Abstract
1 The metabolic fate of doxazosin was investigated in man, mouse, rat and dog using 14C-labelled compound. Bioavailability and pharmacokinetic studies were also conducted with non-labelled drug, using a specific h.p.l.c. method.
2 Following both oral and intravenous administration, the major route of elimination of drug-related compounds was via the faeces for all species studied. Comparison of the oral and intravenous data show that doxazosin is completely absorbed in man, mouse and rat and is moderately well absorbed in dog.
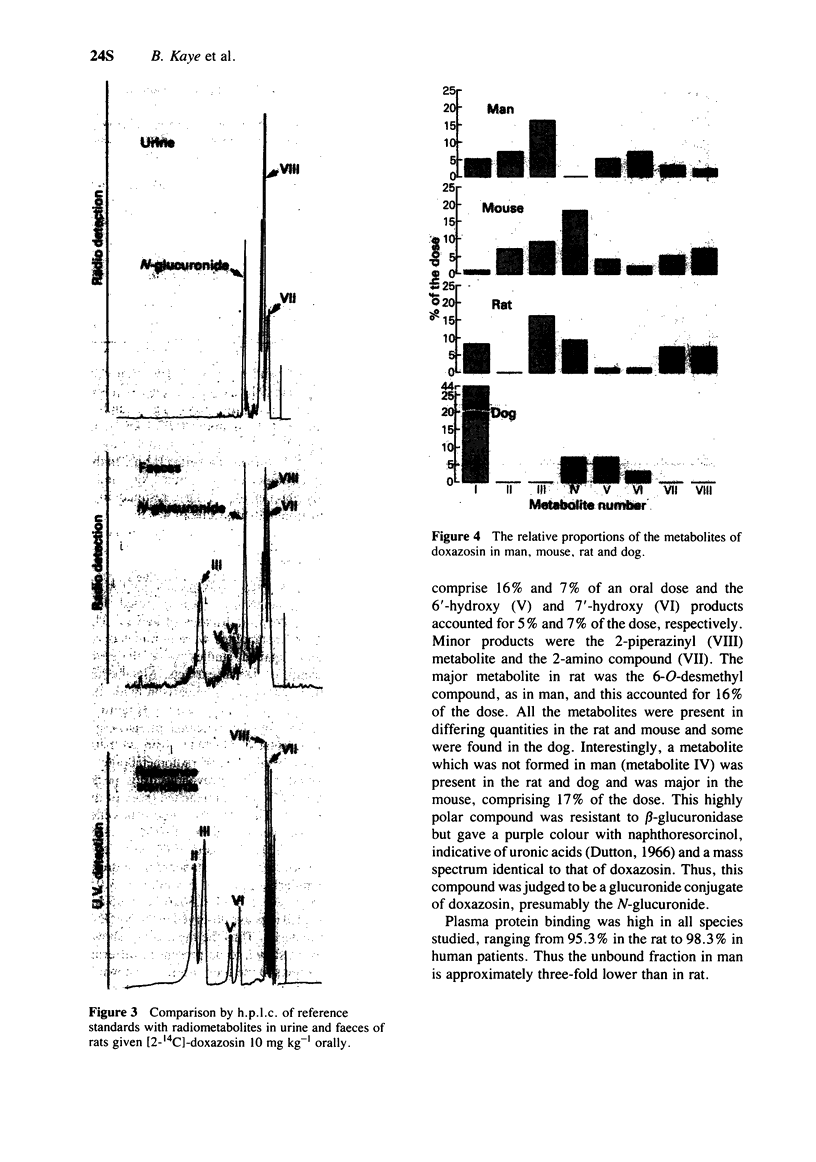
3 The drug is extensively metabolized, e.g. only about 5% of the dose was excreted unchanged in man. Metabolism in man mainly involves 6- and 7- O-demethylation and 6′ and 7′-hydroxylation. These and some minor products were common to the mouse, rat or dog and man.
4 Plasma protein binding was high in all species studied, ranging from 95.3% in the rat to 98.3% in human patients.
5 Oral bioavailability is 60% in dog and approximately 50% in the rat, which is similar to the value of 63% reported for man at therapeutic doses. Mean plasma clearance values were 13 ml min-1 kg-1 (dogs), 30 ml min-1 kg-1 (rats) and 1.2 ml min-1 kg-1 (human subjects). Mean plasma half-life values were 5 h in dogs and 1.2 h in rats: a value of 9 h was reported for human volunteers (cf. 2.5 h for prazosin). The long plasma half-life of doxazosin provides the basis for once-daily dosing.
Keywords: bioavailability, doxazosin, half-life, kinetics, metabolites
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alabaster V. A., Davey M. J. The alpha 1-adrenoceptor antagonist profile of doxazosin: preclinical pharmacology. Br J Clin Pharmacol. 1986;21 (Suppl 1):9S–17S. doi: 10.1111/j.1365-2125.1986.tb02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman D. N., Hobbs D. C., Twomey T. M., Stevens E. A., Rawlins M. D. Prazosin, pharmacokinetics and concentration effect. Eur J Clin Pharmacol. 1979 Sep;16(3):177–181. doi: 10.1007/BF00562058. [DOI] [PubMed] [Google Scholar]
- Elliott H. L., Meredith P. A., Sumner D. J., McLean K., Reid J. L. A pharmacodynamic and pharmacokinetic assessment of a new alpha-adrenoceptor antagonist, doxazosin (UK33274) in normotensive subjects. Br J Clin Pharmacol. 1982 May;13(5):699–703. doi: 10.1111/j.1365-2125.1982.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick M. H., Halttunen P., Himanen P., Huttunen M., Pörsti P., Pitkäjärvi T., Pöyhönen L., Pyykönen M. L., Reinikainen P., Salmela P. A long-term double-blind comparison of doxazosin and atenolol in patients with mild to moderate essential hypertension. Br J Clin Pharmacol. 1986;21 (Suppl 1):55S–62S. doi: 10.1111/j.1365-2125.1986.tb02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway C. V., Stark R. D. Hepatic vascular bed. Physiol Rev. 1971 Jan;51(1):23–65. doi: 10.1152/physrev.1971.51.1.23. [DOI] [PubMed] [Google Scholar]
- Taylor J. A., Twomey T. M., von Wittenau M. S. The metabolic fate of prazosin. Xenobiotica. 1977 Jun;7(6):357–364. doi: 10.3109/00498257709035794. [DOI] [PubMed] [Google Scholar]
- Timmermans P. B., Kwa H. Y., Ali F. K., van Zwieten P. A. Prazosin and its analogues UK-18,596 and UK-33,274: a comparative study on cardiovascular effects and alpha-adrenoceptor blocking activities. Arch Int Pharmacodyn Ther. 1980 Jun;245(2):218–235. [PubMed] [Google Scholar]
- Vincent J., Elliott H. L., Meredith P. A., Reid J. L. Doxazosin, an alpha 1-adrenoceptor antagonist: pharmacokinetics and concentration-effect relationships in man. Br J Clin Pharmacol. 1983 Jun;15(6):719–725. doi: 10.1111/j.1365-2125.1983.tb01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


