Abstract
In the Cf-9/Avr9 gene-for-gene interaction, the Cf-9 resistance gene from tomato confers resistance to the fungal pathogen Cladosporium fulvum, which expresses the corresponding pathogen-derived avirulence product Avr9. To understand R gene function and dissect the signaling mechanisms involved in the induction of plant defenses, we studied Cf-9/Avr9–dependent activation of protein kinases in transgenic Cf9 tobacco cell cultures. Using a modified in-gel kinase assay with histone as substrate, we identified a membrane-bound, calcium-dependent protein kinase (CDPK) that showed a shift in electrophoretic mobility from 68 to 70 kD within 5 min after Avr9 elicitor was added. This transition from the nonelicited to the elicited CDPK form was caused by a phosphorylation event and was verified when antibodies to CDPK were used for protein gel blot analysis. In addition, the interconversion of the corresponding CDPK forms could be induced in vitro in both directions by treatment with either phosphatase or ATP. In vitro protein kinase activity toward syntide-2 or histone with membrane extracts or gel-purified enzyme was dependent on Ca2+ content and was compromised by the calmodulin antagonist N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7) but not by its inactive isoform N-(6-aminohexyl)-1-naphthalenesulfonamide. In these assays, the CDPK activity in elicited samples, reflecting predominantly the phosphorylated 70-kD CDPK form, was greater than in nonelicited samples. Thus, Avr9/Cf-9–dependent phosphorylation and subsequent transition from the nonelicited to the elicited form correlate with the activation of a CDPK isoform after in vivo stimulation. Because that transition was not inhibited by W-7, the in vivo CDPK activation probably is not the result of autophosphorylation. Studies with pharmacological inhibitors indicated that the identified CDPK is independent of or is located upstream from a signaling pathway that is required for the Avr9-induced active oxygen species.
INTRODUCTION
A plant's successful defense against invading microorganisms depends on early perception of the pathogen and initiation of the appropriate signaling processes to recruit the multicomponent defense response. In gene-for-gene interactions, recognition of pathogens requires a plant resistance (R) gene that confers resistance to specific pathogen races that carry the corresponding avirulence (Avr) gene (Flor, 1971). In contrast, in many non-race-specific interactions, the plant recognizes nonspecific elicitors, such as bacterial or fungal oligosaccharides derived from cell wall fragments, peptides, or proteins, released during the infection process. It was anticipated that Avr protein and nonspecific elicitors could function as ligands for a specific membrane-localized or cytosolic receptor, which in the case of the race-specific interactions might be encoded by the R gene itself (Staskawicz et al., 1995).
Many R genes that confer resistance to different pathogens have been cloned from various plant species (Hammond-Kosack and Jones, 1997; Ellis and Jones, 1998). Likewise, elicitor binding proteins have been isolated and characterized (Nürnberger et al., 1994; Umemoto et al., 1997; Nennstiel et al., 1998; Mithöfer et al., 1999). Subsequent signal transduction steps that transmit the recognition event and induce the plant defense are still poorly understood. Some of the activated signaling components appear to be shared in gene-for-gene and nonspecific interactions. Thus, the plant seems to activate its defense against a variety of bacterial, viral, fungal, and nematode pathogens by using and combining a limited number of common mechanisms. Changes in ion fluxes, such as the activation of a Ca2+ and H+ influx or a K+ and Cl− efflux, as well as the production of active oxygen species (AOS) such as O2−, H2O2, or nitric oxide, reportedly occur within minutes after the elicitation process (Richberg et al., 1998). A crucial role for protein phosphorylation has been suggested by the isolation of the Pto gene from tomato that encodes a serine/threonine kinase (Martin et al., 1993; Martin, 1999) and of the Xa21 gene from rice that encodes a leucine-rich–repeat transmembrane receptor kinase (Song et al., 1995). In addition, in vivo phosphorylation experiments and studies with pharmacological inhibitors have demonstrated that protein kinases and phosphatases are crucial for activation of early defense responses (Yang et al., 1997; Scheel, 1998). Recent reports have noted the activation of mitogen-activated protein (MAP) kinases after race-specific and nonspecific elicitation (Ligterink et al., 1997; Zhang and Klessig, 1998; Zhang et al., 1998; Romeis et al., 1999).
The plant defense is triggered by a signaling network of parallel pathways that may be interlinked at single components. The increase in the cytosolic Ca2+ concentration, which occurs within seconds after elicitation, appears to be a master regulator required for many subsequent signaling steps. AOS production, MAP kinase activation, defense gene activation, and phytoalexin production, singly or in combination, were shown to be compromised in the presence of Ca2+-chelating or Ca2+ channel–inhibiting compounds (Scheel, 1998). In addition, an increase in cytosolic Ca2+ was detected after nonspecific elicitation in tobacco plants expressing aequorin as a transgene (Chandra and Low, 1997), and Ca2+-inward channels responding to nonspecific and race-specific elicitors were characterized in parsley and tomato, respectively (Gelli et al., 1997; Zimmermann et al., 1997). However, the corresponding intracellular target proteins that sense and transmit these changes in Ca2+ concentrations are not known.
In animal systems, protein kinase C isotypes and calmodulin-dependent protein kinases have been characterized in detail as Ca2+ modulators. For example, protein kinase C activity is required for induction of the defense signal–related oxidative burst in macrophages (Majumdar et al., 1993; Perkins et al., 1995). Little information is available about the function of protein kinase C in plants, and the possible fulfilling of its role by calcium-dependent (but cal-modulin-independent) protein kinases (CDPKs) has been proposed (Roberts and Harmon, 1992).
CDPKs are a class of serine/threonine protein kinases that are unique to plants and some protists. Conserved in structure, they consist of an N-terminal variable domain, a kinase catalytic domain, and a highly conserved junction domain that functions as autoinhibitor; at the C terminus is a calmodulin-like domain that in most cases contains conserved Ca2+ motifs (Roberts and Harmon, 1992; Satterlee and Sussman, 1998). The large CDPK gene family suggests that isoenzymes confer different specificities and function in multiple signaling pathways (Hong et al., 1996; Hrabak et al., 1996). Although CDPKs have been implicated in response to several environmental stresses, and induction of CDPK mRNA has been reported, there has been no previous evidence that CDPKs participate in activating plant defenses in plant–pathogen interactions.
We have established transgenic tobacco plants that express the Cf-9 disease resistance gene from tomato. This gene confers recognition of the corresponding Avr9 elicitor peptide from the fungus Cladosporium fulvum (Van den Ackerveken et al., 1992; Jones et al., 1994; De Wit, 1997; Hammond-Kosack et al., 1998).
Using this system, we can dissect early signaling events in a gene-for-gene interaction. In impaled Cf9 tobacco guard cells, both a rapid Avr9-induced K+ efflux and an inhibition of K+ influx have been demonstrated (Blatt et al., 1999). Avr9-induced defense responses in Cf9 cell suspension cultures include the production of AOS within 5 min (Piedras et al., 1998), the rapid and transient activation of two MAP kinases, wound-induced protein kinase and salicylic acid–induced protein kinase from tobacco (Romeis et al., 1999), and early changes in gene expression (Durrant et al., 2000). Many of these defense-related responses were dependent on a Ca2+ influx and an upstream phosphorylation event or events. The fact that calmodulin antagonists, which have been shown to inhibit CDPKs, could compromise AOS synthesis and MAP kinase activation led us to conclude that CDPKs may be involved in Avr9/Cf-9–mediated signaling processes.
In this study, we report the identification of a CDPK that undergoes an Avr9/Cf-9–dependent transition from a nonelicited to an elicited form. This transition was detected by an in-gel kinase assay with histone as substrate and by using protein gel blot analysis with antibodies to CDPK. In addition, the phosphorylation-dependent conversion into the elicited 70-kD form was accompanied by an increase in CDPK activity. Our system thus allows us to investigate the activation of a CDPK isoform after an in vivo stimulation. Inhibitor studies demonstrated that the identified CDPK is independent of or located upstream from a signaling pathway that is required for AOS synthesis. We discuss CDPK function and potential in vivo targets that may contribute to the Avr9/Cf-9–mediated induction of the plant defense response.
RESULTS
Avr9/Cf-9–Dependent Activation of a CDPK
By studying the role of protein kinases in the Avr9/Cf-9–dependent defense response, we recently identified two MAP kinases—wound-induced protein kinase and salicylic acid–induced protein kinase—that became activated within a few minutes of elicitation (Romeis et al., 1999). By applying a modified in-gel kinase assay protocol with histone type III-SS as artificial substrate and analyzing solubilized membrane samples, we identified another class of protein kinases that were activated in an Avr9/Cf-9–dependent manner. Tobacco suspension cultures expressing Cf-9 were challenged with intercellular fluid (IF) either containing the Avr9 peptide (IF[Avr9+]) or not (IF[Avr9−]). At different times after elicitation, cells were harvested, solubilized membrane extracts were prepared, and protein kinase activity was determined by the modified in-gel kinase protocol.
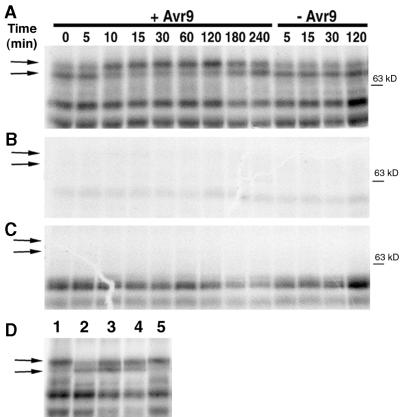
After elicitation with Avr9, a protein kinase of ∼70 kD became activated within 5 min. High activity was maintained between 10 and 60 min after elicitation, whereas during the next 2 hr the activity returned to its basal value (Figure 1A). Intriguingly, an inverse change in the pattern of phosphorylation activity was evident at 68 kD. The signal intensity decreased within 5 to 10 min after elicitation with Avr9, remained low, and returned to its basal value only 2 hr later. These changes in protein phosphorylation did not occur after challenge with IF(Avr9−) (Figures 1A and 1D, lane 2). Also, no changes were observed when the nontransformed parental tobacco cell line Petite Havana was treated with IF(Avr9+) (Figure 1D, lane 3) or IF(Avr9−) (lane 4). Chemically synthesized Avr9 peptide was able to induce the CDPK transition in Cf9 cells, indicating that the Avr9 peptide in the intercellular fluid is responsible for the observed changes (Figure 1D, lane 5).
Figure 1.
Avr9/Cf-9–Dependent Changes in CDPK Histone Phosphorylation.
(A) Transgenic tobacco suspension cultures carrying the Cf-9 resistance gene were treated with IF(Avr9+) or IF(Avr9−), which contains (+Avr9) or does not contain (–Avr9) the Avr9 peptide. Cell samples were harvested at the indicated times (numbers above the gels) after intercellular fluid challenge, and kinase activity in total solubilized membrane extracts was analyzed by an in-gel kinase assay with histone as substrate.
(B) In-gel kinase assay as in (A) except that the kinase reaction was conducted in the presence of 2 mM EGTA.
(C) Control gel without substrate used to test for autophosphorylation. Samples and order of loading are identical to (A).
(D) Gene-for-gene specificity of the changes in CDPK histone phosphorylation. Cf-9 tobacco cells (lanes 1, 2, and 5) or the nontransgenic parental line Petite Havana (lanes 3 and 4) were treated with IF(Avr9+) (lanes 1 and 3), IF(Avr9−) (lanes 2 and 4), or synthetic Avr9 (lane 5). Cells were harvested 15 min after intercellular fluid challenge and analyzed as described in (A).
The sets of two arrows at left indicate changes in the rate of histone phosphorylation at 68 and 70 kD. The position of the 63-kD molecular mass marker is indicated at the right.
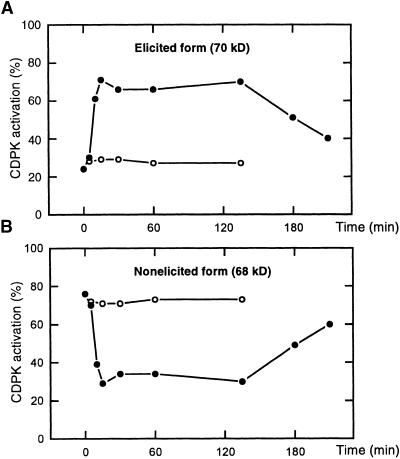
Quantitative analysis (see Methods) revealed an increase of phosphorylation activity at 70 kD from 24% (of total phosphorylation activity at 68 and 70 kD) at time zero to 70% at 15 min after IF(Avr9+) elicitation, whereas in the control experiment with IF(Avr9−), phosphorylation activity remained unaltered at 28% (Figure 2A). Complementary changes were deduced from the phosphorylation signal at 68 kD (Figure 2B). Protein kinase activity decreased from 76% before elicitation to 30% at 15 min after the Avr9 treatment. These experiments were repeated five times; although the overall kinetics remained unchanged, the proportion of phosphorylation activity at the 70-kD signal could vary from 0 to 40% at time zero and from 65 to 100% at 15 min after the addition of Avr9, depending on the cell batch. Interestingly, in contrast to the MAP kinase activation pattern, in which the transient kinase signal clearly peaks at 15 min (Romeis et al., 1999), the “activated state” of the CDPK, once established, appeared constant for nearly 2 hr before gradually returning to the “resting state.”
Figure 2.
Quantitative Analysis of the 68- and 70-kD Phosphorylation Signals.
Cf9 tobacco cells were challenged with IF(Avr9+) (closed circles) or IF(Avr9−) (open circles), and the 68- and 70-kD histone kinase activities were analyzed by the in-gel kinase assays (see Figure 1A). Signal intensities representing kinase activation of the 68- and 70-kD bands were quantitated with a PhophorImager, summed, and set at 100%. The percentage of each form to the total signal was then plotted against time.
(A) Elicited histone kinase activity at 70 kD.
(B) Nonelicited histone kinase activity at 68 kD.
The 68- and 70-kD phosphorylation signals shown in Figure 1A were no longer detectable when the in-gel assay was performed in the presence of 2 mM EGTA (Figure 1B), indicating that both phosphorylation activities are Ca2+ dependent. Also, no protein kinase activity could be seen at 68 or 70 kD when histone was omitted from the gel. This demonstrates that the observed changes in Figure 1A are attributable to phosphorylation of histone used as substrate and not to a kinase autophosphorylation (Figure 1C). Also, with the in-gel kinase protocol used (see Methods), no changes in the protein phosphorylation pattern at 68 and 70 kD were observed when histone was replaced by casein (data not shown).
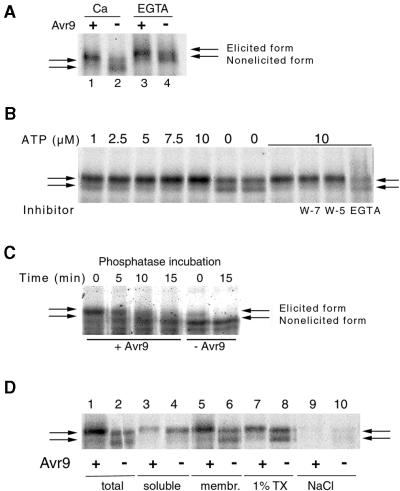
Ca2+ binding protein kinases, such as CDPKs, have been reported to migrate in gels at different rates in the Ca2+-bound versus the Ca2+-free state (Roberts and Harmon, 1992). Therefore, we tested whether the 68- and 70-kD phosphorylation signals displayed such a shift. Extracts from cells challenged with IF(Avr9+) or IF(Avr9−) for 15 min were analyzed by adding 0.5 mM Ca2+ or 1 mM EGTA to the sample buffer just before electrophoresis, and the standard in-gel assay was conducted (Figure 3A). The apparent molecular masses were 70 and 68 kD, respectively, when the sample buffer contained Ca2+. In the presence of EGTA, however, both bands shifted toward 72 and 70 kD, respectively. This suggests that the 68- and 70-kD phosphorylation signal(s) represents CDPK activity.
Figure 3.
Characterization of the 68-/70-kD CDPK.
(A) Ca2+-dependent electrophoretic shift in the in-gel kinase assay. Ca2+ (lanes 1 and 2) or EGTA (lanes 3 and 4), at a final concentration of 0.5 or 1 mM, respectively, was added to solubilized membrane extracts that already were dissolved in SDS sample buffer; the samples were then boiled and analyzed by the in-gel assay with histone. Extracts reflect cells challenged for 15 min with IF(Avr9+) (+, lanes 1 and 3) or IF(Avr9−) (−, lanes 2 and 4).
(B) In vitro interconversion from nonelicited CDPK into elicited CDPK form. Solubilized membrane extracts from nontreated cells were incubated in a kinase buffer supplemented with various concentrations of ATP for 5 min at room temperature in the absence or presence of the inhibitors W-7 (200 μM), W-5 (200 μM), or EGTA (2 mM), as indicated below panel.
(C) Transition from elicited to nonelicited CDPK form by phosphatase treatment. Solubilized membrane extracts originating from cells 15 min after elicitation with IF(Avr9+) (+) or IF(Avr9−) (–) were incubated with nonspecific λ phosphatase for the times indicated, and samples were analyzed using the in-gel kinase assay.
(D) Cell fractionation experiment. Cf9 cells challenged for 15 min with IF(Avr9+) (odd-numbered lanes) or IF(Avr9−) (even-numbered lanes) were broken, and the in-gel kinase activity toward histone was analyzed in corresponding aliquots of total crude extracts (lanes 1 and 2), soluble fractions (lanes 3 and 4), membrane fractions (lanes 5 and 6), and supernatants from membranes solubilized with 1% Triton X-100 (lanes 7 and 8) or treated with 0.5 M NaCl (lanes 9 and 10).
The sets of two arrows at left and right indicate the elicited and nonelicited CDPK forms.
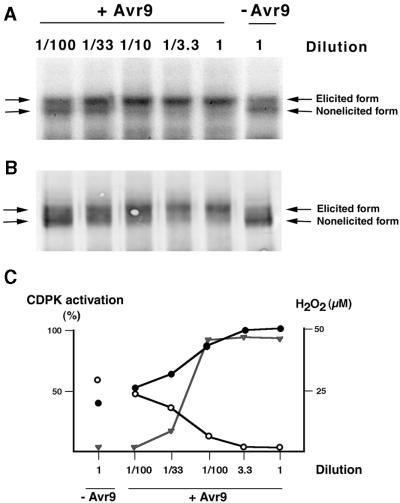
The Avr9/Cf-9–dependent induction of the 70-kD band paralleled by the decrease in the 68-kD signal was confirmed in a dose–response experiment (Figure 4) in which Cf9 cells were challenged with either IF(Avr9−) or various dilutions of IF(Avr9+) for 15 min. The proportion of the 70-kD kinase increased with Avr9 concentrations (Figure 4A), whereas at the same time, no signal was detectable at 68 kD. An immunoblot with affinity-purified antibodies directed against the calmodulin-like domain of soybean CDPKα revealed that both the 68- and 70-kD enzymes cross-react with the antibody (Figure 4B). Remarkably, the staining pattern paralleled the proportions of phosphorylation signals of the in-gel kinase assay (Figure 4A). Increasing the concentration of IF(Avr9+) resulted in a stronger protein signal at 70 kD, whereas the signal at 68 kD concomitantly vanished.
Figure 4.
Dose–Response Curve of CDPK Interconversion.
Cf9 suspension cells were challenged with various dilutions of IF(Avr9+) as indicated above the lanes or with IF(Avr9−), harvested 15 min after elicitation, and treated to prepare solubilized membrane extracts.
(A) CDPK interconversion analyzed by the in-gel kinase assay with histone as substrate. The sets of arrows at left and right indicate the electrophoretic mobilities of nonelicited and elicited CDPK forms.
(B) Immunoblot with antibody to CDPK. Samples identical to those described in (A) were separated on an SDS gel, the proteins were transferred onto nitrocellulose, and the blots were probed with an affinity-purified polyclonal antiserum directed against the calmodulin-like domain from soybean CDPK. Arrows are as described for (A).
(C) Correlation between CDPK activation and AOS production. The relative CDPK activation, given as an increase in the elicited or slower migrating form (filled circles) or a decrease in the nonelicited or faster migrating form (open circles), was determined with a PhosphorImager and plotted against the corresponding dilutions. AOS synthesis (triangles) was detected by the ferricyanide-catalyzed oxidation of luminol.
+Avr9, IF(Avr9+); −Avr 9, IF(Avr9−).
The 68- and 70-kD Kinase Activities Reflect Distinct Forms of a Membrane-Anchored CDPK
The 70- and 68-kD kinase activities may be interpreted as two CDPKs that become activated and inactivated in response to Avr9 elicitation, respectively. Alternatively, because the increase of the 70-kD signal corresponds exactly with the decrease in phosphorylation activity at 68 kD, both may reflect different activation states of one enzyme. As assessed by the in-gel assay, both CDPK forms phosphorylate histone. In samples elicited with Avr9, the electrophoretic mobility of the signal at 68 kD was shifted toward 70 kD. This shift could not be explained by the absence or presence of Ca2+ alone, as shown in Figure 3A, but may reflect a phosphorylation event.
To distinguish between these two interpretations, we incubated solubilized membrane samples from cells elicited for 15 min with IF(Avr9+) or IF(Avr9−) with nonspecific λ phosphatase. With increasing incubation time, the phosphorylation signal shifted from 70 kD back to 68 kD (Figure 3C); however, this return shift was not observed in the presence of the phosphatase inhibitor vanadate (data not shown). In a complementary experiment, a membrane sample from nontreated cells was incubated in a suitable kinase buffer with ATP. In vitro, the nonelicited 68-kD form interconverted into the elicited 70-kD form (Figure 3B); the transition was compromised by 2 mM EGTA but not by the calmodulin antagonist N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7). This confirmed that the transition from nonelicited to elicited CDPK was caused by a phosphorylation event.
A cell fractionation study revealed that substantial amounts of both the elicited and nonelicited CDPK forms were localized in the membrane fraction (Figure 3D, lanes 5 and 6) and could be solubilized by treatment with detergent (lanes 7 and 8) but not with 0.5 M NaCl (lanes 9 and 10). Some histone phosphorylation activity at ∼70 kD was seen in the soluble fraction as well (Figure 3D, lanes 3 and 4), possibly through the action of a CDPK isoenzyme of similar molecular mass that possesses a constitutive, Avr9-independent phosphorylation activity. Additional CDPK signals could be distinguished from the 68-/70-kD enzyme by protein gel blot analysis after separation on a high-resolution SDS gel (data not shown).
Enzymatic Activities of Elicited and Nonelicited CDPK
We next addressed whether the Avr9-induced transition from the nonelicited 68-kD form to the elicited 70-kD form, caused by a phosphorylation event, was accompanied by an increase in catalytic activity. For example, phosphorylation of MAP kinases by an upstream MAP kinase kinase increases the enzymatic activity of the MAP kinase.
Solubilized membrane extracts from cells challenged with or without Avr9 were prepared, and protein kinase activity toward the peptide syntide-2 was determined (Figures 5A and 5B). Syntide-2 was reported previously to be utilized by CDPKs (Huang et al., 1996; Yoo and Harmon, 1996; Lee et al., 1998). No substantial difference in kinase activity between elicited and nonelicited extracts was observed in the absence of Ca2+ or in the presence of EGTA (Figure 5A, bars 1 and 3 and bars 4 and 6). In contrast, adding 1 mM Ca2+ increased the enzyme activity in the elicited extract by >100%, whereas only a slight increase was detectable in the nonelicited extract (bars 2). Remarkably, after preincubation with 10 μM ATP, an increase in the Ca2+-dependent enzyme activity occurred in both samples (Figure 5A, bars 5). As shown in Figure 3B, such ATP preincubation induced a transition from the 68- to the 70-kD CDPK form in nonelicited extracts in vitro. This is consistent with interpreting our kinetic data as showing that the transition between the two CDPK forms is accompanied by an increase in enzymatic activity. Activation of CDPK enzyme activity after ATP preincubation also has been reported by others (Bögre et al., 1988). The kinase assays were conducted in duplicate, and the experiment was repeated independently three times.
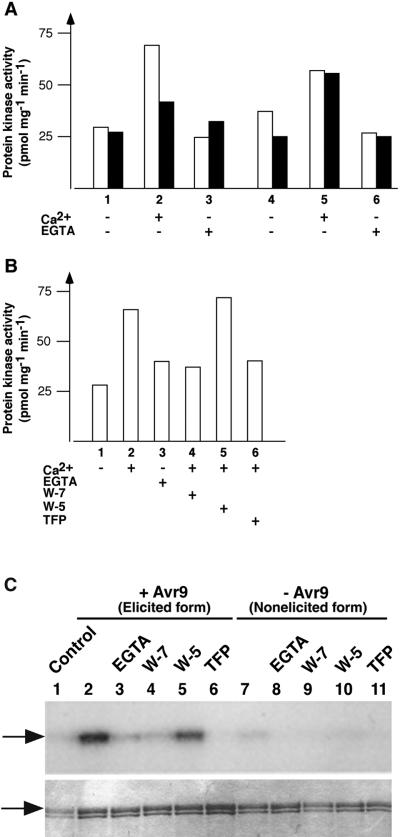
Figure 5.
Ca2+-Dependent Enzyme Activity of Solubilized Membrane Extracts and Gel-Eluted 68-/70-kD CDPK.
Cf9 suspension cells were elicited with IF(Avr9+) (open bars) or IF(Avr9−) (filled bars) for 15 min (as shown in [A] and [B]), and solubilized membrane extracts were prepared.
(A) Kinase activity of solubilized membrane extracts toward the peptide substrate syntide-2 (10 μM) was determined in the presence (+) or absence (–) of Ca2+ (1 mM) and EGTA (2.5 mM) as indicated. Reactions were incubated for 5 min at 29°C in the presence of ATP (10 μM; bars 1 to 3) or after preincubation with ATP for 10 min at room temperature (10 μM; bars 4 to 6). The bars represent the mean value of duplicates.
(B) Effect of inhibitors on the Ca2+-dependent protein kinase activity. Membrane extracts from elicited cells were incubated with Ca2+, EGTA, or the inhibitors W-7 (250 μM), W-5 (250 μM), or TFP (250 μM), as described in (A).
(C) Membrane extracts from elicited and nonelicited samples were separated on an SDS gel, and proteins between 63 and 80 kD, including the 68-/70-kD CDPK, were eluted from cut gel slices. In vitro kinase assays with histone (100 μg/mL) as a substrate were conducted with the eluates alone (lanes 2 and 7) or in the presence of EGTA (2 mM), W-7, W-5, or TFP, as described in (B), and samples were separated on an SDS gel. Arrows indicate the position of histone in the autoradiograph (top) and Coomassie Brilliant Blue R 250 stain (bottom).
Further evidence that the Ca2+-dependent increase in enzymatic activity in the elicited sample was the result of CDPK activity was obtained from studies with various antagonists of protein kinase C and calmodulin, which have been reported also to inhibit CDPKs (Figure 5B; Harmon et al., 1987; Li et al., 1998). In the presence of 250 μM W-7 (Figure 5B, bar 4) or trifluoperazine dimaleate (TFP; bar 6), kinase activity was reduced to its background value (shown without Ca2+ or in presence of EGTA; bars 1 and 3). Ca2+-dependent kinase activity was not compromised with 250 μM N-(6-aminohexyl)-1-naphthalenesulfonamide (W-5), the less active isoform of W-7 (Figure 5B; cf. bars 2 and 5).
To further test Avr9/Cf-9–dependent CDPK activation, we separated elicited and nonelicited solubilized membrane extracts on an SDS gel, proteins between 63 and 80 kD, including the 68-/70-kD CDPK, and eluted them from cut gel slices (see Methods), and the eluates were incubated with histone and γ-32P-ATP for 5 min in the presence of the previously mentioned inhibitors. Histone was chosen as the substrate because gel eluate supernatants prevented binding of the syntide-2 peptide to phosphocellulose paper. Phosphorylation of histone was analyzed by SDS gel electrophoresis and autoradiography, and equal loading was confirmed by protein staining (Figure 5C). Whereas the elicited sample efficiently phosphorylated histone (Figure 5C, lane 2), the nonelicited sample displayed little activity under these assay conditions (lane 7)—differences unlikely to be caused by unequal protein renaturation of the respective CDPK forms. The same protocol and buffers were used for the in-gel kinase assays shown in Figure 1 (except that the incubation time was 90 min), in which both forms showed identical activities (see Discussion). Also, for gel-eluted CDPK, phosphorylation activity was compromised in the presence of 2 mM EGTA (Figure 5C, lanes 3 and 8), 250 μM W-7 (lanes 4 and 9), or TFP (lanes 6 and 11) but not by W-5 (lanes 5 and 10). These data provide additional evidence that the Avr9-induced 68-/70-kD enzyme identified by the in-gel kinase assay is a CDPK and suggest that the elicited 70-kD CDPK form is more active than the nonelicited form.
Avr9/Cf-9–Dependent CDPK Interconversion and AOS Production
Synthesis of AOS generally is considered to be one of the earliest defense responses in plants. Recently, Piedras et al. (1998) reported an Avr9/Cf-9–dependent accumulation of H2O2 in tobacco cell cultures that required Ca2+ influx and was compromised in the presence of the protein kinase inhibitors staurosporin, W-7, and TFP. A dose–response experiment revealed that the Avr9 concentration-dependent increase in the elicited form and a decrease in the nonelicited form were correlated with the synthesis of AOS (Figure 4C).
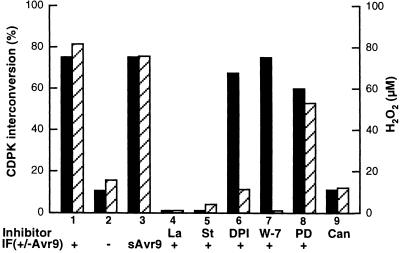
To locate the identified CDPK in an intracellular signaling pathway and to investigate whether the enzyme might be required for the Avr9-induced AOS production, we challenged Cf9 cell cultures with IF(Avr9+), IF(Avr9–), or chemically synthesized Avr9 in the presence of various inhibitors, and samples were analyzed 15 min after elicitation (Figure 6, lanes 1 to 8). Elicitation with IF(Avr9+) or chemically synthesized Avr9 induced CDPK transition into the 70-kD form as well as AOS production (Figure 6, bars 1 and 3). No response could be detected with IF(Avr9–) (Figure 6, bars 2); also, no transition was observed 30 min (data not shown) or 60 min (Figure 6, bars 9) after the phosphatase inhibitor cantharidin was added in the absence of elicitor. La3+, an inhibitor of plasma membrane Ca2+ channels, and the protein kinase inhibitor staurosporine compromised both responses (Figure 6, bars 4 and 5), whereas 2′-amino-3′-methoxyflavone (PD98059), an inhibitor of MAP kinase kinases, had no effect on either of them (bar 8).
Figure 6.
Influence of Inhibitors on the Avr9/Cf-9–Dependent CDPK Interconversion.
Transgenic Cf9 tobacco suspension cells were treated with IF(Avr9+) (+; bars 1 and 4 to 8), IF(Avr9−) (−; bars 2), and synthetic Avr9 peptide (sAvr9; bars 3) in the presence of inhibitors of various signaling processes and were harvested 15 min after elicitation (bars 1 to 8). CDPK interconversion was determined by the in-gel kinase assay and is given as a percentage of the elicited (slower migrating) form (filled bars) of the total activity. AOS synthesis was determined by the ferricyanide-catalyzed oxidation of luminol (striped bars). The inhibitors LaCl (La, 500 μM; bars 4), staurosporine (St, 25 μM; bars 5), and diphenyleneiodonium chloride (DPI, 0.8 μM; bars 6) were added 5 min before elicitation with IF(Avr9+); W-7 (250 μM; bars 7) and PD98059 (PD, 250 μM; bars 8) were added 10 min before elicitation. Cantharidin (Can, 5 μM; bars 9) was added in the absence of IF(Avr9+), and cells were harvested after 60 min. Data reflecting an elicitor/inhibitor pair were repeated in at least three independent experimental series, and although the absolute values of the H2O2 produced and the CDPK activation ratio might vary slightly in different cell batches, activation/transition patterns were always similar.
Diphenyleneiodonium chloride abolished AOS production (Piedras et al., 1998). However, CDPK interconversion still occurred (Figure 6, bars 6). Both inhibited synthesis of AOS and an unaltered CDPK shift were observed in the presence of W-7 (Figure 6, bars 7). Because W-7 is known to inhibit CDPKs, our data therefore suggest that the phosphorylation event causing the Avr9-dependent transition from nonelicited to elicited CDPK form is unlikely to be catalyzed by an Avr9-dependent CDPK autophosphorylation. Furthermore, the fact that the Avr9-induced AOS synthesis as well as activation of MAP kinases was compromised in the presence of W-7 (Piedras et al., 1998; Romeis et al., 1999) seems to locate the 68-/70-kD CDPK upstream from the activation of AOS synthesis. However, W-7, although frequently used for the inhibition of CDPK activity in vitro, also compromised the enzymatic activity of other Ca2+/calmodulin–dependent enzymes and of protein kinase C, and it also may block the function of other, uncharacterized, signaling components that might be required for AOS synthesis. Thus, the 68-/70-kD CDPK also may be located in a signaling pathway independent of that activating the Avr9-induced AOS synthesis.
DISCUSSION
Function of 68-/70-kD CDPK in the Plant Defense Response
We have identified a 68-/70-kD CDPK that is involved in the Avr9/Cf-9 gene-for-gene–dependent signal transduction and thus provides clear evidence that a CDPK isoform participates in defense-related signaling. The enzyme is located in the particulate fraction and can be solubilized from membranes by short treatment with 1% Triton X-100 or other mild detergents (data not shown) but not with 0.5 M NaCl (Figure 3D). This is consistent with the fact that several previously characterized CDPKs from different species contain an N-terminal myristoylation site (Hrabak et al., 1996). In vitro myristoylation was shown for a cloned CDPK from zucchini (Ellard-Ivey et al., 1999). Interestingly, the closest homolog to that enzyme is a CDPK of 70 kD, namely, AtCPK1 from Arabidopsis.
The elucidation of a potential cellular function of the 68-/70-kD CDPK requires identification of its in vivo substrates. The enzyme might phosphorylate transcription factors. A pathogen-induced kinase is responsible for the activation of a basic leucine zipper transcription factor G/HBF-1, enabling its binding to the chalcone synthase chs15 promoter from soybean (Dröge-Laser et al., 1997). Also, phosphorylation—supposedly attributable to a protein kinase C—was reported for nuclear factor PBF-1 from potato, which is required for pathogenesis-related induction of the PR-10a gene (Després et al., 1995; Subramaniam et al., 1997). Furthermore, CDPK isoforms from Arabidopsis promoted the activation of a drought stress–inducible promoter after being transiently expressed in a heterologous maize protoplast system (Sheen, 1996).
Some in vivo substrates of the identified 68-/70-kD CDPK also might be located in the membrane. In vitro experiments with fava bean guard cells demonstrated that a 57-kD CDPK phosphorylated the KAT1 K+ channel (Li et al., 1998). Likewise, a (tonoplast) Cl− channel from the same system could be activated by a recombinant CDPK isoform from Arabidopsis (Pei et al., 1996). Interestingly, impaled guard cells from Cf9 tobacco responded to Avr9 elicitation with a 2.5- to 3.0-fold activation of an outward-rectifying K+ channel and an almost complete inactivation of an inward-rectifying K+ channel. Both responses were prevented in the presence of the protein kinase inhibitor staurosporine (Blatt et al., 1999). However, whereas these ion flux changes became evident within 3 to 5 min after elicitation, activation of the 68-/70-kD CDPK did not occur before 5 min after Avr9 challenge; thus, the K+ channel is less likely to be an in vivo substrate.
Another potential target of the Avr9-induced 68-/70-kD CDPK might be the plasma membrane H+-ATPase. Elicitation of Cf9 tobacco cell cultures with Avr9 resulted in changes of H+ fluxes, detectable as media alkalinization, which could be observed within 20 min after elicitation. These fluxes supposedly are accomplished by way of inactivating an H+-ATPase (Piedras et al., 1998). Reversible phosphorylation of an H+-ATPase in response to a fungal elicitor was described for the same pathosystem (Xing et al., 1996), and H+-ATPase regulation by way of CDPKs has been discussed previously (Schaller et al., 1992; Camoni et al., 1998b; Schaller and Oecking, 1999). Remarkably, regulation of H+-ATPases also appears to depend on the presence or absence of 14-3-3 proteins (Sehnke and Ferl, 1996; Chung et al., 1999; Fuglsang et al., 1999). Members of this highly conserved class of eukaryotic proteins recognize phosphate-bearing amino acids, are transiently associated with enzymes, and regulate enzyme function. The Avr9/Cf-9–dependent accumulation of 14-3-3 transcripts in tomato suggests that these proteins also participate in activation of the gene-for-gene specific defense response (Roberts and Bowles, 1999). Although no in vivo evidence is available, we are tempted to speculate that a functional link for Avr9/Cf-9–dependent 68-/70-kD CDPK, H+-ATPase, and the 14-3-3 protein is required to trigger the plant defense. An in vitro interaction between a (phosphorylated) CDPK (70-kD CPK1) and 14-3-3 isoforms from Arabidopsis (Camoni et al., 1998a) provides additional evidence for such a hypothesis.
Is the 68-/70-kD CDPK Involved in Activation of an NADPH Oxidase?
Cf9 tobacco responds to Avr9 elicitation with a rapid synthesis of AOS (Piedras et al., 1998). We and other groups have shown that the accumulation of superoxide and hydrogen peroxide requires a Ca2+ influx and protein kinase activity (Lamb and Dixon, 1997; Keller et al., 1998; Piedras et al., 1998; Scheel, 1998; Torres et al., 1998; Bolwell, 1999; Romeis et al., 1999). It has been postulated that AOS production is catalyzed by a plant NADPH oxidase, and one might envisage that this enzyme could become phosphorylated by a CDPK. Indeed, our inhibitor studies are consistent with the 68-/70-kD CDPK being located upstream in a signaling pathway that leads to the induction of AOS synthesis. However, because the naphthylsulfonamide W-7 (and W-5) cannot be considered a CDPK-specific inhibitor, a role for other mechanisms in NADPH oxidase activation cannot be ruled out.
Previously, a CDPK was postulated to phosphorylate NADPH oxidase subunits in response to pathogen-related stimuli by way of an activation mechanism identical to that described for the animal system (Xing et al., 1997; Blumwald et al., 1998). The cloning of several plant NADPH oxidases has shown that these enzymes embody different structures and, importantly, that the plant enzymes also possess an N-terminal extension with two (functional) Ca2+ binding motifs (Torres et al., 1998; Keller et al., 1998), so this model might have to be revised. Nevertheless, because CDPK phosphorylation signature sites exist in the derived amino acid sequences of the cloned plant NADPH oxidases, it will be interesting for future experiments to test whether direct phosphorylation accomplished by way of a CDPK is involved in the activation of an NADPH oxidase.
In Vivo Interconversion of the 68-/70-kD CDPK Forms Involves Phosphorylation
By using a modified in-gel kinase assay protocol with histone as an artificial substrate, we were able to follow the in vivo CDPK transition between nonelicited and elicited forms over the time course after challenge with Avr9 (Figure 1A). The transition between the two CDPK forms, accomplished by way of protein phosphorylation, could be mimicked in vitro by either phosphatase treatment (Figure 3C) or incubating the nonelicited form with excess ATP (Figure 3B).
According to the results of the in-gel kinase assay, both CDPK forms appeared to be equally active, as judged by the phosphorylation signal intensity in the in-gel kinase assay before and after the transition (Figure 1A). However, the experimental conditions chosen for the in-gel assay would be sufficient to induce an in vitro transition from the nonelicited to the elicited form, which is similar to what was demonstrated in Figure 3B, and subsequently did not allow the comparison of enzymatic parameters between the two CDPK forms. Protein kinase assays with gel-eluted CDPK revealed increased enzymatic activity of the elicited sample compared with the nonelicited sample. The elicited membrane extract also showed a higher in vitro kinase activity with syntide-2 as artificial CDPK substrate than did the nonelicited form. No difference in Ca2+-dependent and EGTA-inhibitable protein kinase activity between elicited and nonelicited extract was detectable, however, when the samples were preincubated with ATP (Figure 5A). These data suggest that Avr9-induced phosphorylation and subsequent interconversion of CDPK forms are accompanied by an increase in CDPK enzyme activity. However, only the cloning of the corresponding gene coding for the 68-/70-kD CDPK and subsequent experiments with either specific antibodies or tagged enzyme versions will allow investigators to differentiate the contribution of this isoenzyme to various biochemical parameters from those contributed by the other CDPK family members.
As shown by elegant biochemical studies with recombinant enzymes, in its resting state, CDPK is autoinhibited by an interaction of a pseudosubstrate site within its junction domain that blocks the active site of the kinase domain (Huang et al., 1996; Yoo and Harmon, 1996; Lee et al., 1998). Binding of Ca2+ to the calmodulin-like domain of the CDPK causes a conformational change that extends to the adjacent junction domain and finally disengages that autoinhibitor of the active site. Purified and recombinant CDPKs were reported to autophosphorylate at serine and threonine residues (Satterlee and Sussman, 1998), and preincubation of a soybean CDPK with ATP resulted in an increase in enzymatic activity toward histone (Putnam-Evans et al., 1987; Bögre et al., 1988).
When we integrated our data obtained with the Avr9/Cf-9 gene-for-gene interaction system into that model for CDPK activation, the following picture could be envisaged. In nonelicited cells, CDPK remains basically in its 68-kD form. Challenge with Avr9 causes an Avr9/Cf-9–mediated increase in the cytosolic free Ca2+ concentration (M. Blatt, A. Grabov, A. Brearley, and J.D.G. Jones, unpublished data). The conformational change allows the enzyme to become a substrate for phosphorylation and within 5 min after elicitation to interconvert from its 68-kD into the 70-kD form. The fact that the Avr9-induced phosphorylation and subsequent transition into the 70-kD form are not inhibited by W-7, neither in vivo (Figure 6, bars 7) nor in vitro (Figure 3B), argues against CDPK autophosphorylation and suggests that this step is catalyzed by an unknown upstream protein kinase. Also, although CDPK autophosphorylation has been reported for recombinant and purified enzymes, we were unable to detect any 68-/70-kD phosphorylation signals in our in-gel kinase assays, even after prolonged exposure. The 70-kD CDPK then phosphorylates its target protein(s). Inactivation of the CDPK, which occurred 60 to 120 min after Avr9 elicitation, may be achieved by way of a protein phosphatase. The transition back to the 68-kD CDPK form was observed in the in-gel assay (Figure 1A) and during protein gel blot analysis (data not shown) in which the 68-kD protein band became detectable again. Alternately, the elicited CDPK became proteolytically degraded, and the increase in 68-kD signal after 90 min was attributable to newly synthesized CDPK. The presence of conserved motifs for protein degradation (PEST sequences; Rechsteiner and Rogers, 1996), which has been discussed for some of the high molecular mass CDPKs from Arabidopsis (Satterlee and Sussman, 1998), may argue in favor of the latter hypothesis.
In an alternate scenario, the challenge with Avr9 and the subsequent signaling steps presumably could result in the inactivation of a protein phosphatase, which then would enable the phosphorylated 70-kD CDPK form to accumulate. However, the fact that no CDPK interconversion was observed after addition of the phosphatase inhibitor cantharidin renders this hypothesis less likely.
Function of CDPKs in Multiple Signaling Pathways
Environmental stresses, such as drought, salt, and cold exposure, have been shown to result in accumulation of CDPK transcripts (Urao et al., 1994; Tähtiharju et al., 1997). Likewise, an increase in mRNA of some CDPK isoforms appears to occur after nonspecific elicitation in tobacco cell cultures (Yoon et al., 1999). CDPK kinase activity has been correlated with osmotic and metabolic stress (Takahashi et al., 1997; Iwata et al., 1998). However, in only one study to date has a specific CDPK isoform been linked to an in vivo function and shown to induce an environmental stress–related inducible promoter in response to treatment with abscisic acid (Sheen, 1996; Harmon, 1997).
We have identified a 68-/70-kD CDPK that becomes activated in a gene-for-gene–dependent manner, suggesting that it participates in the Avr9/Cf-9–mediated signaling to activate the plant defense. According to preliminary reverse genetics experiments in which virus-induced gene silencing was applied for CDPK analysis, the Avr9/Cf-9–dependent hypersensitive response observed in the CDPK-silenced plants was less than in control plants (T. Romeis and J.D.G. Jones, unpublished data). However, only the cloning of the corresponding gene for this specific enzyme and subsequent rigorous biochemical and genetic analyses will allow definition of the specific role of the enzyme in activating the plant defense response. The reverse genetic analysis of CDPK isoforms was launched by identifying Arabidopsis mutant lines that carry T-DNA insertions (Krysan et al., 1996). Dissecting the role of single members of the CDPK multigene family in specific intracellular signaling processes remains the fascinating and progressively more tractable objective.
METHODS
Tobacco Cell Culture Conditions and Treatments
Suspension cultures from Nicotiana tabacum cv Petite Havana and the derived Cf9 line (Piedras et al., 1998) were subcultured at 2-week intervals by a 1:10 dilution in Murashige and Skoog (1962) medium supplemented with 3% sucrose, B-5 vitamins, 2,4-dichlorophenoxyacetic acid (1 mg/mL), and kinetin (0.1 mg/mL), pH 5.7. Log-phase cells were used 4 days after the dilution. Before the experiment, the cells were washed three times with assay buffer (5 mM Mes, pH 6.0, 175 mM mannitol, 0.5 mM CaCl2, and 0.5 mM K2SO4), with 30-min intervals of shaking between the single washing steps; the cells then were distributed into single flasks (1 g of cells per 20 mL) and allowed to equilibrate in the shaker for 3 hr at 22°C and 180 rpm (Piedras et al., 1998).
For elicitation, cells were challenged with 75 μL of intercellular fluid originating from transgenic tobacco that produces the Avr9 peptide apoplastically (IF[Avr9+]) or with control intercellular fluid lacking Avr9 (IF[Avr9−]) (Hammond-Kosack et al., 1998). At the times indicated, cells were harvested by filtration, immediately frozen in liquid nitrogen, and stored at −70°C.
For studying the effect of various inhibitors, we added the compounds either 5 min before elicitation with Avr9 (0.5 mM lanthanum chloride, 0.8 μM diphenyleneiodonium chloride, or 25 μM staurosporine) or 10 min before elicitation (250 μM N-[6-aminohexyl]-5-chloro-1-naphthalenesulfonamide [W-7] or 250 μM 2'-amino-3'-methoxyflavone [Calbiochem-Novabiochem, San Diego, CA]). Cantharidin was added at 5 μM, and cells were harvested after 60 min. If not otherwise mentioned, all compounds were purchased from Sigma Chemicals (Poole, UK). The synthetic Avr9 peptide was used at a concentration of 10 nM (Piedras et al., 1998).
Determination of Active Oxygen Species
Aliquots (0.2 mL) of all cell samples analyzed for protein kinase activity were tested simultaneously for the production of active oxygen species (AOS) by using the ferricyanide-catalyzed oxidation of luminol as described previously (Schwacke and Hager, 1992; Piedras et al., 1998).
Preparation of Protein Extracts
Cell samples were ground in liquid nitrogen, thawed in two volumes of extraction buffer (50 mM Hepes, pH 7.4, 5 mM EDTA, 5 mM EGTA, 5 mM DTT, 10 mM NaF, 10 mM Na3VO4, 50 mM β-glycerophosphate, 1 mM 4-[2-aminoethyl]-benzenesulfonyl fluoride [AEBSF], 2 μg/mL antipain, 2 μg/mL aprotinin, and 2 μg/mL leupeptin), and centrifuged at 15,000g for 20 min at 4°C in a microcentrifuge. The pellet was resuspended in 2 volumes of elution buffer (20 mM Hepes, pH 7.4, 1 mM MgCl2, 1% Triton X-100, 1 mM NaF, 1 mM Na2VO4, 5 mM β-glycerophosphate, 1 mM AEBSF, 1 μg/mL antipain, 1 μg/mL aprotinin, and 1 μg/mL leupeptin) and incubated for 15 min at 6°C with end-over-end rotating. After centrifugation (as above), the crude solubilized membrane extracts were stored at −70°C.
The protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce, Chester, UK) with BSA as a standard.
In-Gel Kinase Assay
The in-gel kinase assay was conducted primarily as described previously (Romeis et al., 1999). Briefly, 16 μg of total protein per lane was separated on a 10.5% SDS–polyacrylamide gel embedded with 0.25 mg/mL histone (type III-SS; Sigma) as a kinase substrate, and the gels were run for 90 min to ensure high resolution (Mini-Protean II system; Bio-Rad). After electrophoresis, SDS was removed by two washings (30 min each) in buffer A (50 mM Tris-HCl, pH 8.0, and 20% isopropanol), followed by two washings in buffer B (50 mM Tris-HCl, pH 8.0, and 5 mM β-mercaptoethanol). The proteins in the gel were denatured in buffer C (50 mM Tris-HCl, pH 8.0, 5 mM β-mercaptoethanol, and 6 M guanidinium chloride) for 1 hr at room temperature and were renatured overnight at 4°C in buffer D (50 mM Tris-HCl, pH 8.0, 5 mM β-mercaptoethanol, and 0.04% Tween). After equilibration in buffer E (40 mM Hepes, pH 7.4, 2 mM DTT, 15 mM MgCl2, and 0.1 mM EGTA) for 30 min at room temperature, the gel was incubated in 10 mL of buffer E that included 1.85 MBq (50 μCi) γ-33P-ATP (92 TBq/mmol; Amersham Pharmacia Biotech, Little Chalfont, UK) for 90 min at room temperature. For testing whether the in-gel activity was dependent on Ca2+ ions, buffer E also contained 2 mM EGTA.
The reaction was stopped by transferring the gel into a solution of 5% trichloroacetic acid (w/v) and 1% phosphoric acid (v/v). Unincorporated γ-33P-ATP was removed by an intensive washing for 3 hr with at least six changes of the same solution. The gels were dried onto Whatman 3MM paper (Whatman, Maidstone, UK), exposed to Kodak X-OMAT film (Sigma), and analyzed with a PhosphorImager (Stratagene, La Jolla, CA). To determine relative calcium-dependent protein kinase (CDPK) activation, we summed the signal intensities of elicited (slower migrating) and nonelicited (faster migrating) CDPK in each individual sample and considered them as 100%. CDPK activation thus was given as the percentage of elicited signal to total signal. The size of protein kinases was estimated by using various different prestained molecular mass markers.
In Vitro Interconversion Experiments
For the phosphatase (shift down) experiment, cells were treated with IF(Avr9+) or IF(Avr9−) for 15 min, harvested, and broken as described above except that NaF, Na3VO4, and β-glycerophosphate were omitted from the extraction and elution buffers. Twenty-five microliters of solubilized membrane extracts was incubated with 400 units of nonspecific λ phosphatase (New England Biolabs, Beverly, MA) in buffer supplied by the manufacturer at room temperature (30 μL final volume); reactions were stopped at the times indicated by adding sample buffer that included 2 mM Na3VO4 (final concentration). Samples were analyzed by the in-gel kinase assay as described above.
To induce transition from the nonelicited into the elicited CDPK form, we incubated solubilized membrane extracts (20 μg) from untreated cells resuspended in elution buffer (see above) for 5 min at room temperature with 0, 1, 2.5, 5, 7.5, or 10 μM ATP or with 10 μM ATP plus 2 mM EGTA, 250 μM W-7, or 250 μM N-(6-aminohexyl)-1-naphthalenesulfonamide (W-5). Reactions were stopped by the addition of sample buffer and analyzed by the in-gel kinase assay.
Cell Fractionation
Cells (1 g) were treated with IF(Avr9+) or IF(Avr9−) for 15 min, harvested, broken, and resuspended in extraction buffer (0.7 mL) containing 1 mM MgCl but omitting EDTA and EGTA. The supernatant after centrifugation of the crude extract for 30 min at 100,000g and 4°C represents the soluble fraction. The membrane pellet then was resuspended in 0.7 mL of elution buffer without Triton X-100, and aliquots were treated with either 1% Triton X-100 or 0.5 M NaCl for 10 min on ice and centrifuged again. Supernatants of these solubilized membrane extracts were used for analysis.
In Vitro Kinase Assays
Kinase activity was determined in 30 μL of buffer E containing 4 μg of protein from elicited or nonelicited solubilized membrane extract. Reactions were started by adding the substrates syntide-2 (10 μM) and 10 μM ATP plus 74 kBq (2 μCi) of γ-32P-ATP (110 TBq/mmol; Amersham Pharmacia Biotech) for 5 min at room temperature, except as otherwise stated, and stopped by pipetting 20 μL of the reaction mixtures on P81 phosphocellulose paper squares (1.5 × 1.5 cm; Millipore, Bedford, MA). The filter pieces immediately were immersed in 1% phosphoric acid, washed five times for 5 min each, dried, and subjected to scintillation counting. For inhibition studies, enzymatic activity was measured in the presence of 1 mM Ca2+, 2.5 mM EGTA, 250 μM W-7 or W-5, or 250 μM trifluoperazine dimaleate (TFP; Calbiochem). Experiments were performed in duplicate.
For kinase assays with gel-purified CDPK, membrane extracts were separated on an SDS gel as in the in-gel assay protocol (above) except that no kinase substrate was incorporated. After incubation in buffer E, gel slices containing proteins between 63 and 80 kD were cut from the rest of the gel, macerated with a pestle, and incubated with two volumes of buffer E for 15 min at room temperature. After centrifugation for 5 min at 15,000g and room temperature in a microcentrifuge, the supernatant was used for kinase assays. To determine kinase activity, we incubated 40 μL of reaction mixture in buffer E containing 30 μL of enzyme supernatant (corresponding originally to 16 μg of solubilized membrane extract), 100 μg/mL histone, and 0.19 MBq (5 μCi) of γ-32P-ATP for 5 min at room temperature and stopped it by the addition of sample buffer. For inhibition studies, the enzymatic activity was measured in the presence of 2 mM EGTA, W-7 or W-5, or TFP, as described above. Aliquots were separated on a 15% SDS gel, and the phosphorylation of histone was visualized by autoradiography.
Immunoblotting
Crude extracts (16 μg of total protein per lane) were separated on a 10.5% SDS gel, and the proteins were transferred onto nitrocellulose (Amersham) by wet electroblotting (Mini-Protean II system; Bio-Rad). The blots were blocked in TBS buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) with 2% BSA, Fraction V (Sigma), for 1 hr at room temperature and probed with an affinity-purified polyclonal antiserum directed against the calmodulin-like domain from soybean CDPKα (A.C. Harmon, unpublished results) in a 1:2000 dilution in TBS at 4°C overnight. Alkaline phosphatase–conjugated goat anti–rabbit IgG (1:4000 dilution; Sigma) was used as a secondary antibody, and the reaction was visualized by hydrolysis of tetrazolium-5-bromo-4-chloro-3-indolyl phosphate as substrate (Sigma).
Acknowledgments
We thank Alice Harmon (Gainesville, FL) for providing the CDPK antibody. We are grateful to Matthew Smoker for the propagation of cell cultures and to Alan Cavill for assistance with the figures. This work was supported by the Gatsby Charitable Foundation and the Calcium and Activated Oxygens as Signals for Stress Tolerance project (Grant No. BIO-96-0101). T.R. was supported by a European Molecular Biology fellowship.
References
- Blatt, M.R., Grabov, A., Brearley, J., Hammond-Kosack, K., and Jones, J.D.G. (1999). K+ channels of Cf-9-transgenic tobacco guard cells as targets for Cladosporium fulvum Avr9. Plant J. 19, 453–462. [DOI] [PubMed] [Google Scholar]
- Blumwald, E., Aharon, G.S., and Lam, B.C.-H. (1998). Early signal transduction pathways in plant–pathogen interactions. Trends Plant Sci. 3, 342–346. [Google Scholar]
- Bögre, L., Oláh, Z., and Dudits, D. (1988). Ca2+-dependent protein kinase from alfalfa (Medicago varia): Partial purification and autophosphorylation. Plant Sci. 85, 135–144. [Google Scholar]
- Bolwell, G.P. (1999). Role of active oxygen species and NO in plant defense responses. Curr. Opin. Plant Biol. 2, 287–294. [DOI] [PubMed] [Google Scholar]
- Camoni, L., Harper, J.H., and Palmgren, M.G. (1998. a). 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett. 430, 381–384. [DOI] [PubMed] [Google Scholar]
- Camoni, L., Fullone, M.R., Marra, M., and Aducci, P. (1998. b). The plasma membrane H+-ATPase from maize roots is phosphorylated in the C-terminal domain by a calcium-dependent protein kinase. Physiol. Plant. 104, 549–555. [Google Scholar]
- Chandra, S., and Low, P.S. (1997). Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J. Biol. Chem. 272, 28274–28280. [DOI] [PubMed] [Google Scholar]
- Chung, H.-J., Sehnke, P.C., and Ferl, R.J. (1999). The 14-3-3 proteins: Cellular regulators of plant metabolism. Trends Plant Sci. 4, 367–371. [DOI] [PubMed] [Google Scholar]
- Després, C., Subramaniam, R., Matton, D.P.R., and Brisson, N. (1995). The activation of the potato PR-10a gene requires the phosphorylation of the nuclear factor PBF-1. Plant Cell 7, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit, P.J.G.M. (1997). Pathogen avirulence and plant resistance: A key role for recognition. Trends Plant Sci. 2, 452–458. [Google Scholar]
- Dröge-Laser, W., Kaiser, A., Lindsay, W.P., Halkier, B.A., Loake, G.J., Doerner, P., Dixon, R.A., and Lamb, C. (1997). Rapid stimulation of a soybean protein-serine kinase which phosphorylates a novel bZIP DNA-binding protein, G/HBF-1, during the induction of early transcription-dependent defenses. EMBO J. 16, 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant, W.E., Rowland, O., Piedras, P., Hammond-Kosack, K.E., and Jones, J.D.G. (2000). cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell 12, in press. [DOI] [PMC free article] [PubMed]
- Ellard-Ivey, M., Hopkins, R.B., White, T.J., and Lomax, T.L. (1999). Cloning, expression and N-terminal myristoylation of CpCPK1, a calcium-dependent protein kinase from zucchini (Cucurbita pepo L.). Plant Mol. Biol. 39, 199–208. [DOI] [PubMed] [Google Scholar]
- Ellis, J., and Jones, D. (1998). Structure and function of proteins controlling strain-specific pathogen resistance in plants. Curr. Opin. Plant Sci. 1, 288–293. [DOI] [PubMed] [Google Scholar]
- Flor, H.H. (1971). Current status of gene-for-gene concept. Annu. Rev. Phytopathol. 9, 275–296. [Google Scholar]
- Fuglsang, A.T., Visconti, S., Drumm, K., Jahn, T., Stensballe, A., Mattei, B., Jensen, O.N., Aducci, P., and Palmgren, M.G. (1999). Binding of 14-3-3 protein to the plasma membrane H(+)-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J. Biol. Chem. 274, 36774–36780. [DOI] [PubMed] [Google Scholar]
- Gelli, A., Higgins, V.J., and Blumwald, E. (1997). Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiol. 113, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack, K.E., and Jones, J.D.G. (1997). Plant disease resistance genes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 575–607. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack, K.E., Tang, S., Harrison, K., and Jones, J.D.G. (1998). The tomato Cf-9 disease resistance gene functions in tobacco and potato to confer responsiveness to the complementary fungal avirulence gene product Avr9. Plant Cell 10, 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon, A.C. (1997). The calcium connection. Trends Plant Sci. 1, 121–122. [Google Scholar]
- Harmon, A.C., Putnam-Evans, C., and Cormier, M.J. (1987). A calcium-dependent but calmodulin-independent protein kinase from soybean. Plant Physiol. 83, 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Y., Takano, M., Liu, C.-M., Gasch, A., Chye, M.L., and Chua, N.-H. (1996). Expression of three members of the calcium-dependent protein kinase gene family in Arabidopsis thaliana. Plant Mol. Biol. 30, 1259–1275. [DOI] [PubMed] [Google Scholar]
- Hrabak, E.M., Dickmann, L.J., Satterlee, J.S., and Sussman, M.R. (1996). Characterization of eight new members of the calmodulin-like domain protein kinase gene family from Arabidopsis thaliana. Plant Mol. Biol. 31, 405–412. [DOI] [PubMed] [Google Scholar]
- Huang, J.-F., Teyton, L., and Harper, J.F. (1996). Activation of a Ca2+-dependent protein kinase involves intramolecular binding of a calmodulin-like regulatory domain. Biochemistry 35, 13222–13230. [DOI] [PubMed] [Google Scholar]
- Iwata, Y., Muriyama, M., Nakakita, M., Kojima, H., Ohto, M., and Nakamura, K. (1998). Characterization of a calcium-dependent protein kinase of tobacco leaves that is associated with the plasma membrane and is inducible by sucrose. Plant Cell Physiol. 39, 1176–1183. [DOI] [PubMed] [Google Scholar]
- Jones, D.A., Thomas, C.M., Hammond-Kosack, K.E., Balint-Kurti, P.J., and Jones, J.D.G. (1994). Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266, 789–793. [DOI] [PubMed] [Google Scholar]
- Keller, T., Damude, H.G., Werner, D., Doerner, P., Dixon, R.A., and Lamb, C. (1998). A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan, P.J., Young, J.C., Tax, F., and Sussman, M.R. (1996). Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc. Natl. Acad. Sci. USA 93, 8145–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, C., and Dixon, R.A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Lee, J.-Y., Yoo, B.-C., and Harmon, A.C. (1998). Kinetic and calcium-binding properties of three calcium-dependent protein kinase isoenzymes from soybean. Biochemistry 37, 6801–6809. [DOI] [PubMed] [Google Scholar]
- Li, J., Lee, Y.-R., and Assmann, S.M. (1998). Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 116, 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligterink, W., Kroj, T., zur Nieden, U., Hirt, H., and Scheel, D. (1997). Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276, 2054–2057. [DOI] [PubMed] [Google Scholar]
- Majumdar, S., Kane, L.H., Rossi, M.W., Volpp, B.D., Nauseef, W.M., and Korchak, H.M. (1993). Protein kinase C isotypes and signal-transduction in human neutrophils: Selective substrate specificity of calcium-dependent beta-PKC and novel calcium-independent nPKC. Biochim. Biophys. Acta 1176, 276–286. [DOI] [PubMed] [Google Scholar]
- Martin, G.B. (1999). Functional analysis of plant disease resistance genes and their downstream effectors. Curr. Opin. Plant Biol. 2, 273–279. [DOI] [PubMed] [Google Scholar]
- Martin, G.B., Brommonschenkel, S.H., Chunwongse, J., Frary, A., Ganal, M.W., Spivey, R., Wu, T., Earle, E.D., and Tanksley, S.D. (1993). Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262, 1432–1436. [DOI] [PubMed] [Google Scholar]
- Mithöfer, A., Fliegmann, J., and Ebel, J. (1999). Isolation of a French bean (Phaseolus vulgaris L.) homolog to the beta-glucan elicitor-binding protein of soybean (Glycine max L.). Biochim. Biophys. Acta 1418, 127–132. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nennstiel, D., Scheel, D., and Nürnberger, T. (1998). Characterization and partial purification of an oligopeptide elictor receptor from parsley (Petroselinum crispum). FEBS Lett. 431, 405–410. [DOI] [PubMed] [Google Scholar]
- Nürnberger, T., Nennstiel, D., Jabs, T., Sacks, W.R., Hahlbrock, K., and Scheel, D. (1994). High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78, 449–460. [DOI] [PubMed] [Google Scholar]
- Pei, Z.-M., Ward, J.M., Harper, J.F., and Schroeder, J.I. (1996). A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 15, 6564–6574. [PMC free article] [PubMed] [Google Scholar]
- Perkins, R.S., Lindsay, M.A., Barnes, P.J., and Giembycz, M.A. (1995). Early signalling events implicated in leukotriene B4-induced activation of the NADPH oxidase in eosinophils: Role of Ca2+, protein kinase C and phospholipases C and D. Biochem. J. 310, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedras, P., Hammond-Kosack, K.E., Harrison, K., and Jones, J.D.G. (1998). Rapid, Cf-9 and Avr9-dependent, production of active oxygen species in tobacco suspension cultures. Mol. Plant-Microbe Interact. 11, 1155–1166. [Google Scholar]
- Rechsteiner, M., and Rogers, S.W. (1996). PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21, 267–271. [PubMed] [Google Scholar]
- Richberg, M.H., Aviv, D.H., and Dangl, J.L. (1998). Dead cells do tell tales. Curr. Opin. Plant Biol. 1, 480–485. [DOI] [PubMed] [Google Scholar]
- Roberts, D.M., and Harmon, A.C. (1992). Calcium-modulated proteins: Targets of intracellular calcium signals in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 375–414. [Google Scholar]
- Roberts, M.R., and Bowles, D. (1999). Fusicoccin, 14-3-3 proteins, and defense responses in tomato plants. Plant Physiol. 119, 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., Zhang, S., Klessig, D.F., Hirt, H., and Jones, J.D.G. (1999). Rapid Avr9- and Cf-9–dependent activation of MAP kinases in tobacco cell cultures and leaves: Convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlee, J.S., and Sussman, M.R. (1998). Unusual membrane-associated protein kinases in higher plants. J. Membr. Biol. 164, 205–213. [DOI] [PubMed] [Google Scholar]
- Schaller, A., and Oecking, C. (1999). Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, G.E., Harmon, A.C., and Sussman, M.R. (1992). Phosphorylation of the plasma membrane H+-ATPase of oat roots by a calcium-stimulated protein kinase. Biochemistry 31, 1721–1727.1737026 [Google Scholar]
- Scheel, D. (1998). Resistance response physiology and signal transduction. Curr. Opin. Plant Biol. 1, 305–310. [DOI] [PubMed] [Google Scholar]
- Schwacke, R., and Hager, A. (1992). Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein-kinase activity. Planta 187, 136–141. [DOI] [PubMed] [Google Scholar]
- Sehnke, P.C., and Ferl, R.J. (1996). Plant metabolism: Enzyme regulation by 14-3-3 proteins. Curr. Biol. 6, 1403–1405. [DOI] [PubMed] [Google Scholar]
- Sheen, J. (1996). Ca2+-dependent protein kinases and stress signal transduction in plants. Science 274, 1900–1902. [DOI] [PubMed] [Google Scholar]
- Song, W.-Y., Wang, G.-L., Chen, L.-L., Kim, H.-S, Pi, L.-Y., Holsten, T., Gardner, J., Wang, B., Zhai, W.-X., Zhu, L.-H., Fauquet, C., and Ronald, P. (1995). A receptor kinase-like protein encoded by the rice disease resistance gene Xa21. Science 270, 1804–1806. [DOI] [PubMed] [Google Scholar]
- Staskawicz, B.J., Ausubel, F.M., Baker, B.J., Ellis, J.G., and Jones, J.D.G. (1995). Molecular genetics of plant disease resistance. Science 268, 661–667. [DOI] [PubMed] [Google Scholar]
- Subramaniam, R., Després, C., and Brisson, N. (1997). A functional homolog of mammalian protein kinase C participates in the elicitor-induced defense response in potato. Plant Cell 9, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tähtiharju, S., Sangwan, V., Monroy, A.F., Dhindsa, R.S., and Borg, M. (1997). The induction of kin genes in cold-acclimating Arabidopsis thaliana. Evidence for a role for calcium. Planta 203, 442–447. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Isobe, M., and Muto, S. (1997). An increase in cytosolic calcium ion concentration precedes hypoosmotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett. 401, 202–206. [DOI] [PubMed] [Google Scholar]
- Torres, M.A., Onouchi, H., Hamada, S., Machida, C., Hammond-Kosack, K.E., and Jones, J.D.G. (1998). Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 14, 365–370. [DOI] [PubMed] [Google Scholar]
- Umemoto, N., Kakitani, M., Iwamatsu, A., Yoshikawa, N., Yamaoka, N., and Ishida, I. (1997). The structure and function of a soybean β-glucan-elicitor-binding protein. Proc. Natl. Acad. Sci. USA 94, 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao, T., Katagiri, T., Mizoguchi, T., Yamaguchi-Shinozaki, K., Hayashida, N., and Shinozaki, K. (1994). Two genes that encode Ca2+-dependent protein kinases are induced by drought and high-salt stresses in Arabidopsis thaliana. Mol. Gen. Genet. 244, 331–340. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken, G.F.J.M., Van Kan, J.A.L., and De Wit, P.J.G.M. (1992). Molecular analysis of the avirulence gene avr9 of the fungal tomato pathogen Cladosporium fulvum fully supports the gene-for-gene hypothesis. Plant J. 2, 359–366. [DOI] [PubMed] [Google Scholar]
- Xing, T., Higgins, V.J., and Blumwald, E. (1996). Regulation of plant defense response to fungal pathogens: Two types of protein kinases in the reversible phosphorylation of the host plasma membrane H+-ATPase. Plant Cell 8, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, T., Higgins, V.J., and Blumwald, E. (1997). Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH-oxidase to the plasma membrane of tomato cells. Plant Cell 9, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Shah, J., and Klessig, D.F. (1997). Signal perception and transduction in plant defense responses. Genes Dev. 11, 1621–1639. [DOI] [PubMed] [Google Scholar]
- Yoo, B.-C., and Harmon, A.C. (1996). Intramolecular binding contributes to the activation of CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 35, 12029–12037. [DOI] [PubMed] [Google Scholar]
- Yoon, G.M., Cho, H.S., Ha, H.J., Liu, J.R., and Pai Lee, H.-S. (1999). Characterization of NtCDPK1, a calcium-dependent protein kinase gene in Nicotiana tabacum, and the activity of its encoded protein. Plant Mol. Biol. 39, 991–1001. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998). N resistance gene–mediated de novo synthesis and activation of a tobacco MAP kinase by TMV infection. Proc. Natl. Acad. Sci. USA 95, 7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Du, H., and Klessig, D.F. (1998). Activation of the tobacco SIP kinase by both a cell wall–derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10, 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, S., Nürnberger, T., Frachisse, J.-M., Wirtz, W., Guern, J., Hedrich, R., and Scheel, D. (1997). Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc. Natl. Acad. Sci. USA 94, 2751–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]