Abstract
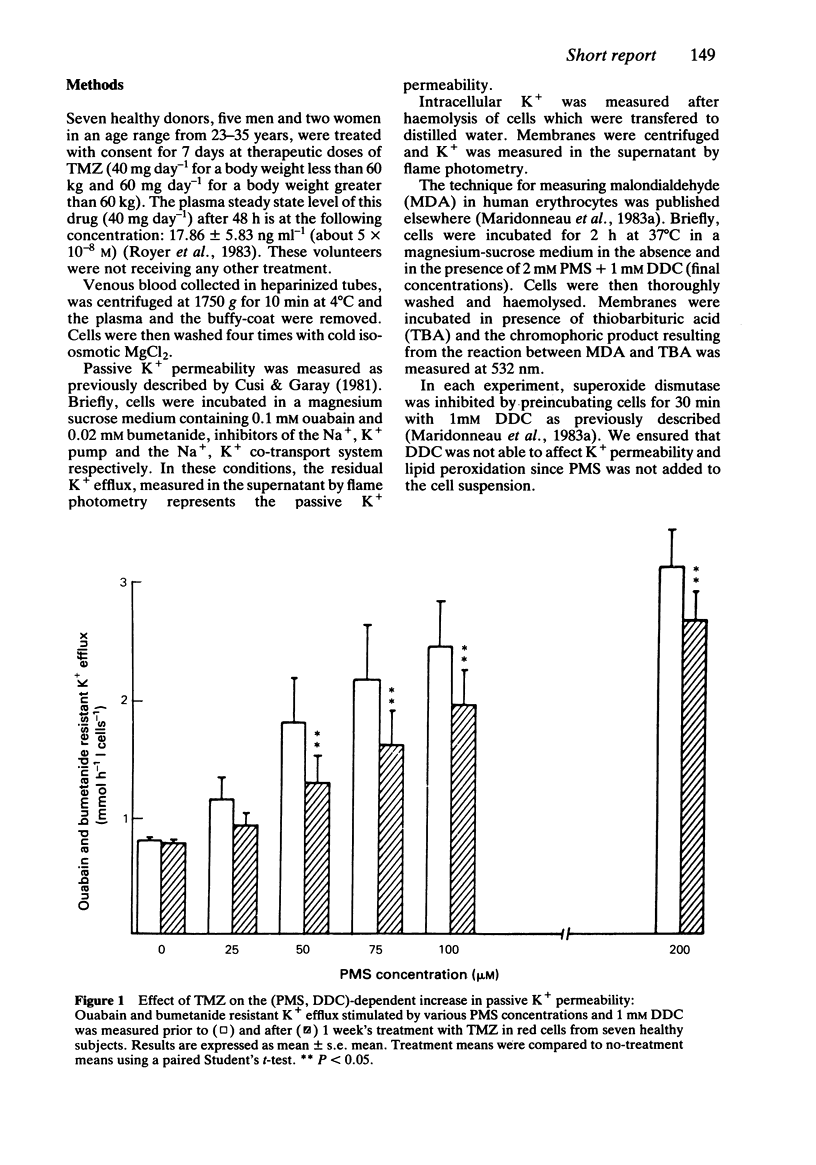
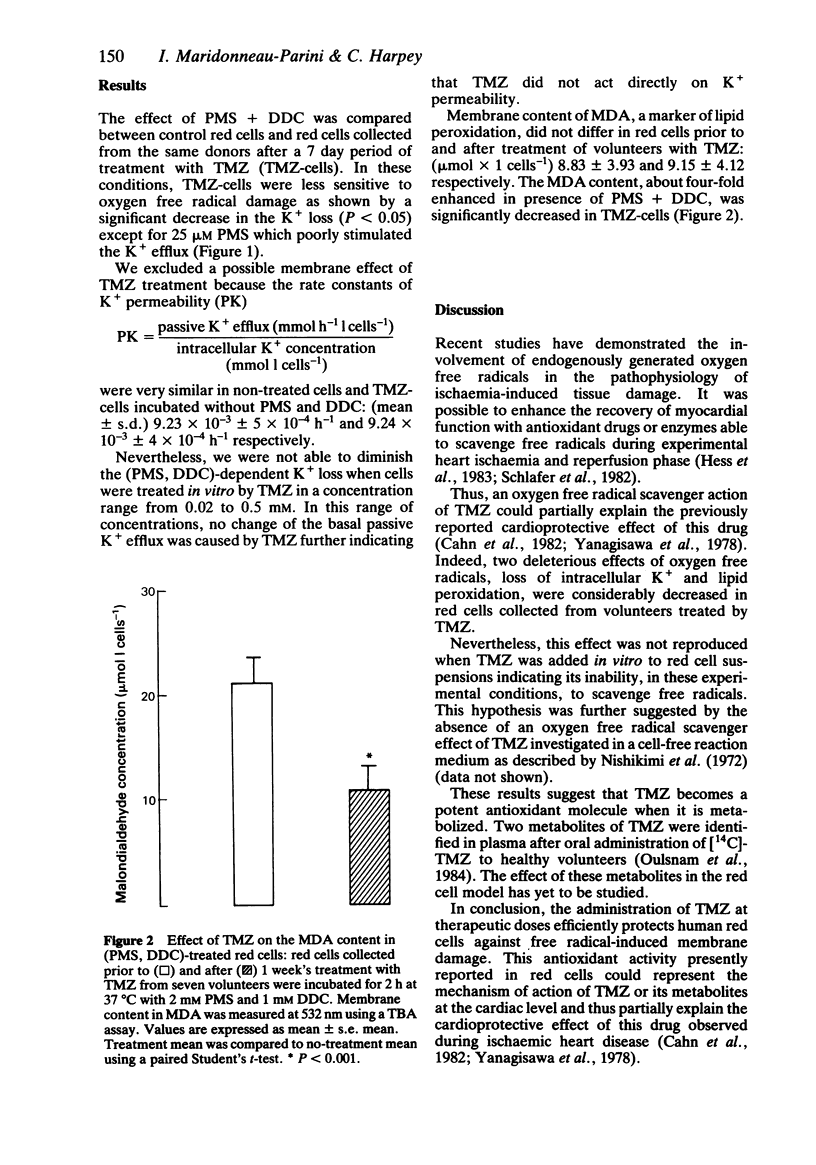
The effect of trimetazidine, 1-(2, 3, 4 trimethoxybenzyl)piperazine di-hydrochloride, on membrane damage induced by oxygen free radicals in red cells was studied in seven healthy volunteers after oral administration. Red cells collected prior to and after a 7 day treatment period with trimetazidine were incubated in the presence of phenazine methosulphate (an intracellular oxygen free radical generator) and diethyldithiocarbamate (a Cu-Zn superoxide dismutase inhibitor). The loss of intracellular K+ induced by oxygen free radicals and the membrane content of peroxidated lipids were significantly reduced in red cells collected after the period of treatment. These results indicate a potent antioxidant activity of trimetazidine which could explain its cardioprotective role during ischaemic and reperfusion phases in which oxygen free radicals are generated and probably implicated in the genesis of cardiac cell injury.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cusi D., Garay R. The effect of tienilic acid on Na+ and K+ transport in human red cells. Mol Pharmacol. 1981 May;19(3):438–443. [PubMed] [Google Scholar]
- Guarnieri C., Flamigni F., Caldarera C. M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol. 1980 Aug;12(8):797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
- Hess M. L., Okabe E., Poland J., Warner M., Stewart J. R., Greenfield L. J. Glucose, insulin, potassium protection during the course of hypothermic global ischemia and reperfusion: a new proposed mechanism by the scavenging of free radicals. J Cardiovasc Pharmacol. 1983 Jan-Feb;5(1):35–43. doi: 10.1097/00005344-198301000-00005. [DOI] [PubMed] [Google Scholar]
- Maridonneau I., Braquet P., Garay R. P. Na+ and K+ transport damage induced by oxygen free radicals in human red cell membranes. J Biol Chem. 1983 Mar 10;258(5):3107–3113. [PubMed] [Google Scholar]
- Maridonneau I., Garay R. P., Braquet P. The effect of lipid peroxidation on transport function in human erythrocytes. Biomed Biochim Acta. 1983;42(11-12):S58–S62. [PubMed] [Google Scholar]
- Nishikimi M., Appaji N., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972 Jan 31;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Kane P. F., Kirsh M. M. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg. 1982 Jun;83(6):830–839. [PubMed] [Google Scholar]
- Vleugels A., Carmeliet E. Hypoxia increases potassium efflux from mammalian myocardium. Experientia. 1976 Apr 15;32(4):483–484. doi: 10.1007/BF01920810. [DOI] [PubMed] [Google Scholar]


