors. J. Biol. Chem. 274:36344-36350. [DOI] [PubMed] [Google Scholar]
- 10.Blackwood, E. M., and R. N. Eisenman. 1991. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251:1211-1217. [DOI] [PubMed] [Google Scholar]
- 11.Bogerd, H. P., R. A. Fridell, R. E. Benson, J. Hua, and B. R. Cullen. 1996. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol. Cell. Biol. 16:4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, M. S., and J. L. Goldstein. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331-340. [DOI] [PubMed] [Google Scholar]
- 13.Brownawell, A. M., G. J. Kops, I. G. Macara, and B. M. Burgering. 2001. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the Forkhead transcription factor AFX. Mol. Cell. Biol. 21:3534-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 15.Brunet, A., F. Kanai, J. Stehn, J. Xu, D. Sarbassova, J. V. Frangioni, S. N. Dalal, J. A. DeCaprio, M. E. Greenberg, and M. B. Yaffe. 2002. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 156:817-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairo, S., G. Merla, F. Urbinati, A. Ballabio, and A. Reymond. 2001. WBSCR14, a gene mapping to the Williams-Beuren syndrome deleted region, is a new member of the Mlx transcription factor network. Hum. Mol. Genet. 10:617-627. [DOI] [PubMed] [Google Scholar]
- 17.de Luis, O., M. C. Valero, and L. A. Jurado. 2000. WBSCR14, a putative transcription factor gene deleted in Williams-Beuren syndrome: complete characterisation of the human gene and the mouse ortholog. Eur. J. Hum. Genet. 8:215-222. [DOI] [PubMed] [Google Scholar]
- 18.Eisenman, R. N. 2001. Deconstructing Myc. Genes Dev. 15:2023-2030. [DOI] [PubMed] [Google Scholar]
- 19.Fu, H., R. R. Subramanian, and S. C. Masters. 2000. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40:617-647. [DOI] [PubMed] [Google Scholar]
- 20.Grandori, C., S. M. Cowley, L. P. James, and R. N. Eisenman. 2000. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16:653-699. [DOI] [PubMed] [Google Scholar]
- 21.Grozinger, C. M., and S. L. Schreiber. 2000. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl. Acad. Sci. USA 97:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu, Y. Z., J. B. Hogenesch, and C. A. Bradfield. 2000. The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40:519-561. [DOI] [PubMed] [Google Scholar]
- 23.Henriksson, M., and B. Lüscher. 1996. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv. Cancer Res. 68:109-182. [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg, S. M., R. Sternglanz, P. F. Cheng, and H. Weintraub. 1995. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol. Cell. Biol. 15:3813-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hope, T. J. 1997. Viral RNA export. Chem. Biol. 4:335-344. [DOI] [PubMed] [Google Scholar]
- 26.Hurlin, P. J., C. Queva, and R. N. Eisenman. 1997. Mnt, a novel Max interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes Dev. 11:44-58. [DOI] [PubMed] [Google Scholar]
- 27.Hurlin, P. J., C. Queva, P. J. Koskinen, E. Steingrimsson, D. E. Ayer, N. G. Copeland, N. A. Jenkins, and R. N. Eisenman. 1995. Mad3 and Mad4: novel Max-interacting transcriptional repressors that suppress c-Myc-dependent transformation and are expressed during neural and epidermal differentiation. EMBO J. 14:5646-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurlin, P. J., E. Steingrimsson, N. G. Copeland, N. A. Jenkins, and R. N. Eisenman. 1999. Mga, a dual-specificity transcription factor that interacts with Max and contains a T-domain DNA-binding motif. EMBO J. 18:7019-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikuta, T., H. Eguchi, T. Tachibana, Y. Yoneda, and K. Kawajiri. 1998. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J. Biol. Chem. 273:2895-2904. [DOI] [PubMed] [Google Scholar]
- 30.Kaffman, A., and E. K. O'Shea. 1999. Regulation of nuclear localization: a key to a door. Annu. Rev. Cell. Dev. Biol. 15:291-339. [DOI] [PubMed] [Google Scholar]
- 31.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi, T., M. Takenoshita, T. Kabashima, and K. Uyeda. 2001. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc. Natl. Acad. Sci. USA 98:13710-13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo, N., S. Khochbin, K. Nishi, K. Kitano, M. Yanagida, M. Yoshida, and S. Horinouchi. 1997. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. J. Biol. Chem. 272:29742-29751. [DOI] [PubMed] [Google Scholar]
- 34.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumagai, A., and W. G. Dunphy. 1999. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 13:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard, W. J., and J. J. O'Shea. 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16:293-322. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Girona, A., B. Furnari, O. Mondesert, and P. Russell. 1999. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397:172-175. [DOI] [PubMed] [Google Scholar]
- 38.Massague, J., and D. Wotton. 2000. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 19:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuire, J., M. L. Whitelaw, I. Pongratz, J. A. Gustafsson, and L. Poellinger. 1994. A cellular factor stimulates ligand-dependent release of hsp90 from the basic helix-loop-helix dioxin receptor. Mol. Cell. Biol. 14:2438-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2001. Identification of a signal-responsive nuclear export sequence in class II histone deacetylases. Mol. Cell. Biol. 21:6312-6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meroni, G., S. Cairo, G. Merla, S. Messali, R. Brent, A. Ballabio, and A. Reymond. 2000. Mlx, a new Max-like bHLHZip family member: the center stage of a novel transcription factors regulatory pathway? Oncogene 19:3266-3277. [DOI] [PubMed] [Google Scholar]
- 42.Meroni, G., A. Reymond, M. Alcalay, G. Borsani, A. Tanigami, R. Tonlorenzi, C. L. Nigro, S. Messali, M. Zollo, D. H. Ledbetter, R. Brent, A. Ballabio, and R. Carrozzo. 1997. Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor. EMBO J. 16:2892-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muslin, A. J., and H. Xing. 2000. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal 12:703-709. [DOI] [PubMed] [Google Scholar]
- 44.Osthus, R. C., H. Shim, S. Kim, Q. Li, R. Reddy, M. Mukherjee, Y. Xu, D. Wonsey, L. A. Lee, and C. V. Dang. 2000. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 275:21797-21800. [DOI] [PubMed] [Google Scholar]
- 45.Pollenz, R. S., and E. R. Barbour. 2000. Analysis of the complex relationship between nuclear export and aryl hydrocarbon receptor-mediated gene regulation. Mol. Cell. Biol. 20:6095-6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prendergast, G. C., D. Lawe, and E. B. Ziff. 1991. Association of Myn, the murine homolog of max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell 65:395-407. [DOI] [PubMed] [Google Scholar]
- 47.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 48.Saltiel, A. R. 2001. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104:517-529. [DOI] [PubMed] [Google Scholar]
- 49.Seimiya, H., H. Sawada, Y. Muramatsu, M. Shimizu, K. Ohko, K. Yamane, and T. Tsuruo. 2000. Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J. 19:2652-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shim, H., C. Dolde, B. C. Lewis, C. S. Wu, G. Dang, R. A. Jungmann, R. Dalla-Favera, and C. V. Dang. 1997. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA 94:6658-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turpin, P., B. Ossareh-Nazari, and C. Dargemont. 1999. Nuclear transport and transcriptional regulation. FEBS Lett. 452:82-86. [DOI] [PubMed] [Google Scholar]
- 52.Vandromme, M., C. Gauthier-Rouviere, N. Lamb, and A. Fernandez. 1996. Regulation of transcription factor localization: fine-tuning of gene expression. Trends Biochem. Sci. 21:59-64. [PubMed] [Google Scholar]
- 53.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 54.Wang, A. H., M. J. Kruhlak, J. Wu, N. R. Bertos, M. Vezmar, B. I. Posner, D. P. Bazett-Jones, and X. J. Yang. 2000. Regulation of histone deacetylase 4 by binding of 14-3-3 proteins. Mol. Cell. Biol. 20:6904-6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, A. H., and X. J. Yang. 2001. Histone deacetylase 4 possesses intrinsic nuclear import and export signals. Mol. Cell. Biol. 21:5992-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolff, B., J. J. Sanglier, and Y. Wang. 1997. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 4:139-147. [DOI] [PubMed] [Google Scholar]
- 57.Yaffe, M. B., K. Rittinger, S. Volinia, P. R. Caron, A. Aitken, H. Leffers, S. J. Gamblin, S. J. Smerdon, and L. C. Cantley. 1997. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91:961-971. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita, H., M. Takenoshita, M. Sakurai, R. K. Bruick, W. J. Henzel, W. Shillinglaw, D. Arnot, and K. Uyeda. 2001. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA 98:9116-9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, J., K. Winkler, M. Yoshida, and S. Kornbluth. 1999. Maintenance of G2 arrest in the Xenopus oocyte: a role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO J. 18:2174-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zervos, A. S., J. Gyuris, and R. Brent. 1993. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell 72:223-232. [DOI] [PubMed] [Google Scholar]
- 61.Ziegelbauer, J., B. Shan, D. Yager, C. Larabell, B. Hoffmann, and R. Tjian. 2001. Transcription factor MIZ-1 is regulated via microtubule association. Mol. Cell 8:339-349. [DOI] [PubMed] [Google Scholar]