Abstract
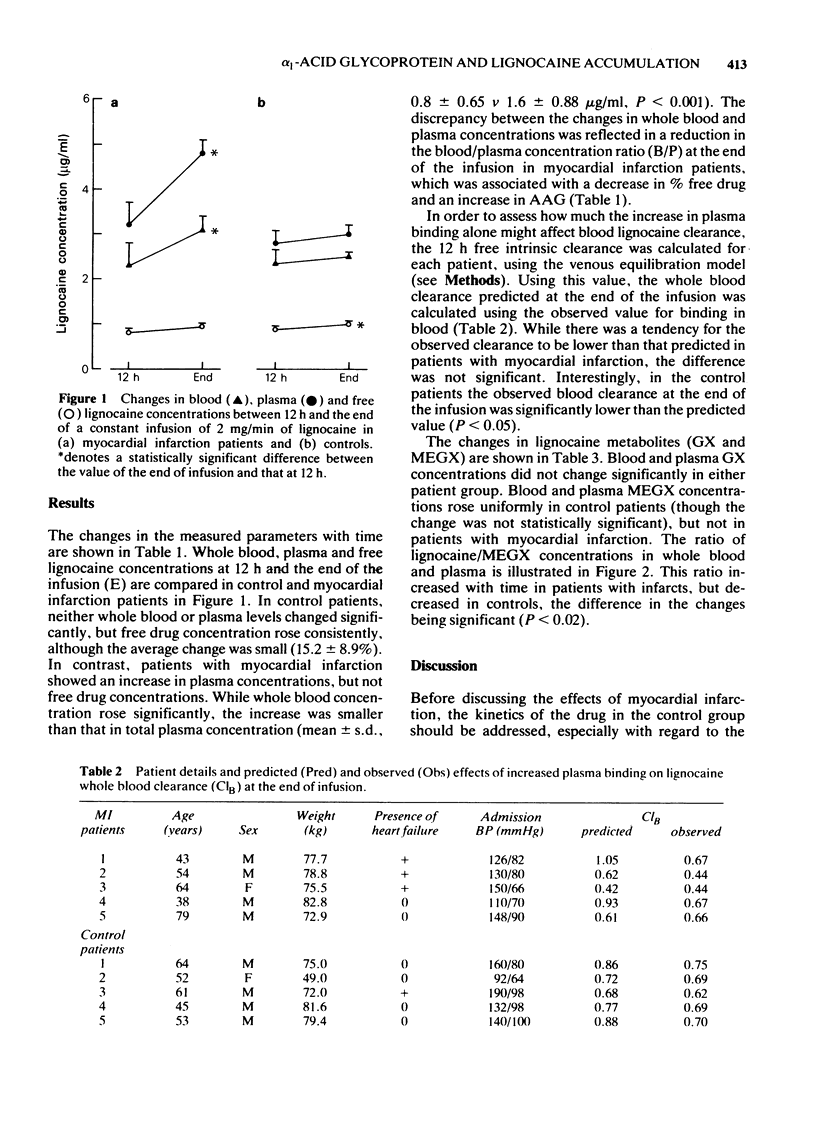
1 Blood plasma and free lignocaine concentrations have been measured 12 h after beginning a constant infusion of 2 mg/min and again at the end of the infusion (36-72 h) in five patients with myocardial infarction (MI) and compared with five control patients who did not develop objective evidence of MI. 2 In MI patients, total plasma concentration rose significantly between 12 h and the end of infusion. Because of an increase in alpha 1 acid glycoprotein (AAG) plasma binding increased, so that free drug concentration did not change. The rise in whole blood concentration was less than that in plasma as a result of drug redistribution out of red cells due to enhanced binding. 3 In control patients, neither blood nor plasma concentrations changed with time and plasma binding remained constant. Free drug concentrations, however, rose slightly. 4 The concentrations of GX and MEGX remained unchanged in all patients, but the ratio of lignocaine/MEGX concentrations fell in controls but rose in MI patients. 5 Pharmacokinetic modelling suggested that at least some of the rise in blood lignocaine concentration was due to reduced clearance resulting from enhanced plasma binding. 6 We conclude that the rise in AAG following MI is responsible for increased plasma binding and drug redistribution within blood. These changes, together with a reduction in lignocaine clearance, can explain much of the phenomenon of lignocaine accumulation in MI.
Full text
PDF

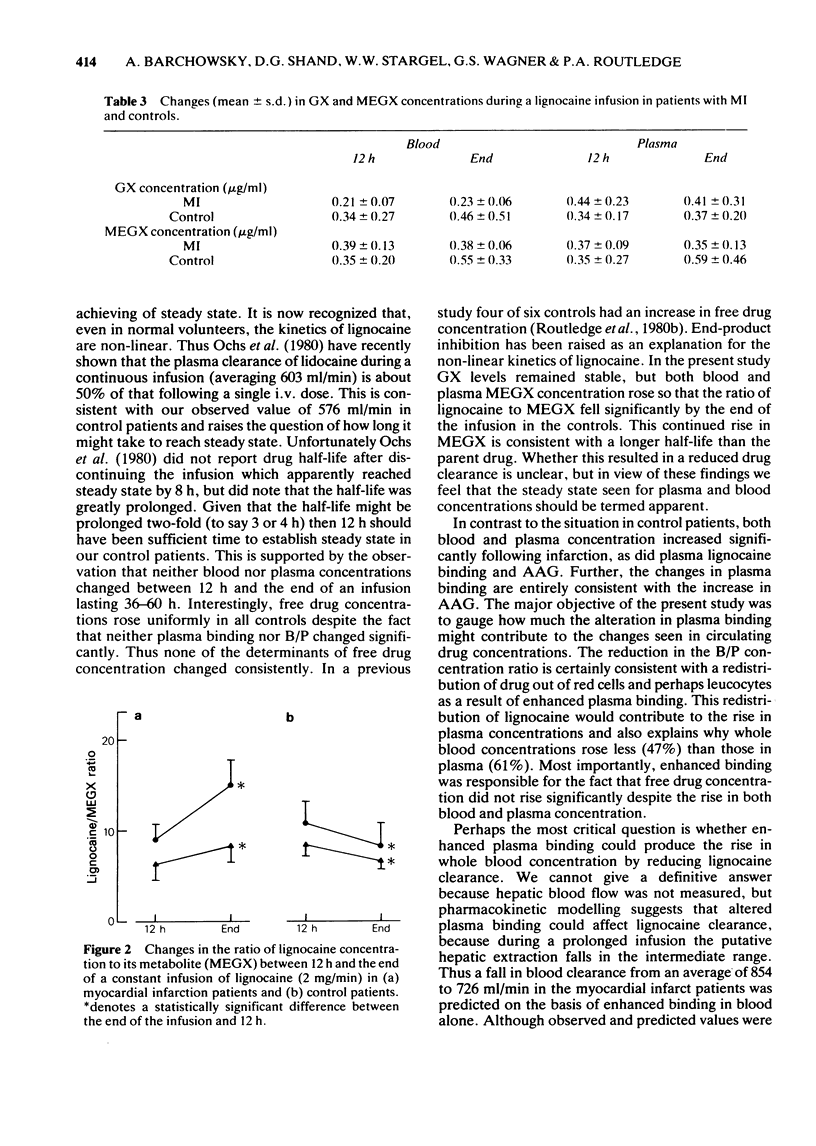

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax N. D., Tucker G. T., Woods H. F. Lignocaine and indocyanine green kinetics in patients following myocardial infarction. Br J Clin Pharmacol. 1980 Oct;10(4):353–361. doi: 10.1111/j.1365-2125.1980.tb01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeLorier J., Grenon D., Latour Y., Caillé G., Dumont G., Brosseau A., Solignac A. Pharmacokinetics of lidocaine after prolonged intravenous infusions in uncomplicated myocardial infarction. Ann Intern Med. 1977 Dec;87(6):700–706. doi: 10.7326/0003-4819-87-6-700. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nation R. L., Peng G. W., Chiou W. L. High-performance liquid chromatographic method for the simultaneous determination of lidocaine and its N-dealkylated metabolites in plasma. J Chromatogr. 1979 Mar 1;162(3):466–473. doi: 10.1016/s0378-4347(00)81538-8. [DOI] [PubMed] [Google Scholar]
- Ochs H. R., Carstens G., Greenblatt D. J. Reduction in lidocaine clearance during continuous infusion and by coadministration of propranolol. N Engl J Med. 1980 Aug 14;303(7):373–377. doi: 10.1056/NEJM198008143030705. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Adjepon-Yamoah K. K., Talbot R. G. Impaired Lignocaine metabolism in patients with myocardial infarction and cardiac failure. Br Med J. 1976 Apr 17;1(6015):939–941. doi: 10.1136/bmj.1.6015.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge P. A., Barchowsky A., Bjornsson T. D., Kitchell B. B., Shand D. G. Lidocaine plasma protein binding. Clin Pharmacol Ther. 1980 Mar;27(3):347–351. doi: 10.1038/clpt.1980.46. [DOI] [PubMed] [Google Scholar]
- Routledge P. A., Stargel W. W., Wagner G. S., Shand D. G. Increased alpha-1-acid glycoprotein and lidocaine disposition in myocardial infarction. Ann Intern Med. 1980 Nov;93(5):701–704. doi: 10.7326/0003-4819-93-5-701. [DOI] [PubMed] [Google Scholar]
- Rowland M., Benet L. Z., Graham G. G. Clearance concepts in pharmacokinetics. J Pharmacokinet Biopharm. 1973 Apr;1(2):123–136. doi: 10.1007/BF01059626. [DOI] [PubMed] [Google Scholar]
- Rowland M. Influence of route of administration on drug availability. J Pharm Sci. 1972 Jan;61(1):70–74. doi: 10.1002/jps.2600610111. [DOI] [PubMed] [Google Scholar]
- Shand D. G., Cotham R. H., Wilkinson G. R. Perfusion-limited of plasma drug binding on hepatic drug extraction. Life Sci. 1976 Jul 1;19(1):125–130. doi: 10.1016/0024-3205(76)90382-9. [DOI] [PubMed] [Google Scholar]
- Stargel W. W., Roe C. R., Routledge P. A., Shand D. G. Importance of blood-collection tubes in plasma lidocaine determinations. Clin Chem. 1979 Apr;25(4):617–619. [PubMed] [Google Scholar]
- Stenson R. E., Constantino R. T., Harrison D. C. Interrelationships of hepatic blood flow, cardiac output, and blood levels of lidocaine in man. Circulation. 1971 Feb;43(2):205–211. doi: 10.1161/01.cir.43.2.205. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. R., Shand D. G. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975 Oct;18(4):377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]


