Abstract
The ability of NR LBDs to transfer repression function to a heterologous DNA binding domain, and the cross-squelching of repression by untethered LBDs, has suggested that repression is mediated by interactions with putative cellular corepressor proteins. The yeast-two hybrid screen for protein interactors has proven to be the key to the isolation and characterization of corepressors. This short review will focus on N-CoR and SMRT.
Background
Hormone binding to nuclear receptors has long been known to activate gene expression. In the case of steroid hormone receptors, hormone triggers dissociation from cytoplasmic chaperones, nuclear localization, and DNA binding. Hence, expression of target genes is neutral in the absence of ligand. The related thyroid hormone receptor (TR) and retinoic acid receptor (RAR) also activate gene expression in the presence of their cognate ligands but, by contrast, these receptors are constitutively nuclear and bind to DNA in the absence of ligand [Samuels et al., 1988]. Molecular analysis has revealed that the ligand binding domains (LBDs) of nuclear receptors (NRs) contain potent transcriptional repression functions [Brent et al., 1989; Graupner et al., 1989]. In addition to TR and RAR, potent repression functions have been identified in the orphan receptors liver X receptor (LXR) [Hu et al., 2003] and RevErb [Harding and Lazar, 1995].
The ability of NR LBDs to transfer repression function to a heterologous DNA binding domain, and the cross-squelching of repression by untethered LBDs, suggested that repression was mediated by interactions with putative cellular corepressor proteins [Baniahmad et al., 1995; Qi et al., 1995]. The yeast-two hybrid screen for protein interactors proved the key to the isolation and characterization of corepressors. The first corepressors identified were named N-CoR (Nuclear Receptor CoRepressor), first identified by Rosenfeld and colleagues [Horlein et al., 1995], and SMRT (Silencing Mediator of Retinoid and Thyroid Receptors, first identified by Evans and colleagues [Chen and Evans, 1995]). Other molecules that may serve as corepressors for nuclear receptors include Alien [Dressel et al., 1999], Hairless [Potter et al., 2001], LCoR [Fernandes et al., 2003], RIP-140 [Cavailles et al., 1995], and SUN-CoR [Zamir et al., 1997].
This short review will focus on N-CoR and SMRT, which have received the most attention because they are structurally related molecules that fulfill two important criteria: 1) they bind to NRs in the absence of ligand, and 2) they possess autonomous, transferable repression domains. N-CoR and SMRT are large proteins, whose NR binding and repression functions are mediated by the carboxyl and amino terminal halves of the molecules, respectively ( Figure 1).
Figure 1. NR corepressors.

See text for details
Nuclear Receptor Binding to N-CoR and SMRT
The major structural change in the NR LBD upon ligand binding is the position of helix 12 (H12), whose importance for coactivator binding has been demonstrated biochemically as well as structurally [Wurtz et al., 1996]. Intriguingly, deletion of H12 actually enhances repression and corepressor binding of several NRs, including TR [Damm et al., 1989; Sap et al., 1989], RAR [Tsai et al., 1992], RXR [Schulman et al., 1997; Zhang et al., 1999] and the orphans PPAR [Gurnell et al., 2000] and ROR [Harding et al., 1997]. Indeed, the orphan NR RevErb is a very potent repressor and does not possess H12 at all [Harding and Lazar, 1995]. The corepressors bind to a surface, composed of residues in NR helices 3, 4 and 5 that is fundamentally similar to that bound by coactivator. This was predicted from biochemical studies, which demonstrated that a "CoRNR box" motif in corepressors, similar to the "NR box" motif in coactivators [Heery et al., 1997; McInerney et al., 1998], was required for NR interaction [Hu and Lazar, 1999; Nagy et al., 1999; Perissi et al., 1999]. This has been recently proven by the first crystal structure of an NR bound to a CoRNR-box containing corepressor- derived peptide [Xu et al., 2002].
Cellular localization of N-CoR and SMRT
N-CoR and SMRT are predominantly nuclear proteins, but recent evidence suggests that changes in signaling at the cell surface can activate second messenger systems leading to protein phosphorylation and nuclear-cytoplasmic shuttling of the corepressors. In the case of SMRT, MAP kinase directed phosphorylation has been implicated [ Hong et al., 2001], For N-CoR the phosphorylation of an associated protein, TAB2, by IKK kinase has been reported to induced nuclear exit [Baek et al., 2002].
N-CoR/SMRT-Containing Repression Complexes
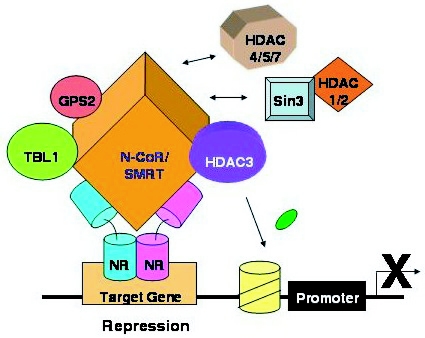
A large number of proteins have been suggested to interact with N-CoR and SMRT, based upon GST-pulldown and yeast two-hybrid studies. Direct biochemical purification of the corepressors by three different groups has demonstrated a major complex involving a WD40-repeat protein called transducin α946;-like protein 1 (TBL1, or a related protein TBL1R) and histone deacetylase 3 (HDAC3) [Guenther et al., 2000; Li et al., 2000; Zhang et al., 2002]. The associated proteins are likely to mediate repression by N-CoR and SMRT, as will be discussed below. This core complex also contains G-protein suppressor 2 (GPS2) [Zhang et al., 2002] and IR-10 [Yoon et al., 2003], as well as a TBL1-related protein (Figure 2). Alternative complexes that include the HDAC1-Sin3 corepressor complex have been reported [Jones et al., 2001; Underhill et al., 2000], although studies of HDAC recruitment by NRs has implicated HDAC3 but not HDACs 1 and 2 [Ishizuka and Lazar, 2003; Li et al., 2002]. Class II HDACs have also been shown to bind strongly to N-CoR and SMRT [Huang et al., 2000; Kao et al., 2000], but their CaM-kinase dependent nuclear-cytoplasmic shuttle [Grozinger and Schreiber, 2000; McKinsey et al., 2000] may limit their interaction with NR corepressors in vivo.
Figure 2. N-CoR/SMRT repression complexes.
See text for details
Mechanisms of Repression by N-CoR and SMRT
Gene expression is regulated by changes in chromatin structure that include DNA unwinding and covalent modification of nucleosomal histones [Jenuwein and Allis, 2001; Kouzarides, 2000; Schreiber and Bernstein, 2002]. SMRT and N-CoR both exist in repression complexes with HDAC enzyme activity, and HDAC3 is largely responsible for this activity [Guenther et al., 2000; Li et al., 2000; Zhang et al., 2002]. Remarkably, the enzyme activity of HDAC3 requires SMRT/N-CoR, which interacts with and activates HDAC3 via a region termed the deacetylase activation domain (DAD) [ Guenther et al., 2001] . The DAD activity of NCoR and SMRT requires the N-terminal SANT1 motif [ Guenther et al., 2001; Zhang et al., 2002], and the downstream SANT2 is part of a histone interaction domain that enhances this activity [Yu et al., 2003]. HDAC3 is required for repression by TR [Ishizuka and Lazar, 2003; Yoon et al., 2003], as is TBL1 which is also a histone binding protein that may function via an HDAC-independent mechanism [Guenther et al., 2000; Yoon et al., 2003].
Biological Functions of N-CoR and SMRT
There are clearly quantitative differences between N-CoR and SMRT binding to NRs both in solution, on DNA, and on target genes in living cells [Hu et al., 2001; Ishizuka and Lazar, 2003; Makowski et al., 2003; Webb et al., 2000; Zamir et al., 1997]. N-CoR and SMRT also function as corepressors for transcription factors other than NRs [Xu et al., 1998] . The best evidence that N-CoR and SMRT have non-redundant functions comes from the knockout of N-CoR, which is embryonic lethal [Jepsen et al., 2000], indicating that SMRT cannot compensate for the lack of N-CoR. NR corepressors have also been implicated in the mechanisms of human diseases, including acute promeyleocytic leukemia due to RAR translocations [Grignani et al., 1998; Guidez et al., 1998; He et al., 1998], acute myeloid leukemia due to the AML1-ETO translocation [Gelmetti et al., 1998; Lutterbach et al., 1998; Wang et al., 1998], thyroid hormone resistance [Tagami et al., 1997; Yoh et al., 1997], and insulin resistance due to mutation in PPARγ [ Gurnell et al., 2000].
Future
Corepressors are complicated molecules, that mediate repression by NRs as well as other transcription factors. Their interactions with NRs are highly specific, and they repress transcription in the context of large, multiprotein complexes with several potential effectors of repression, including potent HDAC activity. These complexes are potential targets of therapy for leukemia, diabetes, and other diseases. Corepressor function may be regulated by extracellular signals, intracellular localization, and cell-specific factors, in addition to the NRs to which they bind. We are rapidly learning more about the composition and regulation of corepressor complexes, and how this regulates NR physiology and function.
Acknowledgments
Work in the author's laboratory is supported by NIDDK grants DK R37 43806 and DK RO1 45586, as well as the NURSA program DK U19 062434.
Abbreviations
- AML
acute myeloid leukemia
- CoRNR box
nuclear receptor interacting domain in corepressors
- GST
glutathione S transferase
- H12
helix in the ligand binding domain of many nuclear receptors
References
- Baek S. H., Ohgi K. A., Rose D. W., Koo E. H., Glass C. K., Rosenfeld M. G. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and β-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Leng X., Burris T. P., Tsai S. Y., Tsai M. J., O'Malley B. W. Mol Cell Biol. 1. Vol. 15. Department of Cell Biology, Baylor College of Medicine, Houston, Texas 77030.: 1995. The tau 4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing; pp. 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent G. A., Dunn M. K., Harney J. W., Gulick T. , Larsen P. R., Moore D. D. Thyroid hormone aporeceptor represses T3-inducible promoters and blocks activity of the retinoic acid receptor. New Biol. 1989;1:329–36. [PubMed] [Google Scholar]
- Cavailles V., Dauvois S., L'Horset F., Lopez G., Hoare S., Kushner P. J., Parker M. G. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. Embo J. 1995;14:3741–51. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Evans R. M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–7. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989;339:593–7. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Dressel U., Thormeyer D., Altincicek B., Paululat A., Eggert M., Schneider S., Tenbaum S. P., Renkawitz R., Baniahmad A. Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:3383–94. doi: 10.1128/mcb.19.5.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes I., Bastien Y., Wai T., Nygard K., Lin R., Cormier O., Lee H. S., Eng F., Bertos N. R., Pelletier N., Mader S., Han V. K., Yang X. J., White J. H. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol Cell. 2003;11:139–50. doi: 10.1016/s1097-2765(03)00014-5. [DOI] [PubMed] [Google Scholar]
- Gelmetti V., Zhang J., Fanelli M., Minucci S., Pelicci P. G., Lazar M. A. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol Cell Biol. 1998;18:7185–91. doi: 10.1128/mcb.18.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989;340:653–6. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Grignani F., De Matteis S., Nervi C., Tomassoni L., Gelmetti V., Cioce M., Fanelli M., Ruthardt M., Ferrara F. F., Zamir I., Seiser C., Lazar M. A., Minucci S., Pelicci P. G. Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukaemia. Nature. 1998;391:815–8. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- Grozinger C. M., Schreiber S. L. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci U S A. 2000;97:7835–40. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther M. G., Barak O., Lazar M. A. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol. 2001;21:6091–101. doi: 10.1128/MCB.21.18.6091-6101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther M. G., Lane W. S., Fischle W., Verdin E., Lazar M. A., Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 2000;14:1048–57. [PMC free article] [PubMed] [Google Scholar]
- Guidez F., Ivins S., Zhu J., Soderstrom M., Waxman S., Zelent A. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91:2634–42. [PubMed] [Google Scholar]
- Gurnell M., Wentworth J. M., Agostini M., Adams M., Collingwood T. N., Provenzano C., Browne P. O., Rajanayagam O., Burris T. P., Schwabe J. W. A dominant-negative peroxisome proliferator-activated receptor γ (PPARgamma) mutant is a constitutive repressor and inhibits PPARgamma-mediated adipogenesis. J Biol Chem. 2000;275:5754–9. doi: 10.1074/jbc.275.8.5754. [DOI] [PubMed] [Google Scholar]
- Harding H. P., Atkins G. B., Jaffe A. B., Seo W. J., Lazar M. A. Transcriptional activation and repression by RORalpha, an orphan nuclear receptor required for cerebellar development. Mol Endocrinol. 1997;11:1737–46. doi: 10.1210/mend.11.11.0002. [DOI] [PubMed] [Google Scholar]
- Harding H. P., Lazar M. A. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol. 1995;15:4791–802. doi: 10.1128/mcb.15.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L. Z., Guidez F., Tribioli C., Peruzzi D., Ruthardt M., Zelent A., Pandolfi P. P. Distinct interactions of PML-RARalpha and PLZF-RARalpha with co-repressors determine differential responses to RA in APL. Nat Genet. 1998;18:126–35. doi: 10.1038/ng0298-126. [DOI] [PubMed] [Google Scholar]
- Heery D. M., Kalkhoven E., Hoare S., Parker M. G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–6. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- Hong S. H., Yang Z., Privalsky M. L. Arsenic trioxide is a potent inhibitor of the interaction of SMRT corepressor with Its transcription factor partners, including the PML-retinoic acid receptor α oncoprotein found in human acute promyelocytic leukemia. Mol Cell Biol. 2001;21:7172–82. doi: 10.1128/MCB.21.21.7172-7182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein A. J., Naar A. M., Heinzel T., Torchia J., Gloss B., Kurokawa R., Ryan A., Kamei Y., Soderstrom M., Glass C. K. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- Hu X., Lazar M. A. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–6. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- Hu X., Li S., Wu J., Xia C., Lala D. S. Liver x receptors interact with corepressors to regulate gene expression. Mol Endocrinol. 2003;17:1019–26. doi: 10.1210/me.2002-0399. [DOI] [PubMed] [Google Scholar]
- Hu X., Li Y., Lazar M. A. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol. 2001;21:1747–58. doi: 10.1128/MCB.21.5.1747-1758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. Y., Zhang J., Miska E. A., Guenther M. G., Kouzarides T., Lazar M. A. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev. 2000;14:45–54. [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T., Lazar M. A. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol. 2003;23:5122–31. doi: 10.1128/MCB.23.15.5122-5131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T., Allis C. D. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jepsen K., Hermanson O., Onami T. M., Gleiberman A. S., Lunyak V., McEvilly R. J., Kurokawa R., Kumar V., Liu F., Seto E., Hedrick S. M., Mandel G., Glass C. K., Rose D. W., Rosenfeld M. G. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102:753–63. doi: 10.1016/s0092-8674(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Jones P. L., Sachs L. M., Rouse N., Wade P. A., Shi Y. B. Multiple N-CoR complexes contain distinct histone deacetylases. J Biol Chem. 2001;276:8807–11. doi: 10.1074/jbc.C000879200. [DOI] [PubMed] [Google Scholar]
- Kao H. Y., Downes M., Ordentlich P., Evans R. M. Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev. 2000;14:55–66. [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? Embo J. 2000;19:1176–9. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lin Q., Wang W., Wade P., Wong J. Specific targeting and constitutive association of histone deacetylase complexes during transcriptional repression. Genes Dev. 2002;16:687–92. doi: 10.1101/gad.962502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang J., Nawaz Z., Liu J. M., Qin J., Wong J. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. Embo J. 2000;19:4342–50. doi: 10.1093/emboj/19.16.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterbach B., Westendorf J. J., Linggi B., Patten A., Moniwa M., Davie J. R., Huynh K. D., Bardwell V. J., Lavinsky R. M., Rosenfeld M. G., Glass C., Seto E., Hiebert S. W. ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol Cell Biol. 1998;18:7176–84. doi: 10.1128/mcb.18.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski A., Brzostek S., Cohen R. N., Hollenberg A. N. Determination of nuclear receptor corepressor interactions with the thyroid hormone receptor. Mol Endocrinol. 2003;17:273–86. doi: 10.1210/me.2002-0310. [DOI] [PubMed] [Google Scholar]
- McInerney E. M., Rose D. W., Flynn S. E., Westin S., Mullen T. M., Krones A., Inostroza J., Torchia J., Nolte R. T., Assa-Munt N., Milburn M. V., Glass C. K., Rosenfeld M. G. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 1998;12:3357–68. doi: 10.1101/gad.12.21.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T. A., Zhang C. L., Lu J., Olson E. N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–11. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L., Kao H. Y., Love J. D., Li C., Banayo E., Gooch J. T., Krishna V., Chatterjee K., Evans R. M., Schwabe J. W. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13:3209–16. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissi V., Staszewski L. M., McInerney E. M., Kurokawa R., Krones A., Rose D. W., Lambert M. H., Milburn M. V., Glass C. K., Rosenfeld M. G. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198–208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter G. B., Beaudoin G. M., 3rd, DeRenzo C. L., Zarach J. M., Chen S. H., Thompson C. C. The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev. 2001;15:2687–701. doi: 10.1101/gad.916701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J. S., Desai-Yajnik V., Greene M. E., Raaka B. M., Samuels H. H. The ligand-binding domains of the thyroid hormone/retinoid receptor gene subfamily function in vivo to mediate heterodimerization, gene silencing, and transactivation. Mol Cell Biol. 1995;15(3):1817–25. doi: 10.1128/mcb.15.3.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Forman B. M., Horowitz Z. D., Ye Z. S. Regulation of gene expression by thyroid hormone. J Clin Invest. 1988;81:957–67. doi: 10.1172/JCI113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Munoz A., Schmitt J., Stunnenberg H., Vennstrom B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989;340:242–4. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Bernstein B. E. Signaling network model of chromatin. Cell. 2002;111:771–8. doi: 10.1016/s0092-8674(02)01196-0. [DOI] [PubMed] [Google Scholar]
- Schulman I. G., Li C., Schwabe J. W., Evans R. M. The phantom ligand effect: allosteric control of transcription by the retinoid X receptor. Genes Dev. 1997;11:299–308. doi: 10.1101/gad.11.3.299. [DOI] [PubMed] [Google Scholar]
- Tagami T., Madison L. D., Nagaya T., Jameson J. L. Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol. 1997;17:2642–8. doi: 10.1128/mcb.17.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S., Bartelmez S., Heyman R., Damm K., Evans R., Collins S. J. A mutated retinoic acid receptor-α exhibiting dominant-negative activity alters the lineage development of a multipotent hematopoietic cell line. Genes Dev. 1992;6:2258–69. doi: 10.1101/gad.6.12a.2258. [DOI] [PubMed] [Google Scholar]
- Underhill C., Qutob M. S., Yee S. P., Torchia J. A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1. J Biol Chem. 2000;275:40463–70. doi: 10.1074/jbc.M007864200. [DOI] [PubMed] [Google Scholar]
- Wang J., Hoshino T., Redner R. L., Kajigaya S., Liu J. M. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc Natl Acad Sci U S A. 1998;95:10860–5. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P., Anderson C. M., Valentine C., Nguyen P., Marimuthu A., West B. L., Baxter J. D., Kushner P. J. The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs) Mol Endocrinol. 2000;14:1976–85. doi: 10.1210/mend.14.12.0566. [DOI] [PubMed] [Google Scholar]
- Wurtz J. M., Bourguet W., Renaud J. P., Vivat V., Chambon P., Moras D., Gronemeyer H. A canonical structure for the ligand-binding domain of nuclear receptors. Nat Struct Biol. 1996;3:87–94. doi: 10.1038/nsb0196-87. [DOI] [PubMed] [Google Scholar]
- Xu H. E., Stanley T. B., Montana V. G., Lambert M. H., Shearer B. G., Cobb J. E., McKee D. D., Galardi C. M., Plunket K. D., Nolte R. T., Parks D. J., Moore J. T., Kliewer S. A., Willson T. M., Stimmel J. B. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 2002;415:813–7. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- Xu L., Lavinsky R. M., Dasen J. S., Flynn S. E., McInerney E. M., Mullen T. M., Heinzel T., Szeto D., Korzus E., Kurokawa R., Aggarwal A. K., Rose D. W., Glass C. K., Rosenfeld M. G. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–6. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- Yoh S. M., Chatterjee V. K., Privalsky M. L. Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptors and transcriptional corepressors. Mol Endocrinol. 1997;11:470–80. doi: 10.1210/mend.11.4.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H. G., Chan D. W., Huang Z. Q., Li J., Fondell J. D., Qin J., Wong J. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. Embo J. 2003;22:1336–46. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Li Y., Ishizuka T., Guenther M. G., Lazar M. A. A SANT motif in the SMRT corepressor interprets the histone code and promotes histone deacetylation. Embo J. 2003;22:3403–10. doi: 10.1093/emboj/cdg326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir I., Dawson J., Lavinsky R. M., Glass C. K., Rosenfeld M. G., Lazar M. A. Cloning and characterization of a corepressor and potential component of the nuclear hormone receptor repression complex. Proc Natl Acad Sci U S A. 1997;94:14400–5. doi: 10.1073/pnas.94.26.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Hu X., Lazar M. A. A novel role for helix 12 of retinoid X receptor in regulating repression. Mol Cell Biol. 1999;19:6448–57. doi: 10.1128/mcb.19.9.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kalkum M., Chait B. T., Roeder R. G. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell. 2002;9:611–23. doi: 10.1016/s1097-2765(02)00468-9. [DOI] [PubMed] [Google Scholar]