Abstract
DNA double strand break (DSB) repair by non-homologous end joining (NHEJ) in mammalian cells requires the Ku70–Ku80 heterodimer, the DNA-PK catalytic subunit DNA-PKcs, as well as DNA ligase IV and Xrcc4. NHEJ of plasmid DSBs in Saccharomyces cerevisiae requires Ku, Xrcc4 and DNA ligase IV, as well as Mre11, Rad50, Xrs2 and DNA damage checkpoint proteins. Saccharomyces cerevisiae Ku is also required for telomere length maintenance and transcriptional silencing. We have characterized NHEJ in Schizosaccharomyces pombe using an extrachromosomal assay and find that, as anticipated, it is Ku70 and DNA ligase IV dependent. Unexpectedly, we find that Rad32, Rad50 (the S.pombe homologues of Mre11 and Rad50, respectively) and checkpoint proteins are not required for NHEJ. Furthermore, although S.pombe Ku70 is required for maintenance of telomere length, it is dispensable for transcriptional silencing at telomeres and is located throughout the nucleus rather than concentrated at the telomeres. Together, these results provide insight into the mechanism of NHEJ and contrast significantly with recent studies in S.cerevisiae.
Keywords: DNA ligase IV/Ku70/Mre11/silencing/telomere length
Introduction
DNA double strand breaks (DSBs) have the potential to disrupt genomic integrity. If the damage is left unrepaired or is misrepaired, it may lead to chromosomal abnormalities or loss of genetic material, events that can cause cell death or ultimately the onset of cancer. Cells have evolved at least two pathways that can repair DSBs: homologous recombination, which repairs the breaks by retrieving genetic information from a sister chromatid or a homologous chromosome, and non-homologous end joining (NHEJ), a process involving direct sealing of the ends utilizing little or no homology.
NHEJ is the major mechanism for the repair of radiation-induced DSBs in higher organisms. Five proteins involved in the process have been identified in mammalian cells (reviewed in Featherstone and Jackson, 1999); the DNA-PK complex, which comprises the Ku70–Ku80 heterodimer and a catalytic subunit (DNA-PKcs), and a DNA ligase IV–Xrcc4 complex. Cells with mutations in any of the genes corresponding to these factors display radiosensitivity and defective rejoining of DSBs as measured by pulsed-field gel electrophoresis. DNA-PKcs-defective mice grow normally (Gao et al., 1998a), Ku70 and Ku80 null mice are viable but show premature senescence (Nussenzweig et al., 1996; Gu et al., 1997), whereas deletion of the gene for either DNA ligase IV or Xrcc4 results in early embryonic lethality (Gao et al., 1998b). Thus, either NHEJ differs in its requirements for these proteins or they have separate functions in addition to their role in NHEJ. All five proteins are involved in V(D)J recombination, a site-specific recombination event responsible for generating antibody and T-cell receptor diversity in mammalian cells. It has been reported that DNA-PK is also necessary for the maintenance of the telomere structure in mammalian cells (Bailey et al., 1999).
Although NHEJ was first characterized in mammalian cells, Saccharomyces cerevisiae has served as a useful model organism to understand NHEJ and to identify additional functions of the proteins. DNA-PKcs has not been identified in the completed sequence of the S.cerevisiae genome, but KU70 and KU80 homologues (named YKU70 and YKU80, respectively) are present and are essential for NHEJ repair of DSBs in plasmids that have been transformed into cells. In addition, budding yeast YKU70 and YKU80 are also required for the maintenance of telomere length, telomere clustering, localization of telomeres to the nuclear periphery and for transcriptional silencing adjacent to telomeres (Boulton and Jackson, 1996a, 1998; Laroche et al., 1998). This demonstrates that in S.cerevisiae, Ku, at least, has roles in addition to NHEJ. Saccharomyces cerevisiae LIG4 and LIF1 (the homologues of DNA ligase IV and Xrcc4, respectively) are also necessary for NHEJ (Teo and Jackson, 1997; Wilson et al., 1997; Herrmann et al., 1998). Several other proteins have also been implicated in NHEJ in S.cerevisiae. These include: members of the Mre11–Rad50–Xrs2 complex (Boulton and Jackson, 1998), which are also involved in DSB repair by homologous recombination (reviewed in Petrini, 1999); DNA integrity checkpoint proteins Rad9, Mec1, Mec3, Rad17 and Rad24 (de la Torre-Ruiz and Lowndes, 2000); and three proteins involved in transcriptional silencing Sir2, 3 and 4 (reviewed in Gartenberg, 2000). It has been shown that the S.cerevisiae Ku and Sir proteins are located predominantly at telomeric regions in undamaged cells and that they become redistributed throughout the nucleus upon induction of DNA damage. This redistribution is dependent on the Mec1/Rad9 checkpoint proteins (Martin et al., 1999; McAinsh et al., 1999; Mills et al., 1999). The model emerging from these studies in S.cerevisiae is that Ku is stored at the telomeres in a pre-formed complex (possibly containing the Sir proteins among other factors). In the presence of DSBs, the DNA damage checkpoint signals the release of Ku and Sir proteins from these telomeric complexes, Ku then relocates to DSBs and enhances repair by DNA end protection and by attracting other NHEJ proteins such as the DNA ligase IV–Xrcc4 complex. Although the Sir proteins are also recruited to DSBs, their role in the repair process remains to be elucidated.
The fission yeast Schizosaccharomyces pombe has also proved to be a good model system for studying many cellular processes. In particular, various aspects of cell cycle regulation, including the DNA damage response in fission yeast, have provided insight into similar pathways in human cells. In this study, we have used S.pombe to study NHEJ and the roles of its component proteins. Here we report the characterization of the homologues of Ku70 and DNA ligase IV in S.pombe (from now on referred to as Pku70 and Lig4, respectively) and show that they are, as expected, required for NHEJ of plasmid DSBs. However, we find that S.pombe cells deleted for either rad32 or rad50 (homologues of MRE11 and RAD50, respectively) as well as a range of DNA damage/replication checkpoint mutants are proficient in NHEJ. Thus, the genetic requirements for NHEJ differ between S.cerevisiae and S.pombe. Further characterization of Pku70 shows that, while it is involved in telomere length maintenance, in marked contrast to S.cerevisiae, it is dispensable for telomeric silencing and is not located predominantly at the telomeres.
Results
Identification of the Ku70 and DNA ligase IV homologues in S.pombe
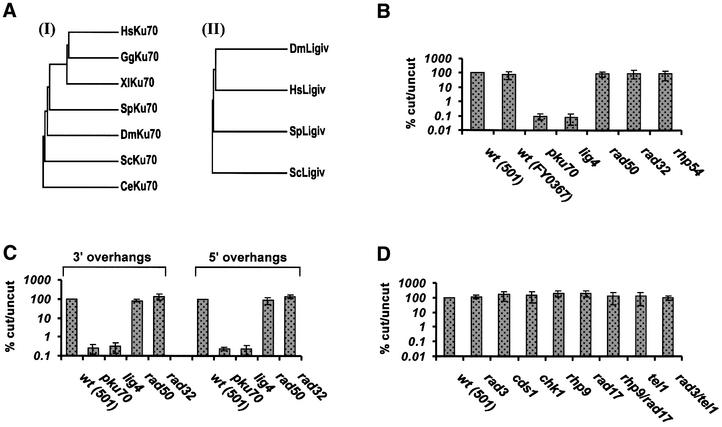
To identify factors involved in NHEJ in S.pombe, we used the sequences of known NHEJ proteins to search the S.pombe database (www.sanger.ac.uk/Projects/S_pombe). Using the TBLASTN program, we identified two open reading frames in cosmids SPCC126 and SPCC1183 with strong homology to Ku70 and DNA ligase IV, respectively. Both are located on chromosome 3. The pku70 gene contains five introns and encodes a protein of 607 amino acids with a predicted mol. wt of 69.1 kDa. The lig4 gene encodes a 923 amino acid protein with a predicted mol. wt of 107.3 kDa and contains nine introns. Both proteins show ∼42% similarity and 30% identity to their respective human homologues. Phylogenetic trees showing the evolutionary relationship of Ku70 and DNA ligase IV proteins from different organisms are shown in Figure 1A.
Fig. 1. Genetic requirements for NHEJ in S.pombe. (A) Phylogenetic trees showing the evolutionary relationship of (I) Ku70 and (II) DNA ligase IV proteins from different organisms. The trees are based on multiple sequence alignments created using the PILEUP program of the GCG software. DDBJ/EMBL/GenBank accession Nos are as follows: HsKu70 (Homo sapiens), P12956; GgKu70 (Gallus gallus), O93257; XlKu70 (Xenopus laevis), BAA76953; SpKu70 (Schizosaccharomyces pombe), CAA22471; DmKu70 (Drosophila melanogaster), Q23976; ScKu70 (Saccharomyces cerevisiae), P32807; CeKu70 (Caenorhabditis elegans), CAB55094; DmLigIV (D.melanogaster), AAF48298; HsLigIV (H.sapiens), NP_002303 with the addition of 67 amino acids at the N-terminus as described in Grawunder et al. (1998); SpLigIV (S.pombe), CAA21085; ScLigIV (S.cerevisiae), Q08387. (B–D) Analysis of NHEJ in S.pombe mutants using a plasmid-based assay: (B and D) blunt DSBs, (C) cohesive DSBs. FY0367 is the pku70 parental strain.
Deletion of genes encoding fission yeast Ku70 or DNA ligase IV abolishes NHEJ
To assess the involvement of Pku70 and Lig4 in DSB repair by NHEJ, we adapted the in vivo plasmid DSB repair assay (see Materials and methods). Plasmid PS, containing a LEU2 selectable marker, has two PvuII and two PstI restriction sites flanking a small fragment. Restriction with either enzyme results in the excision of the fragment and generation of linear molecules with blunt ends, or cohesive ends with 3′ overhangs, respectively. Similarly, an EcoRI fragment was excised from plasmid PI for the generation of substrates with 5′ overhangs. These substrates were then transformed into S.pombe cells. The excision of a fragment allows us to distinguish contaminating uncut plasmids (that retain the fragment) from accurately rejoined ones by restriction digestion. The frequency of NHEJ was determined by measuring the number of leu+ colonies (capable of growth on plates lacking supplementing leucine) arising from cells transformed with linear DNA divided by the colonies arising following transformation of undigested control plasmid. The region in the vicinity of the DSB does not bear homology to any S.pombe genomic sequences and the break can therefore be repaired only by NHEJ. Consistent with previous work (Wilson et al., 1999), we found that S.pombe wild-type cells were equally proficient in the repair of blunt- and cohesive-ended DSBs (data not shown). In contrast, S.cerevisiae wild-type cells are inefficient at repairing blunt-ended DSBs by NHEJ and no further reduction is observed in YKU deletion strains (Boulton and Jackson, 1996b). In agreement with the independence of our NHEJ assay from homologous recombination activities, we found that rhp54Δ, a strain defective in homologous recombination (Muris et al., 1996), is proficient in NHEJ (Figure 1B). We generated pku70 and lig4 deletion strains and found that the frequency of rejoining of blunt DSBs is decreased ∼1000-fold in these cells compared with wild-type cells (isogenic pku70+ and lig4+) (Figure 1B). This demonstrates that repair of blunt-ended DSBs is not only efficient in S.pombe but is also dependent on Ku and DNA ligase IV. pku70Δ and lig4Δ strains displayed an ∼350-fold reduction in the rejoining of cohesive-ended breaks with 3′ or 5′ overhangs compared with the wild type (Figure 1C).
The fidelity of NHEJ was analysed by investigating the nature of the junctions. First, the accuracy of repair was determined by examining the ability of plasmids to be redigested with the original restriction enzyme used for the preparation of the substrate. Using this criterion, in wild-type S.pombe, it was found that ∼40% of blunt DSBs were repaired accurately, whereas rejoining of cohesive DSBs (with either 5′ or 3′ overhangs) was rarely accurate (Table I). This finding contrasts with the error-free rejoining of cohesive DSBs by NHEJ observed in S.cerevisiae wild-type cells, using a similar assay (Boulton and Jackson, 1996b).
Table I. Accuracy of NHEJ.
| Strain | Blunt DSBs |
DSBs with 5′ overhangs |
DSBs with 3′ overhangs |
|||
|---|---|---|---|---|---|---|
| Accurate | Total | Accurate | Total | Accurate | Total | |
| Wild type (501) | 22 | 56 | 2 | 21 | 1 | 13 |
| pku70Δ | 0 | 2a | 0 | 5a | 0 | 3a |
| lig4Δ | 0 | 3a | 0 | 2a | ND | ND |
| rad32Δ | 25 | 42 | 2 | 17 | 0 | 18 |
| rhp50Δ | 15 | 55 | 1 | 20 | 0 | 22 |
aThe small number of junctions obtained in these cases is due to the severe inability of pku70Δ and lig4Δ strains to rejoin linear plasmids.
ND, not done.
Repair in pku70Δ and lig4Δ strains was always associated with loss of the restriction site (Table I). Forty percent of the small number of plasmids recovered from these strains proved to be undigested contaminants and have been eliminated from the calculations since they can be distinguished from accurately repaired junctions, as they give two bands following restriction digestion. Undigested contaminants were never recovered from strains that showed wild-type levels of NHEJ but were recovered from pku70Δ and lig4Δ, presumably because they constitute a high proportion of surviving circular plasmids in these strains.
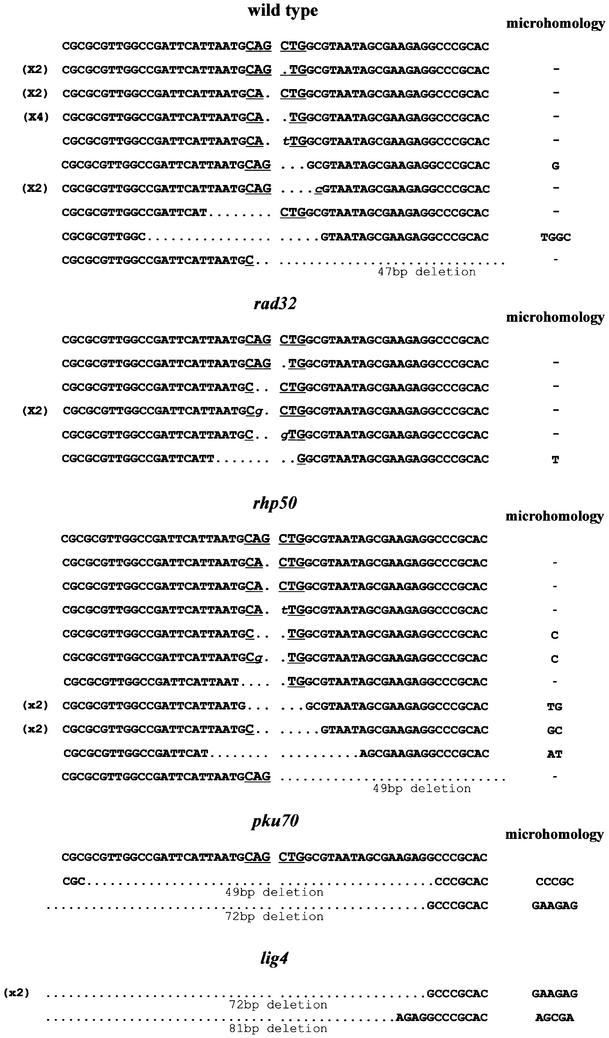
To ascertain the nature of the repair that gave rise to undigestable junctions, some of the PvuII-resistant plasmids were sequenced. All cases involved deletions, which were sometimes associated with single base pair insertions. The number of bases lost during repair in wild-type cells was usually small (1–3 bp) (Figure 2), although there was one junction with a 47 bp deletion. Only two out of 15 junctions involved utilization of short homologies. Similar data were found for PstI junctions (data not shown). Only a small number of junctions were obtained from pku70Δ and lig4Δ strains but all involved large deletions (49–81 bp) and the use of 5 bp or greater microhomology (Figure 2). Taken together, these findings show that in S.pombe, the plasmid rejoining assay monitors a Ku70- and DNA ligase IV-dependent process and that, in the absence of NHEJ, a microhomology-based mechanism is utilized for repair of DSBs.
Fig. 2. Sequence analysis of inaccurately rejoined PvuII junctions. The top sequence in each panel shows the sequence that would result from accurate joining (the PvuII recognition sequence is underlined). Lower case italic letters indicate insertions. Numbers in parentheses on the left of a sequence indicate the number of times the sequence was found.
NHEJ is not impaired in strains lacking rad50 or rad32
We next extended our analysis of the genetic requirements of NHEJ to include other factors that have been implicated in the process. The Mre11–Rad50–Xrs2 complex has been shown to be required for efficient NHEJ of linear plasmids in S.cerevisiae and, therefore, S.pombe strains lacking rad32 or rad50 (the homologues of MRE11 and RAD50, respectively) were investigated. Unexpectedly, rad32Δ and rad50Δ strains were able to repair blunt DSBs proficiently, displaying a range of frequencies similar to those observed for two wild-type strains 501 and FY367 (Figure 1B). Furthermore, NHEJ repair of cohesive-ended DSBs (with either 3′ or 5′ overhangs) was also normal in rad32Δ and rad50Δ (Figure 1C). To examine the fidelity of repair, rejoined plasmids were analysed by restriction digestion and sequencing. The accuracy of blunt end rejoining in rad32Δ was 60% and in rad50Δ 27% (Table I); this is similar to that observed in wild-type cells (40%). Repair of PstI- or EcoRI-generated DSBs was less accurate and resembled the low fidelity cohesive end rejoining observed in wild-type cells (Table I). Moreover, sequence analysis of PvuII junctions showed that the extent of deletions and microhomology utilization was generally small and similar to that of wild-type cells (Figure 2). We conclude that Rad32 and Rad50 are not involved in NHEJ of plasmid DSBs in S.pombe.
Loss of the DNA damage/replication checkpoint does not affect the frequency of NHEJ
The S.cerevisiae DNA damage checkpoint proteins Mec1 and Rad9 have been shown to be necessary for the relocalization of Yku and Sir proteins from telomeres to DSBs in response to DNA damage (Martin et al., 1999; Mills et al., 1999). Furthermore, a modest requirement for Mec3, Rad17 and Rad24 in addition to Mec1 and Rad9 for NHEJ has been demonstrated using a plasmid-based NHEJ assay (de la Torre-Ruiz and Lowndes, 2000). To assess the requirement for proteins involved in the S.pombe DNA damage checkpoints in NHEJ, we analysed strains lacking one of the following genes: rad3, rhp9(crb2), cds1 rad17 and chk1 (the S.pombe homologues of S.cerevisiae MEC1, RAD9, RAD53, RAD24 and CHK1, respectively). The products of these genes are required for the DNA damage and/or replication checkpoint in the fission yeast (reviewed in Caspari and Carr, 1999). All these strains were proficient in the repair of blunt and cohesive DSBs (Figure 1D and data not shown). Saccharomyces cerevisiae cells lacking both RAD9 and RAD24 display a similar reduction in the frequency of NHEJ as strains lacking YKU alone (de la Torre-Ruiz and Lowndes, 2000). We therefore examined the possibility of a synergistic interaction between the S.pombe homologues rhp9(crb2) and rad17 by generating a rhp9Δ/rad17Δ double mutant. In this double mutant, a level of blunt end rejoining similar to that of wild type was observed (Figure 1D). Thus we conclude that NHEJ can operate independently of the DNA damage-sensing activity of the DNA damage/replication checkpoint in S.pombe.
NHEJ can take place in the absence of both rad3 and tel1
Several factors involved in NHEJ in S.cerevisiae (including Yku70 and Yku80) have also been implicated in the maintenance of telomeric length. It has been proposed that the telomeres of S.cerevisiae act as storage sites for NHEJ protein complexes that include the Yku heterodimer and Sir proteins (Martin et al., 1999; Mills et al., 1999). The S.pombe Rad3 and Tel1 proteins are members of the family of phosphatidylinositol 3-kinases that includes DNA-PKcs and ATM. Schizosaccharomyces pombe rad3Δ and tel1Δ strains display telomeric shortening (Dahlen et al., 1998; Naito et al., 1998). Interestingly, when both rad3 and tel1 genes are deleted, cells initially form irregular shaped colonies reflecting cell death due to telomere loss. However, following prolonged culture, regular shaped derivatives arise, which have lost all telomeric sequences resulting in all chromosomes circularizing due to fusion of chromosome ends (Naito et al., 1998). We found that the Tel1 kinase is dispensable for NHEJ using either blunt ends (Figure 1D) or cohesive ends (data not shown). To assess the requirement for telomeric structures for NHEJ, we performed the plasmid assay in rad3Δ/tel1Δ derivative cells and found that they can repair blunt-ended DSBs proficiently (Figure 1D). Therefore, neither the activities of these proteins nor the presence of normal chromosome ends are required for NHEJ in S.pombe.
The effect of DNA-damaging agents on pku70Δ and lig4Δ
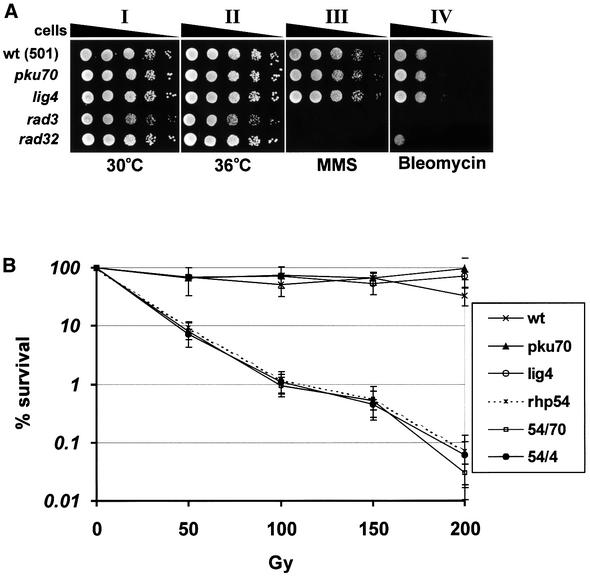
To assess the role of Pku70 and Lig4 in the repair of chromosomal DNA damage, we examined the sensitivity of pku70Δ and lig4Δ strains to DNA-damaging agents. Methyl methanesulfonate (MMS) and bleomycin cause methylation damage and DSBs, respectively. We found that pku70Δ and lig4Δ strains resemble wild type in their sensitivity to 0.005% MMS or 5 µg/ml bleomycin—concentrations that kill most of the rad32Δ and rad3Δ cells (Figure 3A). Both pku70Δ and lig4Δ were also resistant to γ-irradiation (Figure 3B). Therefore, it seems that homologous recombination plays the major role in the repair of genomic DSBs in S.pombe as is the case in S.cerevisiae. Additionally, pku70Δ/rhp54Δ and lig4Δ/rhp54Δ double mutants were no more sensitive to γ-irradiation than the rhp54Δ strain (Figure 3B).
Fig. 3. pku70Δ and lig4Δ are resistant to MMS, bleomycin and γ-irradiation and are not temperature sensitive. (A) A 5 µl aliquot of serial 10-fold dilutions of log-phase cells was spotted onto YEA plates and incubated for 3 days at 30°C (I, III and IV) or 36°C (II). Cells were exposed to 0.005% MMS in III and to 5 µg/ml bleomycin in IV. (B) Survival of strains subjected to γ-irradiation, determined as described in Materials and methods.
Reduced telomere length and linear minichromosome stability in cells lacking pku70
In other systems, Ku70/80 and DNA-PKcs have been implicated in regulating telomere length (Boulton and Jackson, 1996a; Bailey et al., 1999). Since a telomere is a naturally occurring chromosome break, it potentially can bind Ku but its activity must be tempered in a normal telomere environment in order to participate in the formation of a chromosomal cap and not to mediate end to end joining. Deletion of the S.cerevisiae YKU genes results in reduction of telomere length and temperature sensitivity. We found that the average telomere length in pku70Δ cells is clearly shorter compared with wild type (Figure 4). Telomere length was also analysed in rad3Δ and rad32Δ cells (Figure 4) and found to be decreased, at levels similar to those previously published (Dahlen et al., 1998; Wilson et al., 1999). However, as expected, deletion of lig4 did not affect telomere length (Figure 4). Furthermore, pku70Δ and lig4Δ strains did not display a temperature-sensitive phenotype and grow as well as wild-type cells at 36°C (Figure 3A, panel II). The absence of Yku in S.cerevisiae results in further shortening of telomeres at higher temperatures (Boulton and Jackson, 1998) that may lead to activation of a DNA damage sensor checkpoint and cellular arrest/senescence. The resistance of pku70Δ fission yeast strains to increased temperature is consistent with the absence of further telomere shortening under these conditions (data not shown).

Fig. 4. Telomere length is decreased in pku70Δ cells. Genomic DNA was prepared from the indicated strains and analysed by Southern blotting. A fragment consisting of two 250 bp copies of telomere repeats was used as a telomere probe. The FY1870 strain was used as wild type.
To determine if Pku70 has other effects on telomere function, we compared the rate of loss of circular and linear minichromosomes in wild-type and pku70Δ cells. The rate of loss of a linear minichromosome (Ch16) was increased 8-fold from 0.017% per cell per generation observed in wild-type cells to 0.14% in pku70Δ. However, the rate of loss of a circular minichromosome lacking telomeres was only 2-fold higher (0.52% in wild-type cells and 0.88% in pku70Δ). The fact that linear minichromosomes are more unstable than circular minichromosomes in pku70Δ cells indicates that Pku70 might normally act at telomeres to protect and stabilize chromosome ends, and is consistent with the reduced telomere length observed in cells lacking Pku70.
Transcriptional silencing of the subtelomeric region is not alleviated in pku70Δ
When marker genes are placed close to telomeres in S.pombe, their transcription is repressed (Nimmo et al., 1994). Several factors have been identified that contribute to this telomere-mediated transcriptional silencing in S.pombe, including Clr4, Rik1, Swi6, Lot2 and Taz1. Mutation of these factors results in increased expression of telomeric reporter genes (Allshire et al., 1995; Cooper et al., 1997; Nimmo et al., 1998). Since S.cerevisiae Yku proteins participate in the formation of silent telomeric chromatin, the involvement of Pku70 in transcriptional silencing of telomeric ura4 or his3 marker genes was assessed. Surprisingly, in cells lacking Pku70 function, these telomeric marker genes remained transcriptionally silent and no increase in the frequency of colony growth was observed on plates selecting for telomeric marker expression (Figure 5). Furthermore, we were unable to detect the presence of a ura4 transcript from the telomeric ura4 gene in pku70Δ cells by quantitative RT–PCR (data not shown). Likewise, telomeric silencing was not alleviated in rad32Δ, rad3Δ or lig4Δ cells but, as shown previously (Nimmo et al., 1998), it was decreased in the lot2 mutant (Figure 5). If a telomeric pool of Ku and associated proteins is redistributed as a result of DNA damage, then the induction of damage might interfere with telomeric silencing. However, treatment of cells with bleomycin did not alleviate transcriptional silencing at S.pombe telomeres in wild-type or mutant cells (data not shown). The finding that Pku70 does not affect transcriptional silencing adjacent to telomeres suggests that it is not a primary constituent of the chromatin complex that mediates this process.
Fig. 5. Transcriptional silencing of the subtelomeric region is not alleviated in pku70Δ and lig4Δ. (A) Serial dilutions of strains containing a telomeric ura4 marker were spotted onto non-selective (NS) medium, medium containing the drug 5-fluoro-orotic acid (which is toxic to cells expressing uracil) and medium lacking uracil. (B) The expression of a his3 telomeric marker was assessed on medium lacking histidine as well as NS medium.
The S.pombe Ku70 localization is nuclear but not confined to the telomeres
To gain further insight into Pku70 function, we created a C-terminal fusion of Pku70 with 13 Myc epitopes expressed from its own promoter at the pku70 locus. The addition of the Myc epitopes did not affect the telomere maintenance or the NHEJ function of Pku70 (data not shown). Following cell fixation, analysis of Pku70 localization by immunostaining revealed a diffuse pattern of Pku70-Myc that generally coincided with the nuclear 4′,6- diamidino-2-phenylindole (DAPI) staining (Figure 6A, panels a, c and d). This contrasts with the distinct foci of S.cerevisiae Yku80 shown to co-localize with the telomeric Rap1 protein (Martin et al., 1999). The uniform and diffuse localization of Pku70-Myc was confirmed using confocal microscopy (Figure 6B). To assess the localization of Pku70 relative to telomeres, we utilized a fusion of the green fluorescent protein (GFP) with the telomeric protein Taz1. Taz1 is involved in telomere length maintenance (Cooper et al., 1997) and has high affinity for telomeric repeats (Vassetzky et al., 1999; Spink et al., 2000). The Taz1–GFP fusion therefore was utilized to visualize the telomeres. The taz1-gfp strain has normal telomere length (data not shown) and, in wild-type cells, Taz1–GFP is seen to concentrate in 1–3 distinct telomeric foci (Figure 6A, panel b). Since S.pombe has six telomeres, this finding is consistent with there being some clustering of telomeres in interphase nuclei (Funabiki et al., 1993). The strain used to detect Taz1–GFP at telomeres also contains the pku70-myc fusion and allowed us to demonstrate that the diffused Pku70 staining generally does not co-localize with the telomeric Taz1 spots (Figure 6A, panel d). As expected, there is some overlap between the two signals: we found that 53% of Taz1 spots overlap with the diffused Pku70 staining. Treatment of cells with bleomycin or MMS prior to immunofluorescence did not alter the Pku70 staining, which remained diffused throughout the nucleus (data not shown). In addition, the percentage of Taz1 spots that overlap with Pku70 staining remained relatively unchanged after bleomycin or MMS treatment (data not shown). We conclude that Pku70 is not constitutively enriched at the telomeres but is located throughout the nucleus and does not form foci after DNA damage.
Fig. 6. Pku70 is located throughout the nucleus. (A) A logarithmically growing culture of the taz1-gfp/pku70-myc strain was used. (a) Cells stained with DAPI; (b) Taz1–GFP fusion visualized at the fluorescein isothiocyanate (FITC) wavelength; (c) Pku70-Myc visualization at the Texas red wavelength using anti-Myc (primary) and Texas red (secondary) antibodies; (d) overlay of the three signals. Bar length is 2 µm. (B) Confocal intervals of 1 µm of untreated pku70-myc cells. Pku70 was visualized with an FITC-conjugated secondary anti-mouse antibody against the mouse anti-Myc primary antibody used to target Pku70-Myc. Bar length is 1 µm.
Discussion
We have identified homologues of Ku70 and DNA ligase IV in S.pombe. Using a plasmid in vivo NHEJ assay, we demonstrate that these two proteins are essential for NHEJ. Therefore, the basic NHEJ components and their functions are conserved in S.pombe, providing another yeast model in which to investigate this process. Our studies show that NHEJ in S.pombe differs in two important aspects from the process in S.cerevisiae: repair of plasmid DSBs in S.cerevisiae is accurate and functions only to rejoin cohesive-ended breaks, whereas blunt-ended breaks are rejoined by a Ku-independent single strand annealing mechanism (Boulton and Jackson, 1996b). In S.pombe, blunt-ended breaks are rejoined as efficiently as cohesive-ended breaks in a Ku70- and DNA ligase IV-dependent process, and repair of both types of DSBs frequently is associated with small deletions at the junctions. This may reflect greater levels of exonuclease activities in S.pombe that can degrade DNA ends. The single-stranded overhangs of cohesive ends may be especially susceptible to such nucleases since cohesive-ended junctions were associated with deletions more frequently than blunt-ended ones. Significantly, ∼40% of the blunt ends are rejoined accurately, showing that NHEJ can repair DSBs without utilization of complementarity from overlapping sequences and that repair of blunt-ended breaks is not simply the result of nuclease action on these ends. Similar assays in mammalian cells show only a small dependence on known NHEJ components; however, as reported above with S.pombe, blunt ends are rejoined efficiently and the repair of DSBs frequently is associated with deletions (Escarceller et al., 1998; Kabotyanski et al., 1998; K.G.Manolis and P.A.Jeggo, unpublished observations).
Since a DNA-PKcs homologue has not been identified in yeast, we examined strains lacking either or both of the related kinases Rad3 and Tel1. The ability of these strains to carry out NHEJ efficiently showed that they do not act as functional homologues of DNA-PKcs in S.pombe. The absence of telomeres in the rad3Δ/tel1Δ strain indicates that intact telomeres do not play an intrinsic role in NHEJ, e.g. as storage sites or potential loading points for Ku.
Having established an assay for NHEJ and shown that it is dependent upon known components (Pku70 and Lig4), we next examined the requirement for additional proteins reported to function in NHEJ based on studies in S.cerevisiae. Most significantly, we found that loss of Rad32 and Rad50 does not impair either the frequency or accuracy of NHEJ of plasmid DSBs. Saccharomyces cerevisiae strains lacking MRE11 or RAD50 are as defective in plasmid NHEJ as YKU deletion strains. Although it has been reported that the nuclease activity of Mre11 is utilized during NHEJ using an in vitro assay (Paull and Gellert, 1998), an S.cerevisiae strain bearing an mre11 mutation that abolishes its nuclease function shows normal NHEJ of plasmid DSBs in vivo (Moreau et al., 1999). In addition to these reports, a previous study in S.pombe showed a slight reduction (3-fold) in frequency and elevated fidelity of a rad32Δ strain (Wilson et al., 1999). In our study, which involved an extensive examination of junctions formed in rad32Δ as well as rad50Δ, we did not observe any significant difference between these mutants and wild type in either the frequency or fidelity of NHEJ. Furthermore, the 350- to 1000-fold defect of pku70Δ and lig4Δ strains in our study serves as a control to demonstrate a NHEJ-defective phenotype. Taken together, our results suggest that neither of the Mre11 or Rad50 homologues in S.pombe are involved enzymatically in the NHEJ pathway and do not contribute to the end resection that results in the deletion formation observed in the wild-type NHEJ junctions. We also observed that a series of mutants defective in the DNA damage and/or replication cell cycle checkpoint display a normal frequency of NHEJ.
Homologues of Mre11 and Rad50 have been identified in mammalian cells and interact with Nbs1 (a functional homologue of the S.cerevisiae Xrs2). Nbs1 is defective in Nijmegen breakage syndrome (NBS), and defects in Mre11 have been observed in ATLD (ataxia telangiectasia-like disorder) patients (Stewart et al., 1999). Although NBS and ATLD cells are radiosensitive, neither display major defects in DNA DSB rejoining using pulsed-field gel electrophoresis, the hallmark of the NHEJ defect in mammalian cells (Kraakman-van der Zwet et al., 1999; Stewart et al., 1999). Furthermore, MRE11 is not epistatic to KU in chicken cells: the sensitivity to γ-irradiation and the extent of chromosomal aberrations are higher in an MRE11–/–/KU70–/– cell line compared with cell lines deleted for a single one of the two genes (Yamaguchi-Iwai et al., 1999). In contrast, there is mounting evidence that supports the involvement of Mre11 and its partner proteins in meiotic and mitotic homologous recombination (Petrini, 1999; Yamaguchi-Iwai et al., 1999) and sister chromatid recombination (Bressan et al., 1999). Additionally, cells from patients with ataxia telangiectasia (AT) lack checkpoint responses to DSBs but have a largely normal DSB rejoining profile (Blocher et al., 1991). Also, in chicken cells, ATM (the gene mutated in AT) is not epistatic to KU but epistatic to the gene for the homologous recombination protein RAD54 (Morrison et al., 2000). In combination, these results indicate that the genetic requirements for NHEJ in S.pombe closely resemble those in vertebrate cells and underline the usefulness of the fission yeast as a model system for the dissection of this process.
We did not observe any sensitivity of pku70Δ and lig4Δ strains to DNA-damaging agents. Thus homologous recombination is the major pathway for the repair of DSBs in S.pombe. Saccharomyces cerevisiae yku and lig4 mutants are also resistant to γ-irradiation, and sensitivity is only observed in yku/rad52 or lig4/rad52 double deletions (i.e. in the absence of homologous recombination) and only when cells are in a haploid G1 phase (in stationary phase cultures) (Boulton and Jackson, 1996b; Herrmann et al., 1998). We carried out our analysis using logarithmically growing cells since stationary cells in S.pombe are in a diploid G2-like phase. Schizosac-charomyces pombe pku70Δ/rhp54Δ or lig4Δ/rhp54Δ double mutants did not show increased sensitivity to γ-irradiation compared with the rhp54Δ strain. We attribute this difference to the fact that S.pombe cells have a relatively short G1 phase. The investigation of the importance of NHEJ for the repair of genomic DSBs in S.pombe will require further studies.
An intriguing aspect of our studies is the observation that, although Pku70 is required for telomere length maintenance, there is no alleviation of transcriptional silencing adjacent to telomeres in the absence of Pku70. Thus, telomere length regulation and silencing represent two distinct functions for Ku that differ in their evolutionary conservation. Separation of these two processes is consistent with other findings, which demonstrate that their genetic requirements differ. For example, Mre11 is required for telomere length maintenance but not transcriptional silencing in S.pombe and S.cerevisiae (Boulton and Jackson, 1998; Wilson et al., 1999; this work) and active NHEJ per se is not required for telomere length regulation or telomeric silencing. In S.cerevisiae, Yku70 directly associates with silencing proteins such as Sir4 to mediate silencing (Tsukamoto et al., 1997; Boulton and Jackson, 1998), and tethered Yku70 or Yku80 can mediate Sir-dependent silencing (Martin et al., 1999). However, no homologues of Sir3 or Sir4 have been described outside of the budding yeast, and thus the link between Ku and telomeric silencing may not be conserved. It is possible that a distinct class of silencing proteins perform a similar role in other organisms.
Immunostaining showed that the bulk of Pku70 is distributed throughout S.pombe nuclei, in contrast to the high concentration of Yku observed in the vicinity of S.cerevisiae telomeres. Since the high concentration of Ku at or near the telomeres occurs only in S.cerevisiae, this would appear to correlate with a role in transcriptional silencing rather than telomere length maintenance. One possibility would be that these high levels of telomeric Ku are involved in recruiting silencing factors. We surmise that the role of Ku in telomere length maintenance does not require a high concentration of Ku at telomeres. It is likely that a small fraction of Ku is located at or near chromosome ends in S.pombe, although we have been unable to demonstrate this directly. The direct role of S.cerevisiae Yku in telomeric silencing may thus result in its accumulation at telomeres, making it necessary to regulate the redistribution of S.cerevisiae Yku, and potentially other components of the NHEJ machinery, to the sites of DNA damage.
By comparison with S.pombe, it seems likely that in S.cerevisiae the indirect participation of the DNA damage checkpoint sensor proteins and Mre11–Rad50–Xrs2 in NHEJ may be to effect relocalization of Yku. The independence of S.pombe NHEJ for DNA damage checkpoint proteins and Mre11/Rad50 may reflect the fact that Pku is not ‘stored’ at telomeric regions. Significantly, NHEJ of plasmid DSBs in S.cerevisiae mre11, rad50 or checkpoint mutants shows a severe decrease in the frequency of rejoining, but the fidelity of repair is the same as in the parental cells. This suggests that the function of Mre11 and Rad50 in NHEJ in S.cerevisiae is distinct from that of Yku70 and Lig4 and would be consistent with a regulatory role, rather than a direct role in the process. Interactions between the Mre11–Rad50–p95 complex and the DNA damage checkpoint have also been demonstrated in mammalian cells (Lim et al., 2000), which is again consistent with a role in the signalling function in response to DSBs. Such interactions may be conserved throughout eukaryotes and remain to be characterized in S.pombe; however, there is no evidence that they participate in NHEJ in mammalian cells.
The conservation of the NHEJ machinery between S.pombe and mammalian cells suggests that the fission yeast is a good model system for the further characterization of this process. Comparison of the fission yeast NHEJ pathway with that of other eukaryotes will provide increased insight into the functional requirements of DSB repair by NHEJ in the future investigations.
Materials and methods
Genetic manipulations and sensitivity assays
The strains used in this study are listed in Table II. Media and standard genetic techniques were as described previously (Gutz et al., 1974). The pku70 and lig4 deletion strains, the C-terminal tagging of pku70 and the reversion of the ura4 marker to KanR or his3 (in lig4 or pku70, respectively) were created using the PCR-based method (Bahler et al., 1998) and the following primers. For pku70 knockout: Q484, 5′-TTTCTTTTTGTCGTATTTAAAAAGCCAAGGAAATTGTTTTTGATAATCTGTTTTTTACGCTTAGCTACAAATCCCACTGG-3′; and Q485, 5′-ATATCTTTAAGTTGACTAACTTTTAACGCCTTCATATGAACTGTTACTAATCAAGATCTCCAACACCAATGTTTATAACC-3′. For lig4 knockout: UP76F, 5′-TCGGTTATTTATTTGAGAAATATTATTGATCATAAACTGTATTATAAAACGATTGCTTCAAGCCAAAACCTAAGATCGCCAGGGTTTTCCCAGTCACGAC-3′; and DOWN76R, 5′-ATTAACAAATTTGGGTTCAATTTGATATCTATTTGGTTAACCAACAACTCATACTTCTTTCTTTTTGTTTAGCTACAGCGGATAACAATTTCACACAGGA-3′. For pku70-myc tagging: tag70UPF, 5′-TTCTCAGGGATCGTGGACTTAGAGTGAGCGGTAAAAAGGCAGATTTATTAGACAATTAACGAACTATGTCAAAAAATTACGGATCCCCGGGTTAATTAA-3′; and tag70DOWNR, 5′-TGTTTGTCGAAAGTTCAGAGAAAGACGTAGTCAGGTAATAACATATTACATTCTACAATATACACATTATCATTTGCGGCGAATTCGAGCTCGTTTAAAC-3′. For reversion of ura4 to KanR: ura45′, 5′-AAGCTTAGCTACAAATCCCACTGGCTATATGTATGCATTTGTGTTAAAAAAGTTTGTATAGATTATTTAATCTACTCAGCCGGATCCCCGGGTTAATTAA-3′; and ura43′, 5′-AAGCTTGTGATATTGACGAAACTTTTTGACATCTAATTTATTCTGTTCCAACACCAATGTTTATAACCAAGTTTTATCTTGAATTCGAGCTCGTTTAAAC-3′. For reversion of ura4 to his3: P720, CTTCGTCGGCATCTCTGCACATGTCGTGTTTTCTTACCGTATTGTCCTACCAAGAACCTCTTTCCTCTTCAGGTTTCTGA; and P724, GAGAAAAGATTGTGGTAATGTTGTAGGAGCATGTTTAATAAATTACTATAGCAAATTACATGGACTGTTGGCTGTCTTTG. All the genetic alterations were confirmed by PCR. For MMS, bleomycin and temperature sensitivity assays, 5 µl of 10-fold serial dilutions of log-phase cells were spotted on YEA plates or YEA containing 0.005% MMS or 5 µg/ml bleomycin, and incubated at the required temperature. For γ-ray survival assays, logarithmically growing cells were irradiated using a 137Cs source at a dose rate of 12.5 Gy/min. Duplicates of irradiated cells and unirradiated controls were plated on YEA medium plates and incubated at 30°C for 3–4 days. Telomere length detection and transcriptional silencing assays were carried out as described previously (Allshire et al., 1995; Nimmo et al., 1998). The minichromosome loss assays were carried out as described previously (Allshire et al., 1995). At least three separate isolates were examined in each case, and the total number of colonies scored for each experimental point were: 5806 for wild type/linear, 3685 for wild type/circular, 20 308 for pku70Δ/linear, and 4996 for pku70Δ/circular.
Table II. Strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| 501 | ura4.D18 leu1.32 ade6.704 h– | Murray et al. (1992) |
| 972 | wild-type h– | |
| FY0367 | ura4.D18 leu1.32 ade6.210 h+ | Ekwall et al. (1997) |
| lig4Δ | ura4.D18 leu1.32 ade6.704 lig4::ura4+ h– | this study |
| pku70Δ (FY 3506) | ura4.D18 leu1.32 ade6.216 his3.D1 his3-tel pku70::ura4+ | this study |
| pku70-myc | ura4.D18 leu1.32 ade6.704 ku70:myc:KanR h– | this study |
| taz1-gfp (FY 3806) | ura4.D18 leu1.32 ade6.210 his3D1 his3-tel taz1:gfp:ura4+ h90 | R.Allshire, unpublished |
| pku70-myc/taz1-gfp | ura4.D18 leu1.32 ade- ku70:myc:KanR taz1:gfp:ura4+ h90 | this study |
| rhp9Δ | ura4.D18 leu1.32 ade6.704 rhp9::ura4+ | Willson et al. (1997) |
| rad17Δ | ura4.D18 leu1.32 ade6.704 rad17::ura4+ | Griffiths et al. (1995) |
| rhp9Δ/rad17Δ | ura4.D18 leu1.32 ade6.704 rhp9::ura4+ rad17::ura4+ | this study |
| rad32Δ | ura4.D18 leu1.32 ade6.704 arg3.D4 rad32::ura4+ h+ | Tavassoli et al. (1995) |
| rad50Δ | ura4.D18 ade6.210 smt0 rad50::KanR h– | E.Hartsuiker and J.Kohli, unpublished |
| rad50Δ | ura4.D18 leu1.32 ade6.210 smt0 rad50::KanR h– | this study |
| rad3Δ | ura4.D18 leu1.32 ade6.704 rad3::ura4+ h– | Bentley et al. (1996) |
| cds1Δ | ura4.D18 leu1.32 ade6.704 cds1::ura4+ h– | Murakami and Okayama (1995) |
| chk1Δ | ura4.D18 leu1.32 ade6.704 chk1::ura4+ h+ | Al-Khodairy et al. (1994) |
| rhp54Δ | ura4.D18 leu1.32 ade6.704 arg3.D4 rhp54::ura4+ h+ | Muris et al. (1996) |
| tel1Δ | ura4.D18 leu1.32 ade6.704 arg3.D4 tel1::arg3+ h– | N.Bentley, unpublished |
| rad3Δ/tel1Δ | ura4.D18 leu1.32 ade6.704 arg3.D4 rad3::ura4+ tel1::arg3+ h– | this study |
| FY1554 | ura4.D18 leu1.32 ade6.210 his3D1 h– | this study |
| FY1870 | ura4.DS/E leu1.32 ade6.210 his3D1 ade6sphOTR his3-tel | this study |
| FY1872 | ura4.DS/E leu1.32 ade6.210 his3D1 ade6sphOTR ura4-tel h90 | this study |
| pku70-myc (FY4101) | ura4.DS/E leu1.32 ade6.216 his3D1 ura4-tel pku70/myc::KanR h– | this study |
| lig4Δ (FY4102) | ura4.DS/E leu1.32 ade6.210 his3D1 ura4-tel lig4::KanR h– | this study |
| rad3Δ (FY4109) | ura- leu1.32 ade6.210 his3D1 his3-tel rad3::ura4+ h– | this study |
| lig4Δ (FY4110) | ura- leu1.32 ade6.216 his3D1 his3-tel lig4::ura4+ h+ | this study |
| pku70Δ (FY4111) | ura4.DS/E leu1.32 ade6.210 his3D1 ura4-tel pku70::his3+ h– | this study |
| rad50Δ (FY4169) | ura4.DS/E leu1.32 ade6.210 his3D1 ura4-tel rad50::KanR h– | this study |
| rad32Δ (FY4170) | ura- leu1.32 ade6.210 his3D1 his3-tel rad32::ura4+ h- | this study |
| FY3824 | ura4.D18 leu1.32 ade6.210 his3D1 arg3-D4 (Ch16 ade6.216 leu2+) h+ | this study |
| FY757 | ura4DS/E leu1.32 ade6.704 (CM3112 Sup3e) h+ | Allshire et al. (1995) |
| pku70Δ (FY4239/4240) | ura4DS/E leu1.32 ade6.210 (Ch16 ade6.216 leu2+) pku70::ura4+ | this study |
| pku70Δ (FY4241/4242) | ura4DS/E leu1.32 ade6.704 (CM3112 Sup3e) pku70::ura4+ | this study |
| pku70Δ/rhp54Δ | ura4.D18 leu1.32 ade6.704 pku70::his3+ rhp54::ura4+ | this study |
| lig4Δ/rhp54Δ | ura4.D18 leu1.32 ade6.704 lig4::KanR rhp54::ura4+ | this study |
NHEJ assay
Plasmid pAL19 was constructed essentially as described for pUR19 (Barbet et al., 1992) except that LEU2 was used in place of the ura4 marker. Plasmid PS was obtained by cloning an ∼500 bp PstI fragment excised from vector pSTA (Carr et al., 1989) into the unique PstI site of pAL19. Similarly, plasmid PI was created by the insertion of an ∼540 bp fragment into the unique EcoRI site of pAL19. pAL19 was used as the undigested control and PS and PI for the preparation of the linear substrates. The cohesive-ended substrates for the NHEJ experiments were prepared by excision of the PstI fragment from PS or the EcoRI fragment from PI and purification of the remaining linear vector using the Qiagen Gel Extraction Kit. The blunt-ended substrate was prepared by the removal of an ∼700 bp PvuII fragment from PS. Logarithmically growing cells were transformed with equal amounts (1 µg) of undigested or linear DNA using the lithium acetate method (Okazaki et al., 1990). Each experiment was carried out in duplicate and the NHEJ frequency was calculated as the percentage of leu+ colonies arising from cells transformed with linear over those transformed with undigested DNA. This value was normalized to the value obtained from wild-type cells. At least three experiments were performed for each point, and the average and standard deviation are shown on the bar diagrams. For the analysis of the fidelity of repair, plasmids were extracted from leu+ colonies as described previously (Caspari, 1997) and electroporated in XL1-Blue electrocompetent cells. The plasmids were then amplified from ampicillin-resistant colonies using the Qiagen miniprep kit and analysed by restriction digestion or sequenced using an ABI 370A automated sequencer.
Immunofluorescence
Immunofluorescence experiments were carried out essentially as described (Ekwall et al., 1995). For drug treatment, logarithmically growing cells were incubated with 0.03% MMS or 40 µg/ml bleomycin or left untreated for 3 h at 30°C with shaking prior to fixation. The mouse monoclonal c-Myc antibody (Santa Cruz Biotechnologies) was used to target Pku70-Myc. For confocal microscopy, cells were prepared as before and observed using a Biorad MRC 600 confocal microscope.
Acknowledgments
Acknowledgements
We would like to thank all the members of the A.M.C. laboratory and especially T.Caspari for technical advice. We also thank R.Antonelli, S.Macfarlane and G.Hamilton for strain constructions, J.Murray for the ura4 to KanR primers, and N.Bone for assistance with confocal microscopy. Work in the laboratory of P.A.J. contributing to this study has been funded by grants from the Leukaemia Research Foundation, the Human Frontiers Science Programme, the Industry-funded UKCCCR Radiation Research Programme and European Union grant F13PCT920007. Telomere research by E.N. and R.A. is funded by The Cancer Research Campaign project grant SP2345/0102.
REFERENCES
- Al-Khodairy F., Fotou,E., Sheldrick,K.S., Griffiths,D.J.F., Lehmann,A.R. and Carr,A.M. (1994) Identification and characterisation of new elements involved in checkpoints and feedback controls in fission yeast. Mol. Biol. Cell, 5, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire R.C., Nimmo,E.R., Ekwall,K., Javerzat,J.P. and Cranston,G. (1995) Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev., 9, 218–233. [DOI] [PubMed] [Google Scholar]
- Bahler J., Wu,J.Q., Longtine,M.S., Shah,N.G., McKenzie,A., Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Bailey S.M., Meyne,J., Chen,D.J., Kurimasa,A., Li,G.C., Lehnert,B.E. and Goodwin,E.H. (1999) DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc. Natl Acad. Sci. USA, 96, 14899–14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet N.C., Muriel,W.J. and Carr,A.M. (1992) Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene, 114, 59–66. [DOI] [PubMed] [Google Scholar]
- Bentley N.J., Holtzman,D.A., Flaggs,G., Keegan,K.S., DeMaggio,A., Ford,J.C., Hoekstra,M. and Carr,A.M. (1996) The Schizo-saccharomyces pombe rad3 checkpoint gene. EMBO J., 15, 6641–6651. [PMC free article] [PubMed] [Google Scholar]
- Blocher D., Sigut,D. and Hannan,M.A. (1991) Fibroblasts from ataxia telangiectasia (AT) and AT heterozygotes show an enhanced level of residual DNA double-strand breaks after low dose-rate γ-irradiation as assayed by pulsed field gel electrophoresis. Int. J. Radiat. Biol., 60, 791–802. [DOI] [PubMed] [Google Scholar]
- Boulton S.J. and Jackson,S.P. (1996a) Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double-strand break repair and in telomeric maintenance. Nucleic Acids Res., 24, 4639–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton S.J. and Jackson,S.P. (1996b) Saccharomyces cerevisiae Ku70 potentiates illegitimate DNA double-strand break repair and serves as a barrier to error-prone DNA repair pathways. EMBO J., 15, 5093–5103. [PMC free article] [PubMed] [Google Scholar]
- Boulton S.J. and Jackson,S.P. (1998) Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J., 17, 1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan D.A., Baxter,B.K. and Petrini,J.H. (1999) The Mre11–Rad50–xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol., 19, 7681–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A., MacNeil,S.A., Hayles,J. and Nurse,P. (1989) Molecular cloning and sequence analysis of mutant alleles of the fission yeast cdc2 protein kinase gene: implications for cdc2+ protein structure and function. Mol. Gen. Genet., 218, 41–49. [DOI] [PubMed] [Google Scholar]
- Caspari T. (1997) Onset of gluconate-H+ symport in Schizo-saccharomyces pombe is regulated by the kinases Wis1 and Pka1, and requires the gti1(+) gene product. J. Cell Sci., 110, 2599–2608. [DOI] [PubMed] [Google Scholar]
- Caspari T. and Carr,A.M. (1999) DNA structure checkpoint pathways in Schizosaccharomyces pombe. Biochimie, 81, 173–181. [DOI] [PubMed] [Google Scholar]
- Cooper J.P., Nimmo,E.R., Allshire,R.C. and Cech,T.R. (1997) Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature, 385, 744–747. [DOI] [PubMed] [Google Scholar]
- Dahlen M., Olsson,T., Kanter-Smoler,G., Ramne,A. and Sunnerhagen,P. (1998) Regulation of telomere length by checkpoint genes in Schizosaccharomyces pombe. Mol. Biol. Cell, 9, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre-Ruiz M.A. and Lowndes,N.F. (2000) The Saccharomyces cerevisiae DNA damage checkpoint is required for efficient repair of double strand breaks by non-homologous end joining. FEBS Lett., 467, 311–315. [DOI] [PubMed] [Google Scholar]
- Ekwall K., Javerzat,J.P., Lorentz,A., Schmidt,H., Cranston,G. and Allshire,R. (1995) The chromodomain protein Swi6: a key component at fission yeast centromeres. Science, 269, 1429–1431. [DOI] [PubMed] [Google Scholar]
- Ekwall K., Olsson,T., Turner,B.M., Cranston,G. and Allshire,R.C. (1997) Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell, 91, 1021–1032. [DOI] [PubMed] [Google Scholar]
- Escarceller M., Buchwald,M., Jeggo,P.A., Singleton,B.K., Jackson,S.P., Moustacchi,E. and Papadopoulo,D. (1998) Fanconi anemia C gene product plays a role in the fidelity of blunt DNA end-joining. J. Mol. Biol., 279, 375–385. [DOI] [PubMed] [Google Scholar]
- Featherstone C. and Jackson,S.P. (1999) DNA double-strand break repair. Curr. Biol., 90, 759–761. [DOI] [PubMed] [Google Scholar]
- Funabiki H., Hagan,I., Uzawa,S. and Yanagida,M. (1993) Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol., 121, 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Chaudhuri,J., Zhu,C., Davidson,L., Weaver,D.T. and Alt,F.W. (1998a) A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for Ku in V(D)J recombination. Immunity, 9, 367–376. [DOI] [PubMed] [Google Scholar]
- Gao Y. et al. (1998b) A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell, 95, 891–902. [DOI] [PubMed] [Google Scholar]
- Gartenberg M.R. (2000) The Sir proteins of Saccharomyces cerevisiae: mediators of transcriptional silencing and much more. Curr. Opin. Microbiol., 3, 132–137. [DOI] [PubMed] [Google Scholar]
- Grawunder U., Zimmer,D. and Lieber,M.R. (1998) DNA ligase IV binds to XRCC4 via a motif located between rather than within its BRCT domains. Curr. Biol., 8, 873–876. [DOI] [PubMed] [Google Scholar]
- Griffiths D.J.F., Barbet,N.C., McCready,S., Lehmann,A.R. and Carr,A.M. (1995) Fission yeast rad17: a homologue of budding yeast RAD24 that shares regions of sequence similarity with DNA polymerase accessory proteins. EMBO J., 14, 5812–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.S. et al. (1997) Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity, 7, 653–665. [DOI] [PubMed] [Google Scholar]
- Gutz H., Heslot,H., Leupold,U. and Loprieno,N. (1974) Schizosaccharomyces pombe. In King,R.C. (ed.), Handbook of Genetics. Plenum Press, New York, NY, Vol. 1, pp. 395–446.
- Herrmann G., Lindahl,T. and Schar,P. (1998) Saccharomyces cerevisiae LIF1: a function involved in DNA double-strand break repair related to mammalian XRCC4. EMBO J., 17, 4188–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabotyanski E.B., Gomelsky,L., Han,J.O., Stamato,T.D. and Roth,D.B. (1998) Double-strand break repair in Ku86- and XRCC4-deficient cells. Nucleic Acids Res., 26, 5333–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraakman-van der Zwet M. et al. (1999) Immortalization and characterization of Nijmegen breakage syndrome fibroblasts. Mutat. Res., 434, 17–27. [DOI] [PubMed] [Google Scholar]
- Laroche T., Martin,S.G., Gotta,M., Gorham,H.C., Pryde,F.E., Louis,E.J. and Gasser,S.M. (1998) Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr. Biol., 8, 653–656. [DOI] [PubMed] [Google Scholar]
- Lim D.S., Kim,S.T., Xu,B., Maser,R.S., Lin,J., Petrini,J.H. and Kastan,M.B. (2000) ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature, 404, 613–617. [DOI] [PubMed] [Google Scholar]
- Martin S.G., Laroche,T., Suka,N., Grunstein,M. and Gasser,S.M. (1999) Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell, 97, 621–633. [DOI] [PubMed] [Google Scholar]
- McAinsh A.D, Scott-Drew,S., Murray,J.A. and Jackson,S.P. (1999) DNA damage triggers disruption of telomeric silencing and Mec1p-dependent relocation of Sir3p. Curr. Biol., 9, 963–966 [DOI] [PubMed] [Google Scholar]
- Mills K.D., Sinclair,D.A. and Guarente,L. (1999) MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell, 97, 609–620. [DOI] [PubMed] [Google Scholar]
- Moreau S., Ferguson,J.R. and Symington,L.S. (1999) The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol. Cell. Biol., 19, 556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C., Sonoda,E., Takao,N., Shinohara,A., Yamamoto,K. and Takeda,S. (2000) The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J., 19, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H. and Okayama,H. (1995) A kinase from fission yeast responsible for blocking mitosis in S phase. Nature, 374, 817–819. [DOI] [PubMed] [Google Scholar]
- Muris D.F.R., Vreeken,K., Carr,A.M., Smidt,C., Lohman,P.H.M. and Pastink,A. (1996) Isolation of the Schizosaccharomyces pombe RAD54 homolog, rhp54+, a gene involved in the repair of radiation damage and replication fidelity. J. Cell Sci., 109, 73–81. [DOI] [PubMed] [Google Scholar]
- Murray J.M., Doe,C., Schenk,P., Carr,A.M., Lehmann,A.R. and Watts,F.Z. (1992) Cloning and characterisation of the S.pombe rad15 gene, a homologue to the S.cerevisiae RAD3 and human ERCC2 genes. Nucleic Acids Res., 20, 2673–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito T., Matsuura,A. and Ishikawa,F. (1998) Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nature Genet., 20, 203–206. [DOI] [PubMed] [Google Scholar]
- Nimmo E.R., Cranston,G. and Allshire,R.C. (1994) Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J., 13, 3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo E.R., Pidoux,A.L., Perry,P.E. and Allshire,R.C. (1998) Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature, 392, 825–828. [DOI] [PubMed] [Google Scholar]
- Nussenzweig A., Chen,C., da Costa Soares,V., Sanchez,M., Sokol,K., Nussenzweig,M.C. and Li,G.C. (1996) Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature, 382, 551–555. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Okazaki,N., Kume,K., Jinno,S., Tanaka,K. and Okayama,H. (1990) High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res., 18, 6485–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull T.T. and Gellert,M. (1998) The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol. Cell, 1, 969–979. [DOI] [PubMed] [Google Scholar]
- Petrini J.H. (1999) The mammalian Mre11–Rad50–nbs1 protein complex: integration of functions in the cellular DNA-damage response. Am. J. Hum. Genet., 64, 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink K.G., Evans,R.J. and Chambers,A. (2000) Sequence-specific binding of Taz1p dimers to fission yeast telomeric DNA. Nucleic Acids Res., 28, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G.S. et al. (1999) The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell, 99, 577–587. [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Shayegi,M., Nasim,A. and Watts,F.Z. (1995) Cloning and characterisation of the Schizosaccharomyces pombe rad32 gene: a gene required for repair of double strand breaks and recombination. Nucleic Acids Res., 23, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo S.-H. and Jackson,S.P. (1997) Identification of Saccharomyces cerevisiae DNA ligase IV: involvement in DNA double-strand break repair. EMBO J., 16, 4788–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y., Kato,J. and Ikeda,H. (1997) Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature, 388, 900–903. [DOI] [PubMed] [Google Scholar]
- Vassetzky N.S., Gaden,F., Brun,C., Gasser,S.M. and Gilson,E. (1999) Taz1p and Teb1p, two telobox proteins in Schizosaccharomyces pombe, recognize different telomere-related DNA sequences. Nucleic Acids Res., 27, 4687–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson J., Wilson,S., Warr,N. and Watts,F.Z. (1997) Isolation and characterization of the Schizosaccharomyces pombe rhp9 gene: a gene required for the DNA damage checkpoint but not the replication checkpoint. Nucleic Acids Res., 25, 2138–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S., Warr,N., Taylor,D.L. and Watts,F.Z. (1999) The role of Schizosaccharomyces pombe Rad32, the Mre11 homologue, and other DNA damage response proteins in non-homologous end joining and telomere length maintenance. Nucleic Acids Res., 27, 2655–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.E., Grawunder,U. and Lieber,M.R. (1997) Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature, 388, 495–498. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y. et al. (1999) Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J., 18, 6619–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]