Abstract
TASK-1 encodes an acid- and anaesthetic-sensitive background K+ current, which sets the resting membrane potential of both cerebellar granule neurons and somatic motoneurons. We demonstrate that TASK-1, unlike the other two pore (2P) domain K+ channels, is directly blocked by submicromolar concentrations of the endocannabinoid anandamide, independently of the CB1 and CB2 receptors. In cerebellar granule neurons, anandamide also blocks the TASK-1 standing-outward K+ current, IKso, and induces depolarization. Anandamide-induced neurobehavioural effects are only partly reversed by antagonists of the cannabinoid receptors, suggesting the involvement of alternative pathways. TASK-1 constitutes a novel sensitive molecular target for this endocannabinoid.
Keywords: acidosis/CB1 receptor/cerebellum/2P domain K+ channels
Introduction
Leak or background K+-selective channels, as defined by a lack of voltage- and time-dependency, play an essential role in setting the neuronal resting membrane potential and input resistance. The molecular identity of several background K+ channels has now been elucidated (Fink et al., 1996, 1998; Lesage et al., 1996, 2000; Duprat et al., 1997; Leonoudakis et al., 1998; Reyes et al., 1998; Chavez et al., 1999; Kim et al., 1999, 2000; Salinas et al., 1999; Bang et al., 2000; Patel et al., 2000; Rajan et al., 2000). These belong to the family of two pore (2P) domain K+ channels with four transmembrane segments (TMS), two P domains, an extended M1P1 external loop (60–70 residues) and intracellular N- and C-termini (Lesage and Lazdunski, 1999). These channels can be classified into three distinct functional classes: (i) the weak inward rectifiers TWIK-1 and TWIK-2 (Lesage et al., 1996; Chavez et al., 1999; Patel et al., 2000); (ii) the mechano-gated K+ channels TREK-1 [the mammalian functional homologue of the Aplysia S channel (Siegelbaum et al., 1982)], TREK-2 and TRAAK, which are also opened by polyunsaturated fatty acids (Fink et al., 1996, 1998; Patel et al., 1998; Bang et al., 2000; Lesage et al., 2000); and (iii) the acid-sensitive background K+ channels TASK-1, TASK-2 and TASK-3 (Duprat et al., 1997; Leonoudakis et al., 1998; Reyes et al., 1998; Kim et al., 1999, 2000; Rajan et al., 2000).
TASK-1 is differentially expressed in the brain, with high mRNA levels found in cerebellar granule neurons, as well as in brainstem and spinal cord motoneurons (Duprat et al., 1997; Talley et al., 2000). The TASK-1 K+ current is blocked by mild external acidosis in the range of the physiological pH (Duprat et al., 1997; Leonoudakis et al., 1998; Kim et al., 1999). Recently, TASK-1 has been proposed to underlie the acid-sensitive standing-outward background K+ current (IKso) in both rat cerebellar granule cells and hypoglossal motoneurons (Millar et al., 2000; Sirois et al., 2000; Talley et al., 2000). IKso is reversibly inhibited by stimulation of Gαq-coupled receptors (Millar et al., 2000; Talley et al., 2000). In cerebellar granule cells, activation of the muscarinic M3 receptor with acetylcholine inhibits IKso and provokes cell depolarization and action potential firing (Millar et al., 2000). TASK-1 is directly opened by inhalational anaesthetics including halothane and isoflurane (Patel et al., 1999; Sirois et al., 2000). In somatic motoneurons, locus coeruleus cells and type I carotid body cells, anaesthetics open a TASK-1-like background K+ current (Buckler et al., 2000; Sirois et al., 2000). The hyperpolarization induced by volatile anaesthetics is probably an important component to the mechanism of general anaesthesia (Franks and Lieb, 1999). The decrease in cell excitability associated with K+ stimulation in motoneurons is likely to contribute to anaesthetic-induced immobilization, and in the locus coerulus it may support analgesic and hypnotic actions attributed to these neurons (Sirois et al., 2000).
Anandamide, the amide of arachidonic acid isolated in brain (Figure 1A), reproduces most of the effects of Δ9-tetrahydrocannabinol (Δ9-THC), including hypothermia, hypokinesia, analgesia and catalepsy (Dewey, 1986; Di Marzo et al., 1998; Mechoulam et al., 1998; Chaperon and Thiebot, 1999). Anandamide is one member of a putative family of endogenous cannabinoids (CBs), including 2-arachidonyl glycerol, which bind and activate cannabinoid receptors. The two CB receptor subtypes identified so far, CB1 and CB2, belong to the superfamily of G-protein-coupled membrane receptors (Dewey, 1986; Di Marzo et al., 1998; Mechoulam et al., 1998; Chaperon and Thiebot, 1999). Although CB1 receptors are expressed throughout the body, they are particularly abundant in the central nervous system, where no other subtypes have been identified. The high and restricted expression of the CB2 receptor in the immune system suggests that it contributes to the potential immunosuppressant and anti-inflammatory effects of cannabinoids (Klein et al., 1998). Some of the effects of anandamide are independent of CB receptors and are likely to be due to the modulation of other still unknown targets (Crawley et al., 1993; Compton and Martin, 1997; Adams et al., 1998; Chakrabarti et al., 1998; Lichtman et al., 1998; Chaperon and Thiebot, 1999; Jarai et al., 1999; Zygmunt et al., 1999).
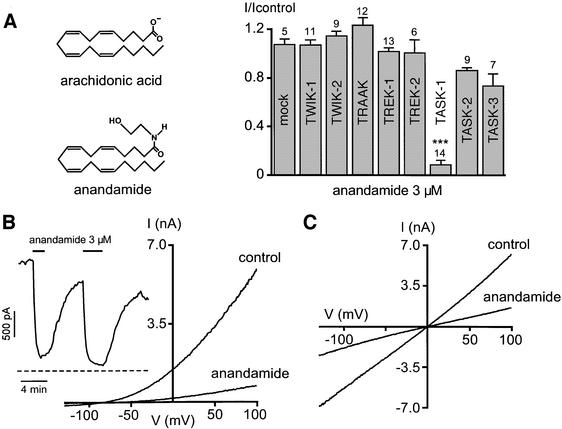
Fig. 1. TASK-1 is selectively inhibited by anandamide. (A) Effects of 3 µM anandamide on the 2P domain K+ channel currents (as indicated) measured at 0 mV. Mock-transfected cells are transfected with an empty expression vector. The arachidonic acid and anandamide molecules are illustrated. (B) Effects of 3 µM anandamide on TASK-1 currents recorded in a physiological K+ gradient (5 mM external K+ and 150 mM internal K+). The inset shows the time course of the effect of 3 µM anandamide on TASK-1 current amplitude measured at 0 mV (different cell from B). The control solution contains 1 mg/ml lipid-free BSA. (C) The same cell as (B), in a symmetrical K+ gradient (155 mM external K+ and 150 mM internal K+). Currents are recorded in transfected COS cells with the whole-cell patch–clamp configuration. The holding potential is –80 mV and cells are stimulated with voltage ramps of 800 ms, duration from –130 mV up to 100 mV. The Student’s t-test was used with p: *<0.05; **<0.01; and ***<0.001. The number of cells studied is indicated in the histogram.
In the present report we provide evidence that sub-micromolar concentrations of anandamide selectively and directly block the background K+ channel TASK-1.
Results
In the present study, we compared the effects of 3 µM anandamide on eight members of the mammalian 2P domain K+ channel family [KCNK7 fails to express a significant current (Salinas et al., 1999)] (Figure 1A). The weak inward rectifiers TWIK-1 and TWIK-2, as well as the mechano-gated K+ channels TREK-1, TREK-2 and TRAAK, are not sensitive to 3 µM anandamide, while the acid-sensitive K+ channel TASK-1 is completely inhibited. The onset effect of anandamide is fast, and block is reversible upon wash with a solution containing lipid-free bovine serum albumin (BSA) (Figure 1B, inset). The effect of anandamide at a concentration of 3 µM is selective, as the other members of the acid-sensitive group, TASK-2 and TASK-3, are not significantly altered by anandamide (Figure 1A). An identical concentration of arachidonic acid has no significant effect on TASK-1 (I/Icontrol: 0.86 ± 0.01, n = 14). Increasing anandamide concentration to 10 µM produced a partial, although significant block of TASK-3 (I/Icontrol: 0.38 ± 0.03, n = 8), but again failed to significantly alter the other 2P domain K+ channels (n = 8). In both physiological and symmetrical K+ gradients, anandamide produces a strong voltage-independent inhibition of TASK-1 (Figure 1B and C).
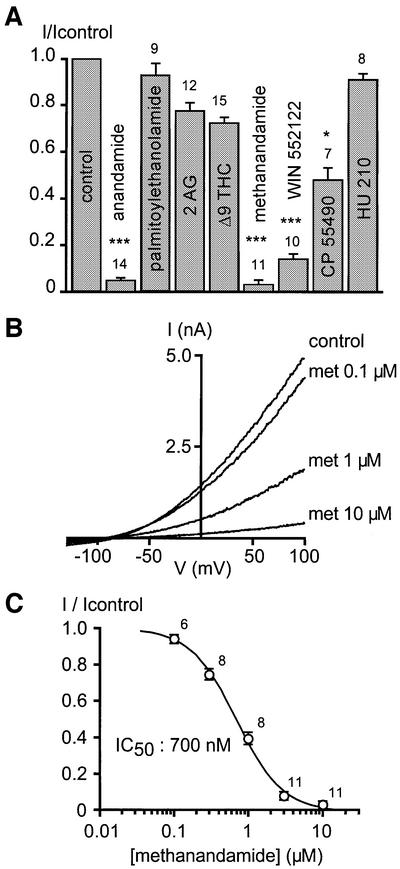
We next investigated the effect of various cannabinomimetic molecules on TASK-1 (Figure 2A). The inhibition of TASK-1 with anandamide is not mimicked by the other endocannabinoid, 2-arachidonyl glycerol (2 AG), nor by the active compound of marijuana, Δ9-THC (Figure 2A). Moreover, TASK-1 is not significantly affected by palmitoylethanolamide (Figure 2A). Anandamide hydrolysis is not involved, as the non-hydrolysable analogue methanandamide is similarly effective (Figure 2A–C). The synthetic CB1/CB2 receptor agonists WIN552122 and CP55940 (Chaperon and Thiebot, 1999) also produce significant inhibition of TASK-1. However, the other powerful CB1/CB2 agonist HU210 (Chaperon and Thiebot, 1999) is not effective (Figure 2A). The threshold concentration for the inhibition of TASK-1 by methanandamide is 100 nM and half inhibition is obtained at 700 nM (Figure 2B and C).
Fig. 2. Submicromolar concentration of anandamide inhibits TASK-1. (A) Effects of various cannabinomimetic molecules at 10 µM (as indicated) on TASK-1 currents measured at 0 mV. (B) Effects of increasing concentrations of methanandamide (met) on TASK-1 currents. (C) Dose–effect curve of methanandamide on TASK-1 measured at 0 mV. The dose–effect curve is fitted with a sigmoidal relationship and the IC50 is 700 nM.
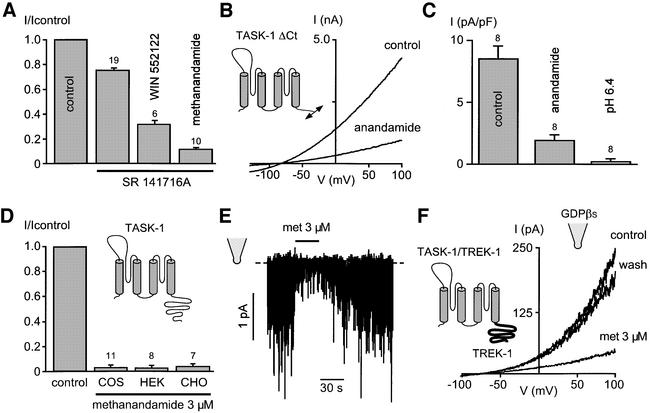
The potent CB1 receptor antagonist SR141716A produces a mild inhibition of TASK-1 at 10 µM but does not prevent the effects of anandamide and WIN552122 (Figure 3A). The lack of effect of several CB1/CB2 agonists, including Δ9-THC, HU210 and 2-AG, and the resistance to the CB1 antagonist SR141716 suggest that anandamide-mediated TASK-1 inhibition is independent of both CB1 and CB2 cannabinoid receptors. Activation of CB receptors modulates various phosphorylation pathways including protein kinase A (PKA) and protein kinase C (PKC), and releases internal Ca2+. A TASK-1 deletion mutant lacking most of the C-terminal region, which comprises all the putative phosphorylation sites, is inhibited by anandamide (Figure 3B–C). Moreover, TASK-1 is not affected by internal Ca2+ (Duprat et al., 1997; Leonoudakis et al., 1998; Kim et al., 1999; our unpublished results). TASK-1 inhibition by anandamide is also observed in CHO and HEK cells, demonstrating that the effect is not restricted to COS cells (Figure 3D). Most importantly, anandamide-induced reversible inhibition of TASK-1 occurs in excised outside-out patch experiments with an internal medium lacking free Ca2+ and nucleotides (Figure 3E) (I/Icontrol: 0.07 ± 0.02, n = 7). In the excised outside-out patch configuration, basal TASK-1 current activity is extremely weak (mean current amplitude at –80 mV in a symmetrical K+ gradient: –0.19 ± 0.03 pA, n = 18) and tends to rundown rapidly. Interestingly, the chimera associating the core of TASK-1 and the entire C-terminus domain of TREK-1 (TA242/TR293) gives robust and stable currents in the excised outside-out patch configuration (mean current amplitude at 0 mV in a physiological K+ gradient: 72.3 ± 17.8 pA, n = 25) (Figure 3F). Methanandamide (3 µM) produces a strong and reversible block of the chimera TA242/TR293 in the outside-out patch configuration (I/Icontrol: 0.24 ± 0.09, n = 5). Intracellular addition of 1 mM GDP-βs, which impairs G-protein functioning, does not prevent anandamide-induced TA242/TR293 channel block (I/Icontrol: 0.19 ± 0.04, n = 10) (Figure 3F). These results strongly suggest that anandamide directly blocks TASK-1, independently of both CB receptors and G-proteins. We swapped the extracellular M1P1 loop of TASK-1 (anandamide sensitive) and TREK-1 (anandamide resistant). The TREK-1 mutant containing the TASK-1 loop (amino acids 68–135 of TREK-1 replaced by amino acids 30–85 of TASK-1) (22.1 ± 7.5 pA/pF at 0 mV in a physiological K+ gradient, n = 11) is not sensitive to 10 µM anandamide (I/Icontrol: 1.18 ± 0.11, n = 7) (data not shown). The corresponding TASK-1 mutant containing the TREK-1 loop is not functional.
Fig. 3. Anandamide directly blocks TASK-1 independently of the CB1/CB2 cannabinoid receptors. (A) Effects of the CB1 receptor antagonist SR141716A, at a concentration of 10 µM, on TASK-1 inhibition induced by 3 µM WIN552122 and 3 µM methanandamide. (B) Effects of 10 µM anandamide on a C-terminally truncated mutant of TASK-1 (TA248 deleted at His280) lacking all phosphorylation sites. Current densities measured at 0 mV in a physiological K+ gradient are 8.4 ± 1.1 pA/pF (n = 15) for ΔCt TASK-1 and 16.9 ± 0.8 pA/pF (n = 119) for TASK-1 WT. (C) Effects of 10 µM anandamide and external acidosis to pH 6.4 on the mutant TASK-1 ΔCt. (D) 3 µM methanandamide produces a similar block of TASK-1 expressed in COS, CHO and HEK transfected cells. TASK-1 currents are measured at 0 mV. The holding potential is –80 mV and cells are stimulated with voltage ramps of 800 ms duration, from –130 mV up to 100 mV. (E) Effects of 3 µM methanandamide (met) on an outside-out patch expressing TASK-1. The holding potential is –80 mV and the external medium contains 155 mM K+. (F) Effects of 3 µM methanandamide (met) on an outside-out patch expressing a chimera between the core of TASK-1 and the entire C-terminal region of TREK-1 (TA242/TR293). The holding potential is –80 mV and cells are stimulated with voltage ramps of 800 ms duration, from –130 mV up to 100 mV. Currents are recorded in a physiological K+ gradient and the intracellular medium contains 1 mM GDP-βs.
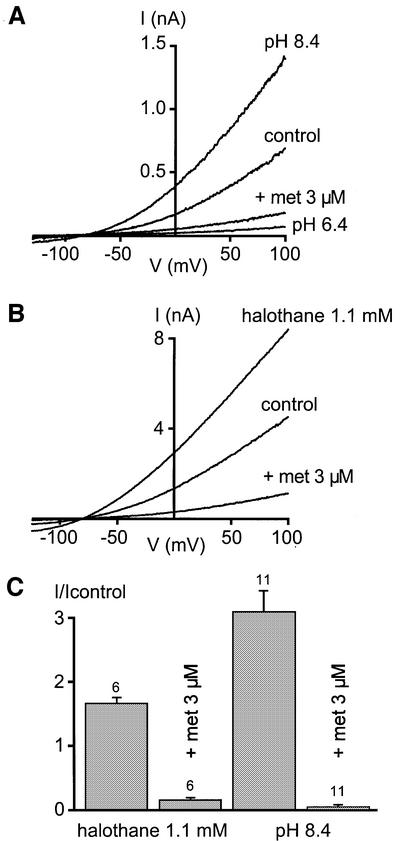
Considering that TASK-1 is an acid-sensitive K+ channel, we investigated whether anandamide inhibition might be affected by pH. Anandamide produces a similar inhibition at pH 7.4 and 8.4, and mimics the effect of an external acidosis to pH 6.4 (Figure 4A and C). We next investigated the effect of anandamide in the presence of halothane, an opener of TASK-1 (Patel et al., 1999; Sirois et al., 2000). Halothane produces a stimulation of TASK-1 current amplitude, which is completely inhibited by anandamide (Figure 4B and C).
Fig. 4. Methanandamide reverses TASK-1 opening by alkaline pH and by the inhalational anaesthetic halothane. (A) Effects of 3 µM methanandamide (met) on TASK-1 currents elicited at pH 8.4. External acidosis to pH 6.4 mimics the effect of methanandamide at pH 8.4. (B) Effects of 3 µM methanandamide (met) on TASK-1 currents in the presence of 1.1 mM halothane. (C) Summary of the effects of 3 µM methanandamide (met) at pH 8.4 and in the presence of 1.1 mM halothane, measured at 0 mV. Currents are recorded in transfected COS cells with the whole-cell patch–clamp configuration. The holding potential is –80 mV and cells are stimulated with voltage ramps of 800 ms duration, from –130 mV up to 100 mV.
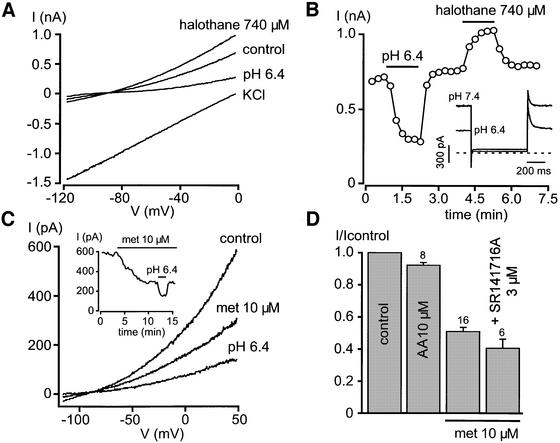
TASK-1 has recently been proposed to encode the standing-outward K+ current IKso in cerebellar granule neurons (Millar et al., 2000). IKso shares the properties of TASK-1 (Millar et al., 2000), as demonstrated by the reversible block by external acidosis, time- and voltage-independence, and the stimulation by inhalational anaesthetics (Figure 5A and B). In a K+-rich solution, IKso is linear over the whole voltage range, demonstrating its typical background TASK-like activity (Figure 5A). About 50% of the pH-sensitive component of IKso is blocked by 10 µM anandamide (Figure 5C and D), a concentration that fully inhibits TASK-1 (Figure 2C). This effect is not mimicked by an equivalent concentration of arachidonic acid (Figure 5D). As observed for TASK-1, anandamide-induced IKso inhibition is insensitive to the CB1 receptor antagonist SR141716A (Figure 5D). Anandamide (10 µM) depolarizes cerebellum granule neurons (from –83 ± 2 mV to –75 ± 2 mV, n = 7) in the presence of the CB1 receptor antagonist SR141716 (3 µM) and mimics part of the effects of an external acidosis to pH 6.4 (from –83 ± 2 mV to –62 ± 1 mV, n = 3).
Fig. 5. Anandamide blocks IKso in cerebellum granule neurons. (A) Identification of IKso in cultured cerebellar granule neurons. Currents are recorded in the perforated patch configuration with voltage ramps of 600 ms duration, applied from a holding potential of 0 mV. IKso is inhibited at pH 6.4 and stimulated by 740 µM halothane. In a symmetrical K+ gradient, the I–V relationship is linear and reverses at 0 mV. (B) Time course of the effects of external acidosis and halothane on IKso recorded at ∼0 mV. The inset shows that external acidosis to pH 6.4 reduces the steady outward current recorded at 0 mV, but fails to affect the current recorded at –80 mV. (C) Effects of 10 µM methanandamide (met) and external acidosis to pH 6.4 on IKso recorded during a voltage ramp from 50 to –120 mV. The holding potential is 0 mV. The time course of the experiment (monitored at 50 mV) is illustrated in the inset. (D) Effects of 10 µM methanandamide (met) on IKso in the absence and in the presence of 3 µM CB1 receptor antagonist SR141716A. The addition of 10 µM arachidonic acid fails to affect IKso. IKso is recorded at 0 mV.
Discussion
Anandamide, the amide of arachidonic acid with ethanolamine, is the first endogenous ligand of CB receptor and was purified from porcine brain (Dewey, 1986; Di Marzo et al., 1998; Mechoulam et al., 1998; Chaperon and Thiebot, 1999). Anandamide reproduces most of the physiological and neurobehavioural effects of the active compound of marijuana, Δ9-THC, including sedation, analgesia and hypothermia (Dewey, 1986; Di Marzo et al., 1998; Mechoulam et al., 1998; Chaperon and Thiebot, 1999). 2-AG has also been shown to behave as a natural functional agonist of CB receptors (Di Marzo et al., 1998). This paper demonstrates that submicromolar concentrations of anandamide directly block the 2P domain K+ channel, TASK-1. This effect is not mimicked by several high affinity synthetic and natural CB1/CB2 receptor agonists, nor is it sensitive to the specific CB1 receptor antagonist SR141716A. Channel block is independent of TASK-1 phosphorylation sites, intracellular Ca2+ and G-proteins, and is observed in the excised patch configuration. These results suggest that anandamide-induced TASK-1 channel block does not involve the CB1/CB2 receptors and is direct. The modulation of TASK-1 by anandamide is specific among other natural agonists, including 2-AG and Δ9-THC, and does not involve hydrolysis.
In cerebellar granule neurons, about half of the acid-sensitive IKso is blocked by 10 µM methanandamide, suggesting that it might be related to TASK-1, as previously proposed (Millar et al., 2000). The time course of IKso inhibition by methanandamide is slower by a factor of 5–10 as compared with inhibition of TASK-1 expressed in COS cells. The peripheral localization of TASK-1 in the diffuse neuropil of the cerebellum (Kindler et al., 2000) might explain the slow time course of inhibition as anandamide was locally applied to the cell body. Two other acid-sensitive 2P domain K+ channels (TASK-2 and TASK-3) have been identified (Reyes et al., 1998; Kim et al., 2000; Rajan et al., 2000). TASK-2 is not expressed in neurons, but TASK-3 is very abundant in the cerebellum (Reyes et al., 1998; Chapman et al., 2000; Kim et al., 2000; Rajan et al., 2000). TASK-3, which is only partially blocked by 10 µM anandamide, might thus encode the acid-sensitive anandamide-resistant component of IKso in cerebellum granule neurons. However, we cannot entirely rule out the participation of other, still unknown acid-sensitive 2P domain background K+ channels in these neurons. The divergence between TASK-1 and TASK-3 (61% similarity) is in the extracellular M1P1 loop as well as in the C-terminal region (Kim et al., 2000; Rajan et al., 2000). Deletion of the C-terminus of TASK-1 does not alter the block by anandamide, demonstrating that this region is probably not involved. The M1P1 loop of TASK-1 is thus a candidate region possibly responsible for the anandamide sensitivity. Fusing the M1P1 loop of TASK-1 to the core of TREK-1 does not transfer anandamide sensitivity, demonstrating that this region of the channel protein might be necessary although not sufficient.
Acid inhibits a TASK-1-like background K+ current in cerebellar granule neurons, somatic motoneurons, locus coeruleus cells, type I carotid body cells and in glomerulosa cells of the adrenal cortex, producing depolarization and an increase in excitability (Kim et al., 1999; Buckler et al., 2000; Czirjak et al., 2000; Millar et al., 2000; Sirois et al., 2000; Talley et al., 2000). High levels of TASK-1 channel expression may suggest a specialized role in acid sensing for these cells (Brown, 2000; North, 2000). This is particularly relevant in type I carotid body cells, the chemoreceptors involved in sensing blood pH (and oxygen) (Buckler et al., 2000).
TASK-1-like background K+ currents in motoneurons and cerebellar granule neurons are inhibited by the activation of several Gαq-coupled receptors, including the muscarinic M3 receptor (Czirjak et al., 2000; Millar et al., 2000; Talley et al., 2000). The fact that CB1 and CB2 receptors are usually not coupled to Gq-proteins is another argument against the possible involvement of CB receptors in the down-modulation of TASK-1 by anandamide (Ho et al., 1999). TASK-1 is insensitive to both PKC and PKA activation, to intracellular Ca2+ and to arachidonic acid (Duprat et al., 1997; Leonoudakis et al., 1998; Kim et al., 1999). The signal transduction pathway by which Gαq-coupled receptors inhibit TASK-1 currents remains unknown. Importantly, the C-terminus of TASK-1, which comprises all the putative phosphorylation sites, is not required, ruling out the involvement of TASK-1 phosphorylation in the mechanism of anandamide inhibition. Anandamide, which is released upon receptor stimulation, could be considered as a candidate second messenger mediating TASK-1 inhibition (Giuffrida et al., 1999). However, a direct demonstration of the involvement of anandamide would require a specific phospholipase D inhibitor, which is unfortunately not available. Closing of TASK-1 channels by neurotransmitters will enhance excitability and amplify synaptic inputs (Brown, 2000; North, 2000). On the contrary, opening of background K+ currents by inhalational anaesthetics will hyperpolarize neurons, reduce excitability and synaptic inputs, and thus contribute to the phenomenon of general anaesthesia (Franks and Lieb, 1999; Patel et al., 1999).
Natural (including anandamide) and synthetic cannabinoids provoke a series of physiological and behavioural effects, including hypothermia, hypokinesia, analgesia and catalepsy (Di Marzo et al., 1998; Mechoulam et al., 1998; Chaperon and Thiebot, 1999). Although anandamide produces most of the effects induced by other natural and synthetic psychoactive cannabinoids, important differences exist (Crawley et al., 1993; Compton and Martin, 1997; Adams et al., 1998; Chakrabarti et al., 1998; Lichtman et al., 1998; Chaperon and Thiebot, 1999; Jarai et al., 1999). For instance, the cognitive and memory processes, anxiety-related behaviour and food consumption observed with Δ9-THC remain unchanged with anandamide (Crawley et al., 1993). In addition, Δ9-THC- but not anandamide-induced hypothermia, antinociception and immobility are reversed by the CB1 receptor antagonist SR141716A in rodents (Compton and Martin, 1997; Adams et al., 1998). These observations suggest that some of the pharmacological effects of anandamide are independent of CB1/CB2 receptors.
Anandamide profoundly affects locomotion and the effect is dose dependent, with a stimulation at low dose but a depression at high dose (Chaperon and Thiebot, 1999). At low dose, anandamide produces ataxia in the mouse strain C57BL/6, which is not related to CB1 gene expression (Chakrabarti et al., 1998). Furthermore, low doses of anandamide stimulate mouse behavioural activities in the open field and on the ring, and aggressive behaviour in timid singly housed mice (Sulcova et al., 1998). The direct modulation of TASK-1 by low doses of anandamide might thus explain some of the bi-phasic effects often observed with anandamide in vivo (Chakrabarti et al., 1998; Sulcova et al., 1998). The anandamide-sensitive channel TASK-1 is highly expressed in both somatic motoneurons and cerebellar granule neurons. These neurons are involved in the control and coordination of motor behaviour. It is thus possible that direct block of TASK-1 and subsequent alteration of excitability might be responsible for some of the non-CB1 receptor effects of anandamide on locomotion. Given the high density of TASK-1 (Talley et al., 2000), the remarkable abundance of anandamide and the scarcity of CB1 receptor (Bisogno et al., 1999) in the brain stem, regulation of TASK-1 by anandamide might thus also be functionally important in this area.
A direct effect of anandamide on ionic channels is not without precedent. Anandamide inhibits Kv channels independently of the CB1 receptors (IC50 of 2.7 µM), although, unlike TASK-1, this effect is mimicked by arachidonic acid (Honoré et al., 1994; Poling et al., 1996). A non-CB receptor mechanism was shown to be responsible for the powerful hypotensive effect of anandamide. Arterial vasorelaxation induced by anandamide is insensitive to the CB1 receptor antagonist SR141716A (Zygmunt et al., 1999). However, it is reversed by capsazepine, a capsaicin receptor antagonist (VR1) (Zygmunt et al., 1999). A direct activation of the cationic channel VR1 by anandamide was shown by patch–clamp, and was proposed to lead to the release of the powerful vaso-relaxing peptide CGRP from activated perivascular capsaicin-sensitive sensory fibres. Anandamide is thus a possible endogenous ligand for VR1 (Zygmunt et al., 1999; Smart et al., 2000), a channel that is of great importance for sensing temperature and pH variations in the pain pathway (Caterina et al., 1997).
The present study identifies the K+ channel TASK-1 as another potentially important molecular target for anandamide. The closing of TASK-1, as well as the opening of VR1, by anandamide will induce depolarization and enhance excitability. This suggests that besides the classical action on G-protein-coupled CB receptors, anandamide can also act at low concentrations on a variety of ionic channels. The direct closing of TASK-1 has to be taken into account when trying to understand the powerful pharmacological and physiological effects of anandamide.
Materials and methods
COS cell culture, transfection, site-directed mutagenesis and electrophysiology procedures have been extensively described elsewhere (Fink et al., 1996, 1998; Duprat et al., 1997; Patel et al., 1999; Lesage et al., 2000).
Cell culture
COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. The 2P domain K+ channel cDNAs hTWIK-1, rTWIK-2, mTREK-1, hTREK-2, mTRAAK, hTASK-1, hTASK-2 and hTASK-3 were subcloned into the pIRES-CD8 vector and transfected using the DEAE dextran procedure. Cells were visualized 48 h after transfection using the anti-CD8 antibody-coated beads method.
Solutions
For whole-cell and outside-out patch experiments with transfected COS cells, bath solution contained 150 mM NaCl, 5 mM KCl, 3 mM MgCl2, 1 mM CaCl2, 10 mM HEPES pH 7.4 (with NaOH), and pipette solution contained 150 mM KCl, 3 mM MgCl2, 5 mM EGTA and 10 mM HEPES pH 7.2 (with KOH). Cerebellar granule cells were isolated from 9-day-old Sprague–Dawley rats and cultured as described (Millar et al., 2000). Whole-cell currents were recorded in the perforated configuration with amphotericin B (240 μg/ml) from neurons between 10 and 14 days in culture. The external solution contained 120 mM NaCl, 2.5 mM KCl, 2 mM MgCl2, 0.5 mM CaCl2, 5 mM glucose, 10 mM HEPES pH 7.4 (with NaOH), and pipette solution contained 125 mM KCl, 5 mM MgCl2, 0.1 mM EGTA and 5 mM HEPES pH 7.2 (with KOH). Cells were continuously superfused with a microperfusion system during the time course of the experiments (0.1 ml/min), as previously described (Patel et al., 1998).
Chemicals
SR141716 [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4- methyl-3-pyrazole-carboxamide] (10 mM in DMSO) was a gift from Sanofi Recherche (Montpellier, France). R(+)-methanandamide and 2-AG were obtained from RBI. Anandamide, palmitoylethanolamide (10 mM in DMSO), WIN552122 (100 mM in DMSO), HU210 (100 mM in DMSO) and CP55940 (100 mM in DMSO) were obtained from TOCRIS. Δ9-THC, arachidonic acid (100 mM in ethanol) and lipid-free BSA were obtained from Sigma. Halothane was obtained from Belamont and concentrations were determined as previously described (Patel et al., 1999). Solvents were included in control solutions.
Acknowledgments
Acknowledgements
We are grateful to F.Lesage and C.Girard for providing us with the 2P domain K+ channels plasmids. We are grateful to SANOFI Recherche (Montpellier, France) for the gift of SR141716A. We wish to thank M.Jodar and V.Lopez for excellent technical assistance. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Association Française contre les Myopathies (AFM) and the Ministére de la Recherche et de la Technologie.
REFERENCES
- Adams I.B., Compton,D.R. and Martin,B.R. (1998) Assessment of anandamide interaction with the cannabinoid brain receptor. J. Pharmacol. Exp. Ther., 284, 1209–1217. [PubMed] [Google Scholar]
- Bang H., Kim,Y. and Kim,D. (2000) TREK-2, a new member of the mechanosensitive tandem pore K+ channel family. J. Biol. Chem., 275, 17412–17419. [DOI] [PubMed] [Google Scholar]
- Bisogno T., Berrendero,F., Ambrosino,G., Cebeira,M., Ramos,J.A., Fernandez-Ruiz,J.J. and Di Marzo,V. (1999) Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem. Biophys. Res. Commun., 256, 377–380. [DOI] [PubMed] [Google Scholar]
- Brown D.A. (2000) Neurobiology: the acid test for resting potassium channels. Curr. Biol., 10, R456–R459. [DOI] [PubMed] [Google Scholar]
- Buckler K., Williams,B. and Honoré,E. (2000) An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol., 525, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher,M.A., Tominaga,M., Rosen,T.A., Levine,J.D. and Julius,D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature, 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Ekuta,J.E. and Onaivi,E.S. (1998) Neurobehavioral effects of anandamide and cannabinoid receptor gene. Brain Res. Bull., 45, 67–74. [DOI] [PubMed] [Google Scholar]
- Chaperon F. and Thiebot,M.H. (1999) Behavioral effects of cannabinoid agents in animals. Crit. Rev. Neurobiol., 13, 243–281. [DOI] [PubMed] [Google Scholar]
- Chapman C.G. et al. (2000) Cloning, localisation and functional expression of a novel human. Brain Res. Mol. Brain Res., 82, 74–83. [DOI] [PubMed] [Google Scholar]
- Chavez R.A., Gray,A.T., Zhao,B.B., Kindler,C.H., Mazurek,M.J., Mehta,Y., Forsayeth,J.R. and Yost,C.S. (1999) TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family. J. Biol. Chem., 274, 7887–7892. [DOI] [PubMed] [Google Scholar]
- Compton D.R. and Martin,B.R. (1997) The effect of the enzyme inhibitor phenylmethylsulfonyl fluoride on thepharmacological effect of anandamide in the mouse model of cannabimimetic activity. J. Pharmacol. Exp. Ther., 283, 1138–1143. [PubMed] [Google Scholar]
- Crawley J.N., Corwin,R.L., Robinson,J.K., Felder,C.C., Devane,W.A. and Axelrod,J. (1993) Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol. Biochem. Behav., 46, 967–972. [DOI] [PubMed] [Google Scholar]
- Czirjak G., Fischer,T., Spat,A., Lesage,F. and Enyedi,P. (2000) TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa. Mol. Endocrinol., 14, 863–874. [DOI] [PubMed] [Google Scholar]
- Dewey W.L. (1986) Cannabinoid pharmacology. Pharmacol. Rev., 38, 151–178. [PubMed] [Google Scholar]
- Di Marzo V., Melck,D., Bisogno,T. and De Petrocellis,L. (1998) Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci., 21, 521–528. [DOI] [PubMed] [Google Scholar]
- Duprat F., Lesage,F., Fink,M., Reyes,R., Heurteaux,C. and Lazdunski,M. (1997) TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J., 16, 5464–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M., Duprat,F., Lesage,F., Reyes,R., Romey,G., Heurteaux,C. and Lazdunski,M. (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J., 15, 6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M., Lesage,F., Duprat,F., Heurteaux,C., Reyes,R., Fosset,M. and Lazdunski,M. (1998) A neuronal two P domain K+ channel activated by arachidonic acid polyunsaturated fatty acid. EMBO J., 17, 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.P. and Lieb,W.R. (1999) Background K+ channels: an important target for volatile anesthetics? Nature Neurosci., 2, 395–396. [DOI] [PubMed] [Google Scholar]
- Giuffrida A., Parsons,L.H., Kerr,T.M., Rodriguez de Fonseca,F., Navarro,M. and Piomelli,D. (1999) Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nature Neurosci., 2, 358–363. [DOI] [PubMed] [Google Scholar]
- Ho B.Y., Uezono,Y., Takada,S., Takase,I. and Izumi,F. (1999) Coupling of the expressed cannabinoid CB1 and CB2 receptors tophospholipase C and G protein-coupled inwardly rectifying K+ channels. Receptors Channels, 6, 363–374. [PubMed] [Google Scholar]
- Honoré E., Barhanin,J., Attali,B., Lesage,F. and Lazdunski,M. (1994) External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc. Natl Acad. Sci. USA, 91, 1937–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z. et al. (1999) Cannabinoid-induced mesenteric vasodilation through an endothelial site. Proc. Natl Acad. Sci. USA, 96, 14136–14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Bang,H. and Kim,D. (1999) TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am. J. Physiol., 277, H1669–H1678. [DOI] [PubMed] [Google Scholar]
- Kim Y., Bang,H. and Kim,D. (2000) TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem., 275, 9340–9347. [DOI] [PubMed] [Google Scholar]
- Kindler C.H., Pietruck,C., Yost,C.S., Sampson,E.R. and Gray,A.T. (2000) Localization of the tandem pore domain K+ channel TASK-1 in the rat. Brain Res. Mol. Brain Res., 80, 99–108. [DOI] [PubMed] [Google Scholar]
- Klein T.W., Newton,C. and Friedman,H. (1998) Cannabinoid receptors and immunity. Immunol. Today, 19, 373–381. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D., Gray,A.T., Winegar,B.D., Kindler,C.H., Harada,M., Taylor,D.M., Chavez,R.A., Forsayeth,J.R. and Yost,C.S. (1998) An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J. Neurosci., 18, 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F. and Lazdunski,M. (1999) Potassium channels with two P domains. In Kurachi,Y. (ed.), Current Topics in Membranes. Academic Press, San Diego, CA, pp. 199–222.
- Lesage F., Guillemare,E., Fink,M., Duprat,F., Lazdunski,M., Romey,G. and Barhanin,J. (1996) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J., 15, 1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Terrenoire,C., Romey,G. and Lazdunski,M. (2000) Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids and Gs, Gi and Gq protein-coupled receptors. J. Biol. Chem., 275, 28398–28405. [DOI] [PubMed] [Google Scholar]
- Lichtman A.H., Wiley,J.L., La Vecchia,K.L., Neviaser,S.T., Arthur, D.B., Wilson,D.M. and Martin,B.R. (1998) Effects of SR 141716A after acute or chronic cannabinoid administration in dogs. Eur. J. Pharmacol., 357, 139–148. [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Fride,E. and Di Marzo,V. (1998) Endocannabinoids. Eur. J. Pharmacol., 359, 1–18. [DOI] [PubMed] [Google Scholar]
- Millar J.A., Barratt,L., Southan,A.P., Page,K.M., Fyffe,R.E., Robertson,B. and Mathie,A. (2000) A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc. Natl Acad. Sci. USA, 97, 3614–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R.A. (2000) Potassium-channel closure taken to TASK. Trends Neurosci., 23, 234–235. [DOI] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Maingret,F., Lesage,F., Fink,M., Duprat,F. and Lazdunski,M. (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J., 17, 4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Lesage,F., Fink,M., Romey,G. and Lazdunski,M. (1999) Inhalational anaesthetics activate two-pore-domain background K+ channels. Nature Neurosci., 2, 422–426. [DOI] [PubMed] [Google Scholar]
- Patel A.J., Maingret,F., Magnone,V., Fosset,M., Lazdunski,M. and Honoré,E. (2000) TWIK-2, an inactivating 2P domain K+ channel. J. Biol. Chem., 275, 28722–28730. [DOI] [PubMed] [Google Scholar]
- Poling J.S., Rogawski,M.A., Salem,N.,Jr and Vicini,S. (1996) Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology, 35, 983–991. [DOI] [PubMed] [Google Scholar]
- Rajan S., Wischmeyer,E., Liu,G.X., Preisig-Muller,R., Daut,J., Karschin,A. and Derst,C. (2000) TASK-3, a novel tandem pore-domain acid-sensitive K+ channel: an extracellular histidine as pH sensor. J. Biol. Chem., 275, 16650–16657. [DOI] [PubMed] [Google Scholar]
- Reyes R., Duprat,F., Lesage,F., Fink,M., Farman,N. and Lazdunski,M. (1998) Cloning and expression of a novel pH-sensitive two pore domain potassium channel from human kidney. J. Biol. Chem., 273, 30863–30869. [DOI] [PubMed] [Google Scholar]
- Salinas M., Reyes,R., Lesage,F., Fosset,M., Heurteaux,C., Romey,G. and Lazdunski,M. (1999) Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J. Biol. Chem., 274, 11751–11760. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S.A., Camardo,J.S. and Kandel,E.R. (1982) Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature, 299, 413–417. [DOI] [PubMed] [Google Scholar]
- Sirois J.E., Lei,Q., Talley,E.M., Lynch,C.,III and Bayliss,D.A. (2000) The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J. Neurosci., 20, 6347–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D., Gunthorpe,M.J., Jerman,J.C., Nasir,S., Gray,J., Muir,A.I., Chambers,J.K., Randall,A.D. and Davis,J.B. (2000) The endogenous lipid anandamide is a full agonist at the human vanilloid. Br. J. Pharmacol., 129, 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulcova E., Mechoulam,R. and Fride,E. (1998) Biphasic effects of anandamide. Pharmacol. Biochem. Behav., 59, 347–352. [DOI] [PubMed] [Google Scholar]
- Talley E.M., Lei,Q., Sirois,J.E. and Bayliss,D.A. (2000) TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron, 25, 399–410. [DOI] [PubMed] [Google Scholar]
- Zygmunt P.M., Petersson,J. Andersson,D.A., Chuang,H., Sorgard,M., Di Marzo,V., Julius,D. and Hogestatt,E.D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamine. Nature, 400, 452–457. [DOI] [PubMed] [Google Scholar]