Abstract
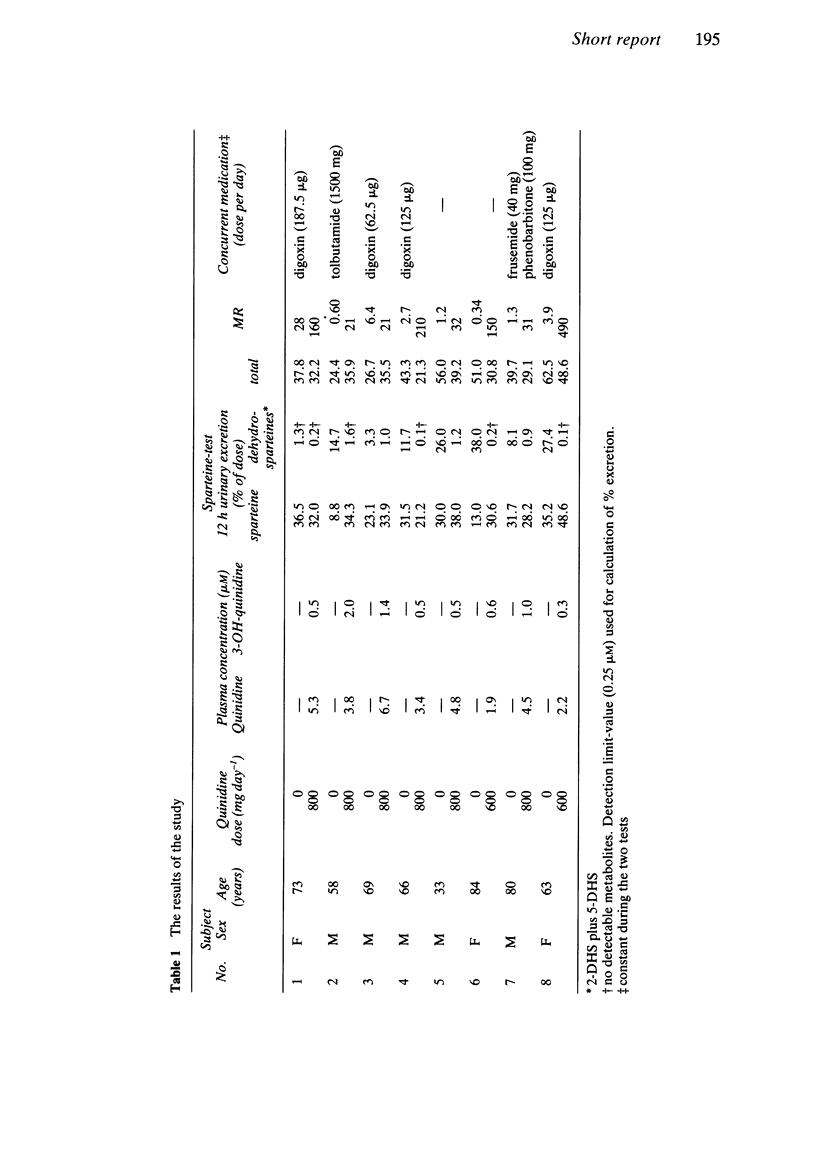
In eight patients a sparteine-test was carried out immediately before and after 1 week of treatment with quinidine 600-800 mg day-1. Before treatment one patient was classified as a poor metaboliser (metabolic ratio: greater than or equal to 20), and seven patients as extensive metabolisers. During quinidine treatment, the formation of sparteine metabolites (2- and 5-dehydrosparteine) was practically abolished. Patients initially classified as extensive metabolisers thus exhibited the phenotype of poor metabolisers during quinidine treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boobis A. R., Murray S., Kahn G. C., Robertz G. M., Davies D. S. Substrate specificity of the form of cytochrome P-450 catalyzing the 4-hydroxylation of debrisoquine in man. Mol Pharmacol. 1983 Mar;23(2):474–481. [PubMed] [Google Scholar]
- Brøsen K., Otton S. V., Gram L. F. Sparteine oxidation polymorphism in Denmark. Acta Pharmacol Toxicol (Copenh) 1985 Nov;57(5):357–360. doi: 10.1111/j.1600-0773.1985.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Clark D. W. Genetically determined variability in acetylation and oxidation. Therapeutic implications. Drugs. 1985 Apr;29(4):342–375. doi: 10.2165/00003495-198529040-00003. [DOI] [PubMed] [Google Scholar]
- Dayer P., Balant L., Courvoisier F., Kupfer A., Kubli A., Gorgia A., Fabre J. The genetic control of bufuralol metabolism in man. Eur J Drug Metab Pharmacokinet. 1982 Jan-Mar;7(1):73–77. doi: 10.1007/BF03189547. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Woolhouse N. M. Inter-ethnic difference in sparteine oxidation among Ghanaians and Germans. Eur J Clin Pharmacol. 1985;28(1):79–83. doi: 10.1007/BF00635712. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Mahgoub A., Sloan T. P., Idle J. R., Smith R. L. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet. 1980 Apr;17(2):102–105. doi: 10.1136/jmg.17.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpfer A., Preisig R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26(6):753–759. doi: 10.1007/BF00541938. [DOI] [PubMed] [Google Scholar]
- Leemann T., Dayer P., Meyer U. A. Single-dose quinidine treatment inhibits metoprolol oxidation in extensive metabolizers. Eur J Clin Pharmacol. 1986;29(6):739–741. doi: 10.1007/BF00615971. [DOI] [PubMed] [Google Scholar]
- Lennard M. S., Jackson P. R., Freestone S., Tucker G. T., Ramsay L. E., Woods H. F. The relationship between debrisoquine oxidation phenotype and the pharmacokinetics and pharmacodynamics of propranolol. Br J Clin Pharmacol. 1984 Jun;17(6):679–685. doi: 10.1111/j.1365-2125.1984.tb02403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard M. S., Silas J. H., Freestone S., Ramsay L. E., Tucker G. T., Woods H. F. Oxidation phenotype--a major determinant of metoprolol metabolism and response. N Engl J Med. 1982 Dec 16;307(25):1558–1560. doi: 10.1056/NEJM198212163072505. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Lennard M. S., Jackson P. R., Tucker G. T., Ramsay L. E., Woods H. F. Timolol and atenolol: relationships between oxidation phenotype, pharmacokinetics and pharmacodynamics. Br J Clin Pharmacol. 1985 Mar;19(3):329–333. doi: 10.1111/j.1365-2125.1985.tb02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin C., Siwers B., Benitez J., Bertilsson L. Plasma concentrations of nortriptyline and its 10-hydroxy metabolite in depressed patients--relationship to the debrisoquine hydroxylation metabolic ratio. Br J Clin Pharmacol. 1985 Jun;19(6):832–835. doi: 10.1111/j.1365-2125.1985.tb02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs H. R., Greenblatt D. J., Woo E. Clinical pharmacokinetics of quinidine. Clin Pharmacokinet. 1980 Mar-Apr;5(2):150–168. doi: 10.2165/00003088-198005020-00003. [DOI] [PubMed] [Google Scholar]
- Otton S. V., Inaba T., Kalow W. Competitive inhibition of sparteine oxidation in human liver by beta-adrenoceptor antagonists and other cardiovascular drugs. Life Sci. 1984 Jan 2;34(1):73–80. doi: 10.1016/0024-3205(84)90332-1. [DOI] [PubMed] [Google Scholar]
- Otton S. V., Inaba T., Kalow W. Inhibition of sparteine oxidation in human liver by tricyclic antidepressants and other drugs. Life Sci. 1983 Feb 14;32(7):795–800. doi: 10.1016/0024-3205(83)90315-6. [DOI] [PubMed] [Google Scholar]
- Raghuram T. C., Koshakji R. P., Wilkinson G. R., Wood A. J. Polymorphic ability to metabolize propranolol alters 4-hydroxypropranolol levels but not beta blockade. Clin Pharmacol Ther. 1984 Jul;36(1):51–56. doi: 10.1038/clpt.1984.138. [DOI] [PubMed] [Google Scholar]
- Vinks A., Inaba T., Otton S. V., Kalow W. Sparteine metabolism in Canadian Caucasians. Clin Pharmacol Ther. 1982 Jan;31(1):23–29. doi: 10.1038/clpt.1982.4. [DOI] [PubMed] [Google Scholar]
- Wedlund P. J., Aslanian W. S., McAllister C. B., Wilkinson G. R., Branch R. A. Mephenytoin hydroxylation deficiency in Caucasians: frequency of a new oxidative drug metabolism polymorphism. Clin Pharmacol Ther. 1984 Dec;36(6):773–780. doi: 10.1038/clpt.1984.256. [DOI] [PubMed] [Google Scholar]
- von Bahr C., Spina E., Birgersson C., Ericsson O., Göransson M., Henthorn T., Sjöqvist F. Inhibition of desmethylimipramine 2-hydroxylation by drugs in human liver microsomes. Biochem Pharmacol. 1985 Jul 15;34(14):2501–2505. doi: 10.1016/0006-2952(85)90533-7. [DOI] [PubMed] [Google Scholar]


