Abstract
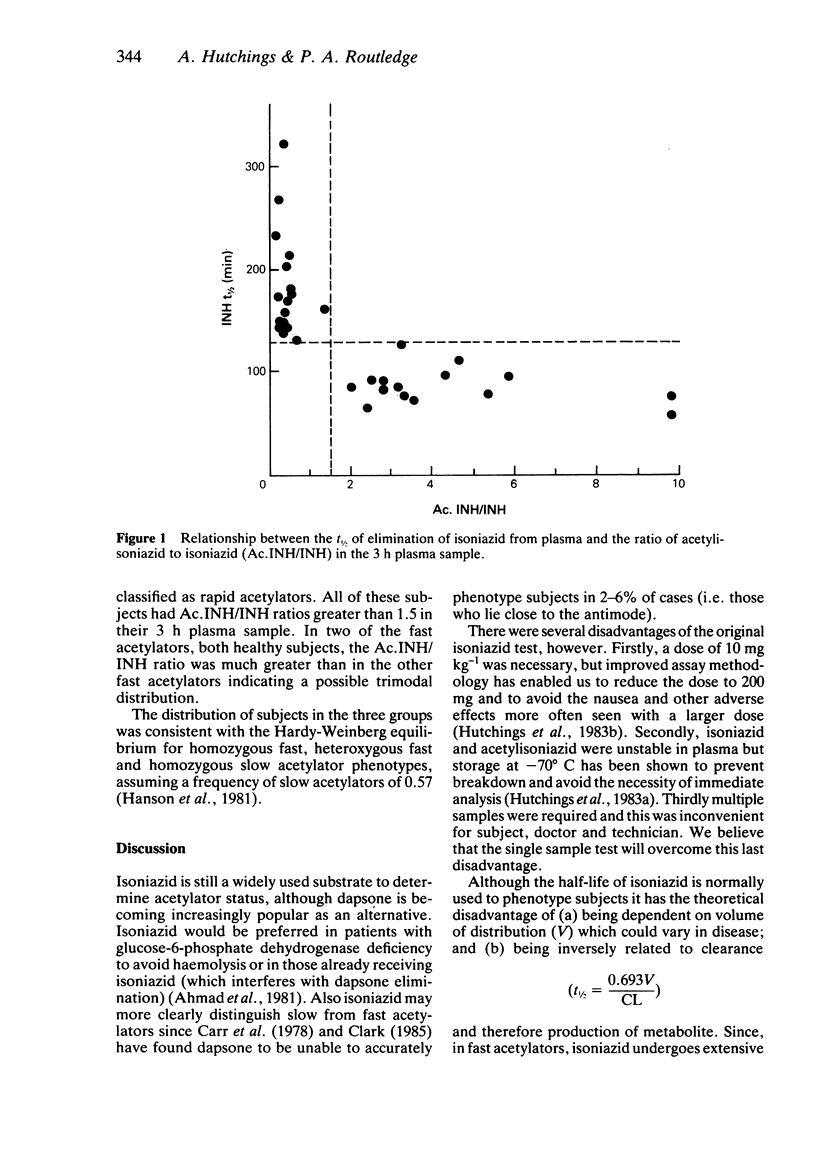
A comparison was made between the results of acetylator phenotyping by isoniazid (INH) half-life measurements based on samples taken for 6 h after a single oral dose (200 mg), and by determination of the ratio of acetylisoniazid (Ac.INH) to isoniazid in the 3 h samples. In the 32 subjects, examined, there was complete agreement about classification of the subject as a fast (t1/2 less than 130 min; Ac.INH/INH greater than 1.5) or slow acetylator (t1/2 greater than 130 min; Ac.INH/INH less than 1.5). The single sample test appears to be as reliable as the more time-consuming isoniazid half-life method.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad R. A., Rogers H. J., Vandenburg M., Wright P. Effects of concurrent administration of other substrates of N-acetyltransferase on dapsone acetylation. Br J Clin Pharmacol. 1981 Jul;12(1):83–86. doi: 10.1111/j.1365-2125.1981.tb01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr K., Oates J. A., Nies A. S., Woosley R. L. Simultaneous analysis of dapsone and monoacetyldapsone employing high performance liquid chromatography: a rapid method for determination of acetylator phenotype. Br J Clin Pharmacol. 1978 Nov;6(5):421–427. doi: 10.1111/j.1365-2125.1978.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. W. Genetically determined variability in acetylation and oxidation. Therapeutic implications. Drugs. 1985 Apr;29(4):342–375. doi: 10.2165/00003495-198529040-00003. [DOI] [PubMed] [Google Scholar]
- EVANS D. A., MANLEY K. A., McKUSICK V. A. Genetic control of isoniazid metabolism in man. Br Med J. 1960 Aug 13;2(5197):485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A., Melander A., Wåhlin-Boll E. Acetylator phenotyping: a comparison of the isoniazid and dapsone tests. Eur J Clin Pharmacol. 1981;20(3):233–234. doi: 10.1007/BF00544604. [DOI] [PubMed] [Google Scholar]
- Hutchings A., Monie R. D., Spragg B., Routledge P. A. A method to prevent the loss of isoniazid and acetylisoniazid in human plasma. Br J Clin Pharmacol. 1983 Feb;15(2):263–266. doi: 10.1111/j.1365-2125.1983.tb01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings A., Monie R. D., Spragg B., Routledge P. A. High-performance liquid chromatographic analysis of isoniazid and acetylisoniazid in biological fluids. J Chromatogr. 1983 Oct 14;277:385–390. doi: 10.1016/s0378-4347(00)84863-x. [DOI] [PubMed] [Google Scholar]
- Weber W. W., Hein D. W. Clinical pharmacokinetics of isoniazid. Clin Pharmacokinet. 1979 Nov-Dec;4(6):401–422. doi: 10.2165/00003088-197904060-00001. [DOI] [PubMed] [Google Scholar]


