Abstract
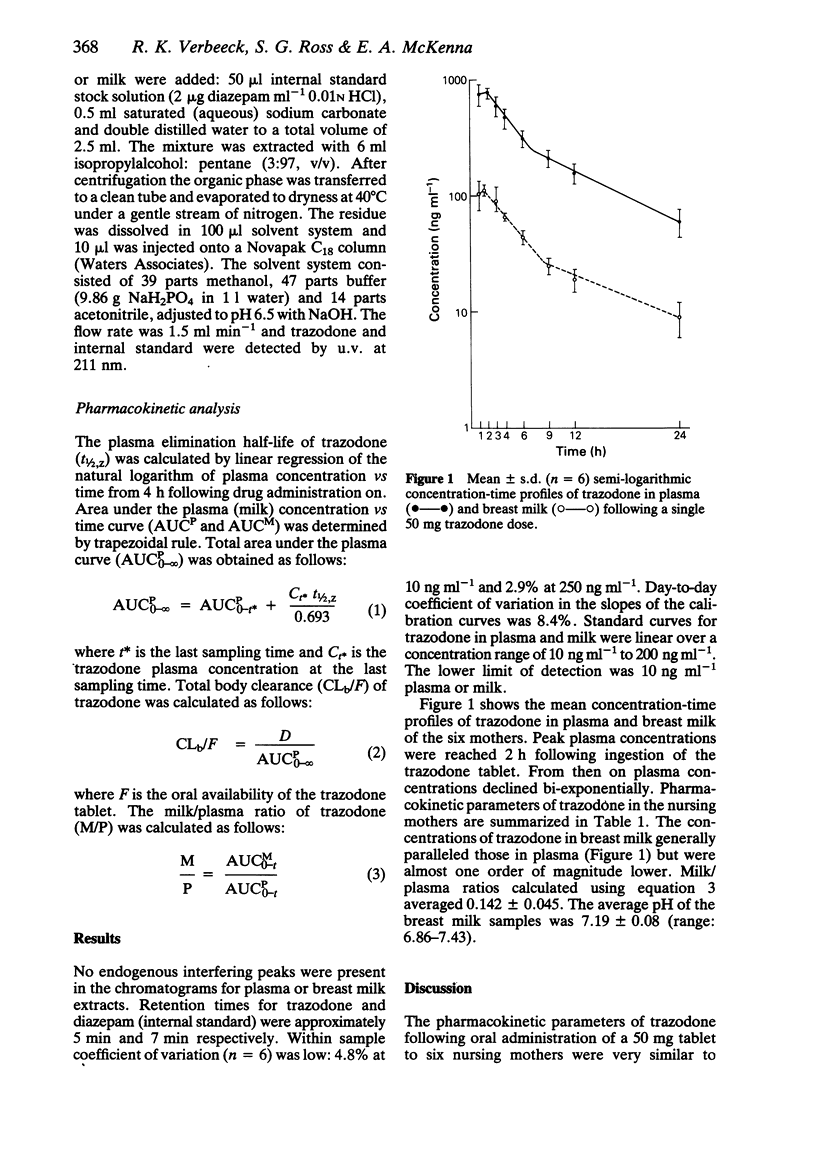
The excretion of breast milk was studied in six lactating women following the oral administration of a single trazodone tablet (50 mg). The milk/plasma ratio of trazodone based on area under the plasma and milk curves was small: 0.142 +/- 0.045 (mean +/- s.d.). Assuming that the babies would drink 500 ml 12 h-1, they would be exposed to less than 0.005 mg kg-1 as compared to 0.77 mg kg-1 for the mothers. It is concluded that exposure of babies to trazodone via breast milk is very small.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer A. J., Pathy M. S., Ankier S. I. Pharmacokinetic and pharmacodynamic characteristics of trazodone in the elderly. Br J Clin Pharmacol. 1983 Oct;16(4):371–376. doi: 10.1111/j.1365-2125.1983.tb02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R. N., Heel R. C., Speight T. M., Avery G. S. Trazodone: a review of its pharmacological properties and therapeutic use in depression and anxiety. Drugs. 1981 Jun;21(6):401–429. doi: 10.2165/00003495-198121060-00001. [DOI] [PubMed] [Google Scholar]
- Caccia S., Ballabio M., Samanin R., Zanini M. G., Garattini S. (--)-m-Chlorophenyl-piperazine, a central 5-hydroxytryptamine agonist, is a metabolite of trazodone. J Pharm Pharmacol. 1981 Jul;33(7):477–478. doi: 10.1111/j.2042-7158.1981.tb13841.x. [DOI] [PubMed] [Google Scholar]
- Gammans R. E., Mackenthun A. V., Russell J. W. Comparative bioavailability of trazodone formulations using stable isotope methodology. Br J Clin Pharmacol. 1984 Sep;18(3):431–437. doi: 10.1111/j.1365-2125.1984.tb02485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon S., Newton R. Lack of anticholinergic side effects with a new antidepressent--trazodone. J Clin Psychiatry. 1980 Mar;41(3):100–104. [PubMed] [Google Scholar]
- Syversen G. B., Ratkje S. K. Drug distribution within human milk phases. J Pharm Sci. 1985 Oct;74(10):1071–1074. doi: 10.1002/jps.2600741010. [DOI] [PubMed] [Google Scholar]
- Welch R. M., Findlay J. W. Excretion of drugs in human breast milk. Drug Metab Rev. 1981;12(2):261–277. doi: 10.3109/03602538108994032. [DOI] [PubMed] [Google Scholar]
- Whitehead R. G., Paul A. A. Infant growth and human milk requirements. A fresh approach. Lancet. 1981 Jul 25;2(8239):161–163. doi: 10.1016/s0140-6736(81)90352-4. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Brown R. D., Cherek D. R., Dailey J. W., Hilman B., Jobe P. C., Manno B. R., Manno J. E., Redetzki H. M., Stewart J. J. Drug excretion in human breast milk: principles, pharmacokinetics and projected consequences. Clin Pharmacokinet. 1980 Jan-Feb;5(1):1–66. doi: 10.2165/00003088-198005010-00001. [DOI] [PubMed] [Google Scholar]
- Yamato C., Takahashi T., Fujita T. Studies on metabolism of trazodone. III Species differences. Xenobiotica. 1976 May;6(5):295–306. doi: 10.3109/00498257609151641. [DOI] [PubMed] [Google Scholar]


