Abstract
We show that p27 localization is cell cycle regulated and we suggest that active CRM1/RanGTP-mediated nuclear export of p27 may be linked to cytoplasmic p27 proteolysis in early G1. p27 is nuclear in G0 and early G1 and appears transiently in the cytoplasm at the G1/S transition. Association of p27 with the exportin CRM1 was minimal in G0 and increased markedly during G1-to-S phase progression. Proteasome inhibition in mid-G1 did not impair nuclear import of p27, but led to accumulation of p27 in the cytoplasm, suggesting that export precedes degradation for at least part of the cellular p27 pool. p27-CRM1 binding and nuclear export were inhibited by S10A mutation but not by T187A mutation. A putative nuclear export sequence in p27 is identified whose mutation reduced p27-CRM1 interaction, nuclear export, and p27 degradation. Leptomycin B (LMB) did not inhibit p27-CRM1 binding, nor did it prevent p27 export in vitro or in heterokaryon assays. Prebinding of CRM1 to the HIV-1 Rev nuclear export sequence did not inhibit p27-CRM1 interaction, suggesting that p27 binds CRM1 at a non-LMB-sensitive motif. LMB increased total cellular p27 and may do so indirectly, through effects on other p27 regulatory proteins. These data suggest a model in which p27 undergoes active, CRM1-dependent nuclear export and cytoplasmic degradation in early G1. This would permit the incremental activation of cyclin E-Cdk2 leading to cyclin E-Cdk2-mediated T187 phosphorylation and p27 proteolysis in late G1 and S phase.
INTRODUCTION
The Cdk inhibitor p27 is an important regulator of G1 progression. It is highly expressed in G0, where it binds tightly and inhibits cyclin E-Cdk 2 (Hengst et al., 1994; Polyak et al., 1994; Slingerland et al., 1994). In mid-G1, p27 also plays a role in the assembly and nuclear import of D-type cyclin-Cdk complexes (LaBaer et al., 1997; Cheng et al., 1999). p27 levels are regulated by translational controls and by proteolysis, and decrease as cells progress from G1 to S phase (Hengst and Reed, 1996; Millard et al., 1997; Slingerland and Pagano, 2000). The ubiquitin-dependent proteolysis of p27 (Pagano et al., 1995) is regulated by its phosphorylation at threonine 187 (T187) by cyclin E-Cdk 2 in late G1 and S phase (Sheaff et al., 1997; Vlach et al., 1997; Montagnoli et al., 1999). T187 phosphorylation allows recognition of p27 by its SCF-type E3 ligase, comprised of Skp1, Cul1, and the F-box protein, Skp2 and Roc1 and the Cks1 cofactor (Carrano et al., 1999; Ohta et al., 1999; Sutterluty et al., 1999; Tsvetkov et al., 1999; Ganoth et al., 2001; Spruck et al., 2001). Recent evidence suggests that p27 proteolysis is regulated by at least two distinct mechanisms, with mitogenic signaling conditioning p27 for degradation in early G1 in a manner independent of T187 phosphorylation (Hara et al., 2001; Malek et al., 2001), whereas Skp2-dependent cyclin E-Cdk 2-mediated degradation occurs in S phase after T187 phosphorylation (Malek et al., 2001).
Although p27 is detected in the nuclei of most normal quiescent cells (Slingerland and Pagano, 2000), the relationship between its intracellular localization and proteolysis has afforded some controversy. Efficient degradation of mammalian p27 and of its Xenopus homolog, Xic1, requires nuclear import to allow its phosphorylation by cyclin E-Cdk2 (Guan et al., 2000; Swanson et al., 2000). Interaction of p27 with the nuclear pore protein, Nup50/NPAP60 (Guan et al., 2000; Muller et al., 2000), may play a role in p27 import. A nuclear localization signal has been identified and import may also involve the binding of p27 by other import mediators (Reynisdottir and Massague, 1997; Zeng et al., 2000). Nup50 antibodies have been shown to block nuclear export but not import (Guan et al., 2000), thus a potential role for p27-Nup50 interaction in p27 export cannot be excluded (Smitherman et al., 2000). p27 can interact with the Jun activation domain-binding protein 1 (p38Jab1). Cotransfection of p27 together with Jab1 led to accelerated proteolysis of p27. The observation that the cytotoxin leptomycin B (LMB) inhibited Jab1-mediated p27 proteolysis suggested that CRM1-dependent nuclear export mechanisms influence p27 degradation (Tomoda et al., 1999) and provided a potential link between p27 turnover and nuclear export. In contrast, the Xenopus homolog of p27, Xic1, is ubiquitinated in the nucleus and its proteolysis is not impaired by LMB (Swanson et al., 2000).
The nuclear pore has an estimated mass of ≈125 MDa and controls nucleocytoplasmic protein exchange. Although the pore diameter permits molecules of up to 50 kDa to diffuse freely across the nuclear envelope, the localization of many small proteins is actively regulated (Mattaj and Engimeier, 1998; Gorlich and Kutay, 1999). Proteins whose function is spatially and temporally regulated, such as cyclin D1 (Alt et al., 2001), cyclin B1 (Pines and Hunter, 1991, 1994; Jin et al., 1998; Yang et al., 1998), IκB (Huang et al., 2000), and mitogen-activated protein kinase (MAPK; Adachi et al., 2000), are actively transported between the nucleus and cytoplasm. This process involves a number of nucleocytoplasmic shuttling proteins. Two proteins, CRM1/exportin 1 (Fukuda et al., 1997; Fornerod et al., 1997; Stade et al., 1997) and the small ras family GTPase, Ran (Melchior et al., 1993; Moore and Blobel, 1993), both play prominent roles in the regulation of nuclear protein export. Ran is abundant and exists in GDP- or GTP-bound forms depending on its cellular location (Melchior and Gerace, 1998; Macara, 1999; Azuma and Dasso, 2000; Sacer and Dasso, 2000). Cytoplasmic RanGDP plays a key role in nuclear import. In the nucleus, RanGDP is converted to RanGTP by the guanine nucleotide exchange factor RCC1, and becomes competent to bind export cargo before shuttling back to the cytoplasm (Nigg, 1997; Mattaj and Engimeier, 1998; Gorlich and Kutay, 1999). The exportin protein, CRM1, binds export cargo proteins and RanGTP in the nucleus to form an export complex that is subsequently translocated to the cytoplasm (Fornerod et al., 1997; Stade et al., 1997; Kehlenbach et al., 1998). At the cytoplasmic face, the complex is dissociated by RanGAP, in combination with either RanBP1 or RanBP2 (Nigg, 1997; Mattaj and Engimeier, 1998; Gorlich and Kutay, 1999).
In the present study, we demonstrate that nuclear export of the Cdk inhibitor, p27, is actively regulated by CRM1/RanGTP binding. Nuclear export of p27 is time, temperature, and energy dependent. Although p27 binding to CRM1 is cell cycle regulated, it is LMB insensitive and LMB has no apparent effect on CRM1-mediated p27 nuclear export either in vitro or in vivo. A putative nuclear export sequence (NES) in p27 is identified. Mutation of this NES reduced nuclear export, impaired p27-CRM1 interactions, and increased p27 stability.
MATERIALS AND METHODS
Cell Culture
MCF-7 cells were grown in improved modified Eagle's medium (IMEM) supplemented with 5% (vol/vol) fetal bovine serum (FBS). Cells were synchronized in G0 by estradiol depletion as described by Cariou et al. (2000). Cells were released from quiescence by the addition of 10−8 M 17β-estradiol (Sigma, St. Louis, MO).
Plasmids and Transfections
A vector encoding wild-type p27 fused to an enhanced yellow-green variant of the Aequorea victoria green fluorescent protein (YFPp27 WT) was prepared by insertion of the full-length wild-type human p27 cDNA sequence into the pEYFP-C1 vector (Clontech, Palo Alto, CA). Double L41A/L45A mutations were introduced into the YFPp27 WT vectors using a Quickchange site-directed mutagenesis kit (Stratagene, La Jolla, CA) to generate the YFPp27NES vector. All p27 cDNA constructs were sequenced fully to ensure the absence of cloning artifacts. Asynchronous MCF-7 cells were transfected with the different YFPp27 vectors (10 μg) using lipofectamine PLUS (GIBCO, Grand Island, NY) and were then estradiol deprived for 48 h. Assays of YFPp27 nuclear export and binding to CRM1 were carried out at 48 h posttransfection. Equal transfection efficiency was verified by direct visualization of p27 by fluorescence microscopy. For the heterokaryon assays, we used a green fluorescence protein tagged p53 construct kindly provided by G. Wahl (Stommel et al., 1999).
Indirect Immunofluorescence of p27 and BrdU
MCF-7 cells were grown on glass slides, arrested in G0 by estradiol depletion (48 h), and then induced to reenter the cell cycle by the addition of 10−8 M 17β-estradiol. Cells were also labeled with 5-bromo-2-deoxy uridine (BrdU) to monitor the timing of S phase entrance. In G0 and at intervals after estradiol addition, cells were fixed in 4% paraformaldehyde and 0.2% Triton X-100, blocked in 3% H2O2 and 10% goat serum, and then incubated with a mouse mAb for p27 (BD Transduction Laboratories, Lexington, KY) and a rabbit polyclonal anti-BrdU antibody (CalTag Laboratories, Burlingame, CA). Fluorescein (FITC)-conjugated anti-mouse and Texas red-conjugated anti-rabbit secondary antibodies allowed visualization of p27 protein and BrdU incorporation, respectively. Cells were visualized by confocal fluorescence microscopy and photographed.
Detection of p27-CRM1 Complexes in Cell Lysates
At indicated times after G0 release, p27 was immunoprecipitated from 1 mg of cellular lysate using the C-19 (Santa Cruz Biotechnology, Santa Cruz, CA). Complexes were resolved and associated CRM1 was detected by immunoblotting with polyclonal CRM1 antibodies (kindly provided by R. Kehlenbach and L. Gerace or by M. Fornerod; Fornerod et al., 1997; Kehlenbach et al., 1998). p27 was detected in CRM1 immunoprecipitates from 1 mg of cell lysate using the Gerace laboratory anti-CRM1 antibody for immunoprecipitation.
Detection of p27-Cyclin E1-Cdk2 Complexes
To test the effect of NES mutation on the association of p27 with cyclin E1-cdk2, cells were transfected with either YFPp27WT or YFPp27NES and cyclin E1 or YFP proteins immunoprecipitated. Complexes were resolved by SDS-PAGE and immunoblotted to detect associated proteins (cyclin E1 or p27).
Subcellular Fractionation and In Vitro Nuclear Export
Cells in G0 and early G1 were harvested and resuspended in 300 μl of an isotonic transport buffer (20 mM HEPES, pH 7.3, 110 mM KAcetate, 5 mM NaAcetate, 2 mM MgAcetate, 1 mM EGTA, and 2 mM dithiothreitol) containing protease inhibitors, 1 mM NaVO3, and 50 mM NaF. Digitonin (20–30 μg/ml final concentration) was added until 90%–95% of the cells exhibited trypan blue staining. Cells were then centrifuged and the supernatant (cytoplasmic fraction) was recovered. The pellet (nuclear fraction) was washed and resuspended in 300 μl of transport buffer. The intact nuclei were then used for nuclear export assays or for immunoblotting. To assay nuclear export of p27, digitonin-permeabilized cells were incubated with fractionated cytosolic protein (75 μg), an ATP regenerating system (5 mM ATP, 5 mM creatine phosphate, and 20 U/ml creatine phosphokinase), and 2 mM GTP at room temperature for up to 30 min (Adam et al., 1992). Nuclear export was stopped by centrifugation, and the supernatant was removed. p27 was immunoprecipitated from both nuclear and supernatant fractions, resolved by SDS-PAGE, and nuclear and exported p27 was detected by immunoblotting. For controls, export assays were conducted at 4°C and in the absence of cytosolic proteins, an ATP regenerating system, or both.
For detection of nuclear p27 export using indirect immunofluorescence, cells were grown on glass slides and permeabilized by incubation with 25 μg/ml digitonin for 3 min at 4°C. The cells were washed and incubated with cytosolic proteins (2.5 mg/ml) and an ATP regenerating system (as above) at room temperature for 10, 20, and 30 min. Nuclear p27 was detected by indirect immunofluorescence and fluorescence intensity quantified using laser scanning software (LSM 510; Carl Zeiss, Jena, Germany) and confocal microscopy.
To assay the expression and nuclear export of green-yellow fluorescent-tagged p27, MCF-7 cells were grown on glass slides and were transfected with wild-type or NES YFPp27 vectors. Export of p27 from digitonin-permeabilized cells was as above. Nuclear export of p27 was determined by measuring the decline in nuclear YFP fluorescence by direct photomicroscopy using a digital camera, and fluorescence intensity was quantified using Laser Scanning Software (LSM 510; Carl Zeiss).
Heterokaryon Assay
Nucleocytoplasmic shuttling of YFP-p27 and GFP-p53 was detected in HeLa/NIH 3T3 cell heterokaryons. Hela cells were grown on glass coverslips in Dulbecco's MEM (DMEM) containing 10% fetal calf serum and was transfected using Superfect reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions. Cells were washed four times with DMEM medium 12 h after transfection, and NIH 3T3 cells were seeded. Cells were treated with cycloheximide (75 μg/ml) for 30 min before fusion. Cell fusion was induced by the addition of 50% (wt/vol) polyethylenglycol in DMEM for 2 min in the presence of 50 μg/ml cycloheximide. After washing four times with PBS, cells were further incubated in DMEM containing 50 μg/ml cycloheximide for 2 h to allow shuttling. To the indicated samples, LMB (final concentration 50 ng/ml) was added 2 h before cell fusion.
After washing with PBS, cells were fixed with 3.7% formaldehyde in PBS (5 min at 20°C, 10 min at 4°C). Cells were permeabilized by treatment with 0.1% Triton X in PBS (4°C) for 5 min. After washing in PBS, cells were incubated with PBS supplemented with 2% bovine serum albumin (BSA) at room temperature for 10 min. Actin was stained with 100 μl of Texas-red phalloidin (Sigma) diluted 1:2000 in PBS/2% BSA. After washing with PBS, DNA was stained with 1% Hoechst 33258 (Molecular Probes, Eugene, OR) diluted 1:2000 in PBS. Coverslips were then washed and mounted on glass slides.
p27 Nuclear Import
Quiescent MCF-7 cells were digitonin permeabilized and incubated with 4 μg/μl (final concentration) cytosolic proteins and an ATP-regenerating system at room temperature. His-tagged p27 was added, and the reactions incubated for 60 min. Reactions were stopped by centrifugation and were separated into nuclear and supernatant fractions. Where indicated, nuclei were preincubated with LMB (50 ng/ml), N-acetyl-leu-leu-norleucinal (LLnL; 2.5 μM), or wheat germ agglutinin (WGA; 20 μg/ml).
Recombinant Protein Assays
p27 was immunoprecipitated from 1 mg of cell lysate recovered 6 h after estradiol stimulation of quiescent MCF-7 cells. After 5 min incubation at 95°C to denature any endogenous p27-associated proteins, the supernatant containing heat-stable p27 was incubated with recombinant CRM1 in the presence or absence of GTP-loaded Ran or GDP-loaded Ran for 20 min at 4°C (Askjaer et al., 1999). p27 was immunoprecipitated and the associated proteins were separated by SDS-PAGE. To assess if p27-CRM1/Ran complexes could be dissociated in vitro, they were incubated with recombinant RanGAP and RanBP1 for 30 min at 30°C. p27 immune complexes were then centrifuged, and dissociation of CRM1 or Ran into the supernatant was assayed by immunoblotting both fractions. Similar experiments were conducted using cells transfected with the wild-type and mutant YFPp27 constructs.
Similar experiments were carried out entirely with recombinant proteins. Recombinant His-tagged p27 prepared in Escherichia coli or baculovirus-produced cyclin D1 proteins were mixed with recombinant CRM1 for 60 min at 4°C with or without pretreatment of CRM1 with LMB 100 ng/ml for 30 min. p27 or cyclin D1 was immunoprecipitated, complexes were resolved, and associated CRM1 was detected by immunoblotting.
To test the effect of HIV-1 Rev on p27-CRM1 and cyclin D1-CRM1 complexes, the HIV-1 Rev NES peptide, NH2-CLPPELERLTL-COOH (Kudo et al., 1998), was synthesized and purified over reverse-phase high-performance liquid chromatography (HPLC) on a C8 column and was verified by mass spectrophotometry. A molar excess of peptide was preincubated with recombinant CRM1 for 30 min at 4°C before the addition of RanGTP and p27 or cyclin D1 proteins for an additional 60 min. p27 or cyclin D1 immune complexes were then assayed for associated CRM1 by IP blots.
Microinjection of Nuclei and Assays of Nuclear Export of p27-NES-Peptide Coupled to BSA
Export ligand preparation followed published procedures (Melchior, 1998). In brief, fatty acid-free BSA (Boehringer Mannheim, Indianapolis, IN) was conjugated with FITC isomer I (Molecular Probes) and was purified via gel filtration. Peptides containing the putative p27 nuclear export sequence (CRNLFGPVDHEELTRDLE) were coupled to FITC-BSA via their N-terminal cysteine using the cross-linker, Sulfo-SMCC (Pierce, Rockford, IL). Microinjection of FITC-BSA-NES and FITC-BSA (0.1 mg/ml) into nuclei of adherent HeLa cells was performed with an Eppendorf Femtojet. Cells were kept at 37°C, and photomicrographs were taken at different times after injection using an inverted fluorescence microscope (IX70; Olympus, Melville, NY) and a back-illuminated charged-coupled device camera (Princeton Instruments, Trenton, NJ).
RESULTS
p27 Localization Is Cell Cycle Regulated
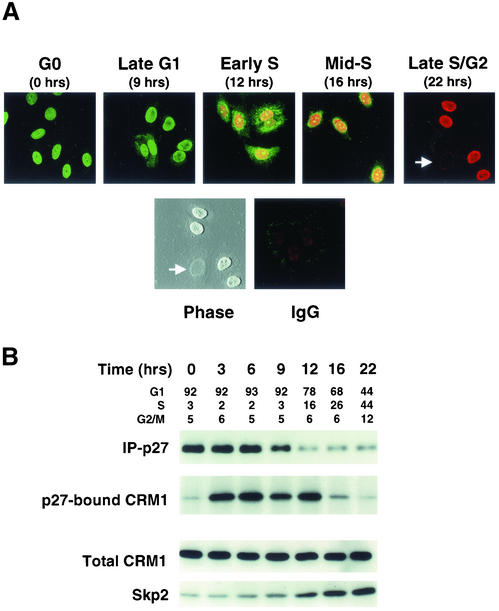
p27 localization was assayed at intervals across the cell cycle in synchronized MCF-7 cells (Cariou et al., 2000). p27 levels and BrdU uptake were monitored by indirect immunofluorescence labeling and confocal microscopy. Quiescent MCF-7 cells exhibited strong nuclear p27 expression (Figure 1A, G0). Nuclear p27 intensity fell progressively as cells moved through G1. At 9 h after estradiol addition, late G1 cells showed predominantly nuclear p27, with some cells showing both cytoplasmic and nuclear p27 localization. Twelve hours after G0 release, at a time when p27 is known to undergo rapid proteolysis (Malek et al., 2001), early S phase cells exhibiting both nuclear and cytosolic p27 were consistently detected, suggesting either delayed import or that nuclear p27 export exceeds import (Figure 1A, early S). Because new p27 synthesis is dramatically decreased within hours of G0 exit (Hengst and Reed, 1996; Millard et al., 1997; S. Cariou and J.M. Slingerland, unpublished MCF-7 data), the latter may be more likely. During S phase (t = 16–22 h), p27 became progressively undetectable in cells staining positive for BrdU uptake. In late S/early G2 cell populations, some cells were negative for both p27 staining and BrdU uptake (Figure 1A, see dual negative cell indicated by white arrow). Flow cytometry of cells at each time point in Figure 1A is shown in Figure 1B.
Figure 1.
p27 localization and CRM1-binding are cell cycle dependent. Cell cycle entry of quiescent MCF-7 cells was induced by the addition of 17β-estradiol at time 0. Cells were assayed at intervals thereafter for p27 localization (A) or for protein assays and cell cycle profiles (B). (A) MCF-7 cells grown on glass slides were arrested in G0 by estrogen depletion. After stimulation with estradiol, p27 levels (green) and BrdU uptake (red) were visualized by confocal fluorescence microscopy at intervals across the cell cycle. Cells negative for both p27 and BrdU staining were evident in the late S phase/G2 population (white arrow). These unstained cells are apparent on phase contrast imaging of the same field. Control cells stained with nonspecific control IgG followed by FITC and Texas Red conjugated antibodies are shown (IgG). (B) Transient binding of p27 to CRM1 occurs early in G1. p27 was immunoprecipitated at intervals across the cell cycle, p27 complexes were resolved, and immunoblots were probed for p27 and CRM1. The same cell lysates were immunoblotted for CRM1 and Skp2. The cell cycle profile at each time point was assayed by dual propidium iodide/BrdU labeling and flow cytometry.
p27-Bound CRM1 Increases during G1 Progression
A major mechanism of nuclear export involves binding of the export cargo protein to the exportin CRM1. Cells were synchronized in parallel with those assayed in Figure 1A above. Immunoprecipitation of cellular p27 at intervals between G0 and S phase revealed an association between p27 and CRM1 that increased during G1 as p27 levels decreased (Figure 1B). p27 was also found to interact with Ran in early G1 in coimmunoprecipitation experiments (M. Connor and J. Slingerland, unpublished results). CRM1 levels were constant across the cell cycle. Given the reduction in p27 in the 6- to 12-h time points, the increase in p27-bound CRM1 is dramatic as cells move through G1 toward late G1/S. The increase in levels of p27-bound CRM1 is temporally associated with the activation of p27 proteolysis demonstrated earlier as cells move from G0 to G1 and S phase (Pagano et al., 1995; Hengst and Reed, 1996; Malek et al., 2001). The onset of this transient binding of p27 to CRM1 occurs before the increase in Skp2 protein levels observed 12 h after the addition of estradiol (Figure 1B). In addition, the onset of p27-CRM1 binding occurs before activation of cyclin E-Cdk2 in this cell line (Cariou et al., 2000).
Nuclear Export of p27 Is Actively Regulated
The cell cycle-dependent changes in localization of endogenous p27 led us to investigate how nuclear export of p27 is regulated. To assay nuclear export of p27, MCF-7 cells were grown on glass slides, synchronized in quiescence, and released into the cell cycle. In mid-G1 (6 h after release from quiescence), cells were treated with digitonin at concentrations that selectively permeabilize the cytoplasmic membrane and leave the nuclear membrane intact. Nuclear export of cellular p27 was detected by indirect immunofluorescence at intervals after the addition of ATP, an ATP-regenerating system, and cytosolic proteins (Figure 2A). A second method was used to demonstrate and quantitate p27 export. Nuclei were separated from cytosolic proteins by digitonin-permeabilization of cells in mid-G1. In vitro export of nuclear p27 was assayed by immunoblotting of nuclear p27 and p27 exported into the supernatant over time (Figure 2B). Passive diffusion of p27 from the nuclei was not observed in the absence of ATP or at 4°C in either of these assays, indicating that p27 export is actively regulated (Figure 2, A and B). In vitro export of p27 from G0 nuclei proceeded at a rate 50% slower than that from mid-G1 nuclei (Figure 2B). Thus, as cells progress from G0 into mid-G1, p27 or other cofactors may be modified to facilitate nuclear export. The rate of p27 export assayed by densitometric analysis of p27 blots in Figure 2B was similar to that measured by the decay in nuclear p27 fluorescence quantitated by confocal microscopy using laser scanning software (Carl Zeiss) in Figure 2A.
Figure 2.
Active nuclear export of p27. (A) Cells were grown on glass slides and synchronized as in Figure 1. Six hours after induction of cell cycle entry by 17β-estradiol addition, cells were digitonin permeabilized and subjected to export assays, fixed, and nuclear p27 visualized by indirect immunofluorescence. (B) Cells were recovered in either mid-G1 or G0 and nuclear export of p27 after digitonin permeabilization assayed as described in “Materials and Methods.” At indicated times, reactions were stopped by centrifugation and p27 was assayed in nuclei (N) and supernatant (S) fractions. p27 export was minimal after 30 min in the absence of ATP (-ATP), cytosol (-CYT), or both (-ATP/–CYT).
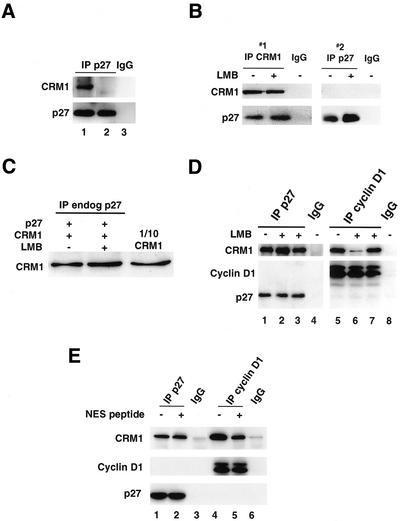
p27 Interacts with CRM1 In Vitro
When CRM1 binds to export cargo in association with nuclear RanGTP, cytoplasmic dissociation of the complex is stimulated by GTP hydrolysis. To test the specificity of p27-CRM1 interactions, formation of CRM1-Ran-p27 complexes was assayed in vitro. p27 was immunoprecipitated from mid-G1 cells, boiled to dissociate heat labile proteins, and was then incubated with recombinant CRM1 and either GTP-loaded Ran (Figure 3A, lane 1) or GDP-loaded Ran (Figure 3A, lane 2). p27-CRM1-Ran complexes were readily detected in the presence of RanGTP, but were significantly reduced in the presence of RanGDP. Nonspecific binding of CRM1 or Ran to p27 antibody-bound protein A beads was not evident (Figure 3A, lane 3). p27 binding to CRM1 alone was also detected and p27-CRM1-RanGTP complexes dissociated after incubation with RanBP1 and RanGAP (M. Connor and J.M. Slingerland, unpublished results). These data support the specific association of p27, CRM1, and Ran complex formation in early G1 regulating nuclear export of p27.
Figure 3.
p27 binds CRM1 in vitro and LMB does not impair p27-CRM1 binding. (A) p27 was immunoprecipitated from cells in early G1 and incubated at 95°C for 5 min to denature heat labile-associated proteins. The supernatant containing p27 was then incubated with recombinant CRM1 together with either GTP-loaded Ran (lane 1) or GDP-loaded Ran (lane 2) for 30 min at 4°C followed by immunoprecipitation of p27. p27 antibody-bound protein A Sepharose beads did not show any nonspecific interaction with recombinant Ran or CRM1 (lane 3). (B) CRM1 was first immunoprecipitated from cells recovered in midG1 with or without prior treatment with LMB (IP #1). The supernatant was recovered and p27 was then immunoprecipitated from the CRM1-depleted lysates (IP #2). CRM1- and p27-bound proteins were immunoblotted for CRM1 and p27. Antibody only controls are shown for IP #1 and #2 (IgG). (C) The effect of LMB on binding of recombinant CRM1 to cellular p27 was assayed as in A. Equal amounts of heat stable p27 recovered from midG1 cells were reacted with recombinant RanGTP and CRM1 without (lane 1) or with (lane 2) pretreatment of the CRM1 with LMB. p27 was then immunoprecipitated and complexes were resolved and blotted for associated CRM1. One-tenth of the input recombinant CRM1 was loaded in the lane on the right. Equal amounts of p27 were immunoprecipitated in each lane (not shown). (D) Recombinant his-tagged p27 (lanes 1 through 3) or flag-tagged T286-phosphorylated cyclin D1 (lanes 5 through 7) were incubated with RanGTP and CRM1 either without (lanes 1 and 5) or with (lanes 2 and 6) pretreatment of the CRM1 with LMB. LMB was also added to the reaction mixture after complex formation (lanes 3 and 7). Antibody control lanes are also shown (lanes 4 and 8). (E) CRM1 binding assays were carried out as in D. CRM1 was preincubated with a peptide corresponding to the NES of the HIV-1 Rev protein (NES peptide) before the addition of p27 (lane 2) or cyclin D1 (lane 5).
LMB Increases p27 Levels But Does Not Disrupt p27-CRM1 Binding
Treatment of cells with LMB increased p27 levels (Figure 3B, right panel and Figure 4C). Densitometric analysis of repeat experiments showed that ∼18% of total cellular p27 was detected in CRM1 complexes at 9 h after release from G0. However, LMB treatment did not appear to impair endogenous cellular p27-CRM1 interaction (Figure 3B). To further explore this unexpected result, we assayed p27-CRM1 binding in vitro by two different methods. Pretreatment of recombinant CRM1 with a molar excess of LMB in vitro did not impair the binding of CRM1 to heat stable p27 isolated from cells in mid-G1 (Figure 3C), nor did it impair CRM1 binding to His-tagged recombinant p27 protein (Figure 3D, lane 1 versus lane 2). In contrast, CRM1 binding to cyclin D1 was impaired by LMB. CRM1 binding to cyclin D1 was assayed using flag-tagged recombinant cyclin D1, phosphorylated at T286 in vitro with GSK-3β to promote binding to CRM1 (Alt et al., 2001). Cyclin D1-bound CRM1 was substantially reduced by pretreatment of the recombinant CRM1 with LMB (Figure 3D, lane 5 versus lane 6). LMB was unable to dissociate CRM1-bound p27 or cyclin D1 when it was added to these respective complexes subsequent to mixing of the recombinant proteins (Figure 3D, lanes 3 and 7).
Figure 4.
Both LLnL and LMB increase nuclear and cytoplasmic p27 levels. (A) Mid-G1 cells were recovered at 9 h after G0 release, with or without 6 h of LLnL treatment immediately before harvesting. Lysates were immunoblotted for p27. (B) Cells were also treated as in A above and nuclear (N) and cytoplasmic (C) fractions immunoblotted for p27. Immunoblots were probed for the nuclear protein RCC1 to verify the lack of leakage of nuclear proteins into the cytoplasm. (C and D) At 6 h after G0 release, cells were incubated either with or without LMB for an additional 6 h and whole cell lysates (C) or nuclear and cytosolic fractions (D) were immunoblotted as shown. RCC1 probing verified adequacy of fractionation (not shown).
HIV-1 Rev NES Peptide Competes with CRM1 Binding to Cyclin D1 But Not to p27
Active nuclear export involves binding of an exportin to a NES motif on the export substrate (Mattaj and Engimeier, 1998; Gorlich and Kutay, 1999). The exportin, CRM1 binds to classical NES-containing proteins via an LMB-sensitive domain. The failure of LMB to inhibit CRM1 binding to p27 both in vivo and in vitro raised the possibility that p27 interacts with CRM1 in a novel manner. To test this, we assayed the effect of prebinding CRM1 to the classical NES motif of the HIV-1 Rev protein on the association between p27 and CRM1 and between cyclin D1 and CRM1 in vitro. CRM1 was preincubated with a 10 amino acid peptide corresponding to the HIV Rev NES (NH2-CLPPLRLTL-COOH) for 30 min before the addition of RanGTP and either p27 or cyclin D1. Preincubation of CRM1 with the HIV-1 Rev NES peptide did not reduce the amount of CRM1 that bound to p27 (Figure 3E, lane 1 versus lane 2). In contrast, the NES peptide reduced cyclin D1/CRM1 interaction (Figure 3E, lane 4 versus lane 5).
Proteasome Inhibition and LMB Increase Detectable Cytoplasmic p27
Recent reports suggest that p27 degradation is complex and may involve both T187-dependent and -independent proteolytic mechanisms (Sheaff et al., 1997; Malek et al., 2001; Ishida et al., 2002). If even a portion of cellular p27 undergoes nuclear export before cytosolic degradation, proteasome inhibition should lead to accumulation of p27 within the cytoplasm. As shown previously (Pagano et al., 1995), proteasome inhibitors increased p27 protein levels (Figure 4A). Nuclear-cytoplasmic fractionation confirmed the data in Figure 1A that in G0, p27 is almost exclusively nuclear (Figure 4B). A modest reduction of p27 was notable by 9 h after G0 release, with a minor amount of p27 in the cytoplasm. LLnL treatment in mid-G1 led to increases in both nuclear and detectable cytoplasmic p27 levels. The nuclear:cytoplasmic ratios of p27 were 9.2:1 and 4.5:1 for cells in mid-G1 in the absence or presence of LLnL, respectively. Because LLnL appears not to delay either export or import of p27 (see below, Figure 5), these data are consistent with degradation of at least part of the cellular p27 pool occurring in the cytoplasm.
Figure 5.
LMB does not prevent p27 nuclear export in vitro or in vivo. (A) MCF-7 cells were transfected with YFPp27WT and arrested in quiescence. Cells were digitonin permeabilized and incubated with 2.5 mg/ml cytosolic proteins and an ATP regenerating system. Incubations were carried out for the indicated times at room temperature. Where indicated, cells were pretreated with LMB or LLnL to assess the affects of these drugs on p27 nuclear export. (B) The decay of nuclear p27 fluorescence in A was visualized by direct fluorescence microscopy, photographed with a digital camera, quantitated using Carl Zeiss laser scanning software (LSM) 510, and graphed as a function of time. (C) Hela cells transfected with expression vectors for either YFPp27 or GFPp53 were fused to nontransfected NIH 3T3 cells (white arrow) in the presence or absence of LMB. The localization of either YFPp27 or GFPp53 (green) was visualized by fluorescence microscopy. Cells were fixed and stained for actin (red). Nuclei were visualized by staining the DNA with Hoechst 33258 (black and white panels). (D) p27 nuclear import was assessed by the addition of his-tagged p27 (His-p27) to digitonin-permeabilized cells in the presence of cytosolic proteins (4 μg/μl) and an ATP regenerating system. Reactions were centrifuged and nuclear (N) and supernatant (S) fractions were immunoblotted for p27. Nuclei preincubated with 200 μg/ml WGA showed no import of p27.
To further investigate the effect of LMB on p27 levels and localization, MCF-7 cells were released from quiescence for either 12 or 6 h followed by addition of 200 ng/ml LMB for an additional 6 h and nuclear and cytoplasmic fractions were isolated. LMB treatment in mid-G1 inhibited the reduction in p27 levels that occurred in when cells progress into early S phase (Figure 4, C and D). Although LMB caused an accumulation of p27, it did not sequester p27 exclusively within the nucleus (Figure 4D). p27 levels were increased in both the nucleus and in the cytoplasm, exhibiting a pattern similar to that observed after inhibition of proteolysis by LLnL (Figure 4B). Immunoblotting for RCC1 showed no escape of nuclear protein into the cytoplasmic fractions (M. Connor and J.M. Slingerland, unpublished results).
LLnL and LMB Do Not Prevent Nuclear Export of p27 In Vitro or In Vivo
In the context of Jab-1 overexpression, LMB has been shown to inhibit the cytoplasmic accumulation of p27 and its proteolysis (Tomoda et al., 1999). Theoretically, the detection of cytoplasmic p27 in both LLnL- and LMB-treated cells (Figure 4, B and D) could reflect accelerated p27 export, impaired p27 import, or impaired proteolysis of exported protein. To test this, the effects of both LMB and LLnL on nuclear p27 export were assayed (Figures 5, A-C). MCF-7 cells were grown on glass slides and were transfected with a vector encoding wild-type p27 linked to a yellow fluorescence protein (YFPp27WT). Export of YFPp27WT from the nuclei of digitonin-permeabilized cells was assayed as in Figure 2A. A progressive reduction in nuclear YFPp27WT >30 min was quantitated by fluorescence confocal microscopy using scanning laser microscopy software (Figure 5B). The addition of LMB did not delay YFPp27WT nuclear export. Similar results were evident when export was assayed in the presence of LLnL (Figure 5, A and B). Nuclear export assays of endogenous p27 using the same method as in Figure 2B confirmed the results above, with no inhibition of p27 nuclear export evident in the presence of either LMB or LLnL (M. Connor and J.M. Slingerland, unpublished data). Thus, neither LMB nor LLnL had a measurable effect on the rate or extent of p27 nuclear export.
Because LMB did not prevent p27-CRM1 interaction in vivo or in vitro, and LMB did not inhibit nuclear export of p27 in vitro, we next assayed the effect of LMB on nuclear to cytoplasmic shuttling of p27 in vivo in heterokaryons. Heterokaryon assays were conducted in which Hela cells, transfected with either YFPp27WT or green fluorescence protein linked p53 (GFPp53) vectors, were fused to untransfected NIH 3T3 cells (Figure 5C). In the heterokaryons, the consistent appearance of YFPp27WT in the NIH 3T3 nucleus (Figure 5C, white arrow) indicated that the YFPp27 was being exported from the Hela cell nucleus. Pretreatment with 50 ng/ml LMB for 2 h and cycloheximide for 30 min before heterokaryon fusion had no effect on p27 shuttling (Figure 5C, p27, -LMB, and +LMB). All heterokaryons showed p27 shuttling in the absence of LMB, and 95% of the heterokaryons pretreated with LMB displayed p27 shuttling. In contrast, as shown by Stommel et al. (1999), the export of GFPp53 from the Hela cell nucleus was inhibited by LMB, with 90% of the heterokaryons showing shuttling without LMB, and only 25% showing shuttling in the presence of LMB (Figure 5C, p53 -LMB versus +LMB).
Having shown that neither LLnL nor LMB appears to affect p27 export, the cytoplasmic accumulation of p27 after treatment with either drug could reflect impaired p27 nuclear import. Thus, we assayed the effect of these drugs on nuclear import of p27. After a 60-min incubation with 4 μg/μl cytosolic proteins and an ATP regenerating system, recombinant His-p27 was imported into the nuclei of digitonin-permeabilized cells (Figure 5D, 60 min). No import occurred at 4°C, in the absence of ATP and cytosol (M. Connor and J.M. Slingerland, unpublished data), or in the presence of WGA (Figure 5D). Neither LMB nor LLnL impaired His-p27 nuclear import. Thus, LMB and LLnL do not impair either p27 import or export as measured by these assays, and the appearance of cytoplasmic p27 after treatment with these drugs is consistent with delayed degradation of exported protein.
p27 Contains an Atypical NES
We identified a putative NES within the Cdk-binding domain of p27 between amino acids 32 and 45 (Figure 6A) based on the homology of leucine spacing to a cryptic NES identified in the equine infectious anemia virus (EIAV) Rev protein (Mancuso et al., 1998). The spacing of the three leucines in this region of p27 is highly conserved between species.
Figure 6.
p27 nuclear export involves a nuclear export sequence. (A) The classical and nonclassical NES of the HIV-1Rev and EIAV Rev proteins and the sequence (amino acids 32 through 45) containing the conserved leucines in the putative p27 NES from different species are shown. (B) FITC-BSA (right panel) or FITC-BSA coupled to peptides containing the putative p27 NES (two left panels) was microinjected into the nuclei of adherant HeLa cells. Pictures at 0 and 45 min after injection were taken with identical exposure times. (C) NES mutation delays p27 export. Cells were grown on glass slides and were then transfected with YFPp27WT, YFPp27NES, YFPS10A, or YFPp27T187A. Forty-eight hours after transfection, cells were digitonin permeabilized and p27 export assayed. Nuclear export of p27 was visualized directly by photomicroscopy with a digital camera. (D) The intensity of nuclear p27 fluorescence was quantitated using Carl Zeiss Laser Scanning Software (LSM) 510 and graphed as a percentage of the maximum intensity measured at t = 0 min for each p27 allele product (p27WT, and the NES, S10A, and T187A p27 mutants).
To obtain additional evidence that amino acids 32 through 45 in p27 comprise a functional NES, we tested its ability to mediate nuclear export of an unrelated protein. For this, peptides containing the putative p27 NES (CRNLFGPVDHEELTRDLE) were coupled to FITC-labeled BSA and were microinjected into nuclei of adherent HeLa cells. As is shown in Figure 6B, a significant fraction of p27NES-FITC-BSA translocated into the cytoplasm within 45 min. In contrast, the nuclear localization of FITC-BSA remained unchanged. Although p27-NES mediated export of p27NES-FITC-BSA is not very efficient, possibly due to competing events such as the observed accumulation in nuclear speckles, or due to rate-limiting binding partners, these findings support the interpretation that amino acids 32 through 45 in p27 function as an NES.
Two leucines within this region were mutated (L41A and L45A) and effects on p27 export were examined. MCF-7 cells were transiently transfected with vectors encoding YFPp27WT or the putative NES mutant p27 linked to YFP (YFPp27NES), and nuclear export of p27 from digitonin-permeabilized cells was quantitated over time. Nuclear export of YFPp27NES was slower than that of YFPp27WT (Figure 6, B and C). The export kinetics of YFPp27WT were similar to those of the endogenous p27 in Figure 2.
Mutation of S10 But Not of T187 Affects Nuclear Export of p27
p27 contains several sites whose phosphorylation could influence its nuclear export. Ishida et al. (2000) recently identified serine 10 (S10) as a major phosphorylation site in G0 arrested cells. Mutation of S10 to alanine strongly inhibited p27 export (Figure 6, B and C), suggesting that phosphorylation at this site is essential for nuclear export of p27. The nuclear export of YFPp27S10D was similar to that observed for YFPp27WT (M. Connor and J.M. Slingerland, unpublished results). Because p27 degradation in late G1 involves its phosphorylation at T187 by cyclin E-cdk 2, nuclear export of YFPp27T187A was assayed. The mutation of T187 to alanine had no effect on p27 export, suggesting that phosphorylation at this site is not necessary for p27 nuclear export (Figure 6).
p27-CRM1 Interaction Is Reduced by NES and S10A But Not by T187A Mutation
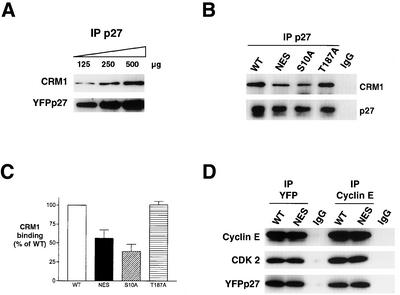
In vitro binding assays were conducted to test whether these p27 mutations affect p27-CRM1 interaction. To demonstrate the linearity of the p27-CRM1 binding reaction, increasing amounts of immunoprecipitated YFPp27WT (125–500 μg) were incubated with recombinant CRM1 and RanGTP, both in molar excess of p27 (Figure 7A). p27-associated CRM1 increased in proportion to the amount of input p27 in the reactions. The NES mutant p27 (L41A/L45A) bound 44% less CRM1 in vitro than did YFPp27WT (Figure 7, B and C). Thus, the reduction in p27 export conferred by the NES mutation (Figure 6) is associated with impaired binding to CRM1. Mutation of S10 to alanine also reduced p27-CRM1 binding to 40% that of p27WT. The T187A mutation of p27 did not affect either its rate of nuclear export or its binding to CRM1 (Figure 7, B and C).
Figure 7.
Effects of p27 NES mutation on CRM1- and Cyclin E-binding. (A) To show the linearity of WT p27-CRM1 binding assays, increasing amounts of heat-stable YFP-p27 (125–500 μg) were incubated with fixed amounts of CRM1 and RanGTP. p27 was immunoprecipitated and immunoblots probed for associated CRM1. (B) YFPp27 was immunoprecipitated from 300 μg of lysate from cells transfected with WT, NES, S10A, or T187A YFPp27, released from protein A beads by boiling for 10 min, and incubated with recombinant CRM1 and RanGTP. p27-complexes were probed for CRM1 and p27. CRM1 binding was corrected for differences in the amount of YFPp27 expressed in the different transfectants and graphed. (C) Graphical quantification of YFP-bound CRM1. Results represent the mean ± SEM of four independent experiments. (D) MCF-7 cells were transfected with YFPp27WT or YFPp27NES expression vectors. YFP and cyclin E immunoprecipitates were assayed for associated cyclin E, Cdk 2, or p27 by immunoblotting.
Reduced Export of the p27NES Mutant Is Not Due to Decreased Cyclin E-Cdk 2 Binding
Because the p27 NES resides within its cyclin-binding domain, the increased stability of p27NES could theoretically reflect reduced binding to cyclin E-Cdk2 and thus reduced cyclin E-Cdk2-Skp2-dependent p27 proteolysis. To evaluate this, MCF-7 cells were transfected with YFPp27WT and YFPp27NES, and lysates were immunoprecipitated with either YFP or cyclin E antibodies and associated proteins were detected by immunoblotting (Figure 7D). The amount of p27NES bound to cyclin E-Cdk2 was similar to cyclin E-bound p27WT.
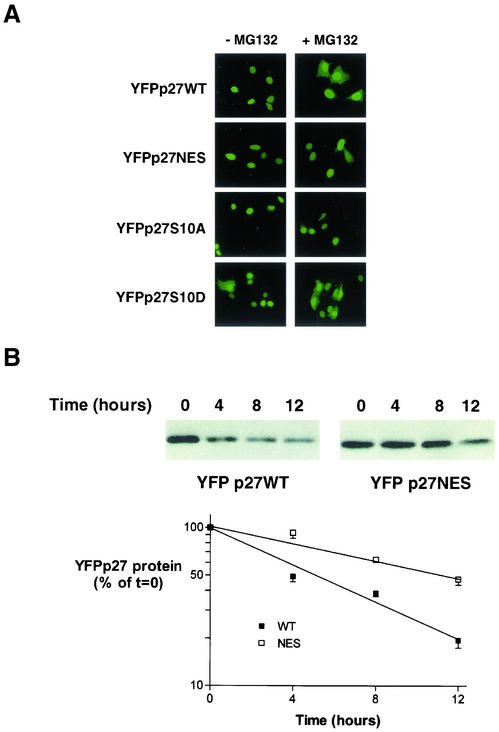
p27NES Shows Delayed Cytoplasmic Accumulation after Proteasome Inhibition
Cytoplasmic p27 is detected when proteasomal degradation is impaired (Figure 4B). We reasoned that if the NES mutations delay p27 export in vivo, and if some p27 undergoes cytoplasmic degradation, p27NES should show a reduced or delayed appearance in the cytoplasm after proteasome inhibition. YFPp27NES and YFPp27WT were transfected into MCF-7 cells. Newly synthesized p27 was detected exclusively in the nuclei in >90%-95% of all cell populations between 16 and 24 h posttransfection (Figure 8A, -MG132). The proteasome inhibitor, MG132 (25 μM), was added to cells at 16 h posttransfection. Direct fluorescence microscopy at intervals after MG132 addition showed a progressive cytoplasmic accumulation of p27WT, with 44% of cells showing cytoplasmic p27 by 8 h after MG132 addition (Figure 8A, + MG132). YFPp27NES showed a significant delay in redistribution of from nucleus to cytoplasm compared with that of p27WT, with only 18% of the p27NES transfected cells displaying cytoplasmic p27 at the same 8-h time point. S10A mutation severely impaired p27 export, with only 7% of transfected cells exhibiting cytoplasmic p27 after 8 h of MG132. In contrast, the S10D mutation did not affect the accumulation of cytoplasmic p27 (Figure 8A).
Figure 8.
Effects of p27 mutation on localization and half-life. (A) Cells were grown on glass slides and transfected with either WT, NES, S10A, or S10D YFPp27 vectors for 16 h and then treated with the proteasome inhibitor MG132 (+MG132) or without (-MG132) for an additional 8 h before fixation and photomicroscopy. (B) Cells were transfected with YFPp27WT or YFPp27NES constructs and cycloheximide (100 μg/ml) was added at 48 h posttransfection. Cells were harvested 4, 8, and 12 h after cycloheximide addition, and p27 was detected by immunoblotting with YFP-specific antibody. The decay of the p27 signal is graphed as a function of time postcycloheximide addition. Linear regression curves were fitted to calculate the half-lives of each of the mutant p27 proteins using data from repeat experiments. SE bars are shown.
Delayed Nuclear Export Is Associated with Increased p27 Protein Stability
The p27 protein half-lives (t1/2) of p27WT and p27NES were compared by cycloheximide chase (Figure 8B). At 48 h posttransfection, both the YFPp27WT and YFPp27NES induced G0/G1 arrest as assayed by dual BrdU/propidium iodide staining and flow cytometry (M. Connor and J.M. Slingerland, unpublished data). Wild-type p27 exhibited a t1/2 of 5.7 h. The p27NES mutant was over twice as stable as p27WT, with a t1/2 of 11.7 h.
DISCUSSION
The reduction of p27 levels is critical for cyclin E-Cdk2 activation and G1-to-S phase progression. As cells exit G0, p27 synthesis is rapidly reduced (Hengst and Reed, 1996; Millard et al., 1997) and its proteolysis is increased (Pagano et al., 1995). The half-life of p27 is maximal in G0 and is reduced in asynchronous cells (Pagano et al., 1995; Hengst and Reed, 1996). Indeed, in G1 and S phase cells, the p27 half-life is reduced fivefold compared with that in quiescence (Malek et al., 2001). Although much is known regarding mechanisms of ubiquitin-dependent SCFSKP2-mediated p27 proteolysis, the relationship between p27 localization and its degradation has been unclear.
Although molecules of up to 50 kDa can diffuse freely through nuclear pores, p27 is largely bound to multiprotein complexes. The present data suggests that both p27 import into and export from the nucleus are actively regulated. Moreover, we provide evidence that for at least part of the nuclear p27 pool, CRM1-dependent nuclear export may precede degradation. Detectable p27 is exclusively nuclear in G0 and early G1, with transient appearance in both the nucleus and cytoplasm as cells progress through G1, before its disappearance in late S phase. The dramatic increase in CRM1-p27 binding during G1 progression and the transient appearance of cytosolic p27 at the G1/S transition suggested a link between nuclear export of p27 and its degradation. Furthermore, proteasome inhibition in G1 led to the appearance of cytoplasmic p27 at a time when new p27 synthesis is minimal. The timing of cellular p27-CRM1 interaction and the observation that p27 is exported more rapidly from G1 nuclei than from G0 nuclei suggest that p27, the CRM1-ran export machinery, or both may undergo periodic posttranslational changes to facilitate p27 export in early G1. p27 phosphorylation appears to play a critical role in this process. Not only does p27 phosphorylation differ between the nucleus and cytoplasm (M. Connor and J.M. Slingerland, unpublished data), we and others have shown that the phosphorylation status of serine 10 (S10) critically regulates p27 export (Rodier et al., 2001; Ishida et al., 2002; Boehm et al., 2002).
CRM1 mediates nuclear export by binding to a leucine-rich NES motif in the export substrate. The first NES identified was that of the HIV-1 Rev protein, and a classical NES consensus sequence has been identified based on a conserved clustering of leucine residues (Bogerd et al., 1996). We propose that amino acids 32 through 45 constitute a NES for p27. The spacing of the leucine residues in this putative p27 NES is identical to that in the NES of the EIAV Rev protein. This EIAV Rev sequence is functionally homologous to the NES of the HIV-1 Rev (Mancuso et al., 1998). Although the leucines in the EIAV and p27 NES are less tightly clustered than those in the HIV-1 Rev NES, their spacing is completely conserved in the p27 sequence of all known species. When a peptide comprised of amino acids 32 through 45 of p27 was linked to FITC-tagged BSA, the peptide directed export of this construct to the cytoplasm. The ability of this p27 peptide to direct the nuclear export of a heterologous protein is consistent with this sequence functioning as an NES. In control experiments, FITC-BSA without the p27 NES remained nuclear.
Mutations converting two of these three NES leucines to alanine reduced CRM1 binding, impaired export in vitro, and prolonged the half-life of the mutant p27 protein. Moreover, the p27NES mutant showed delayed and reduced accumulation in the cytoplasm after proteasome inhibition. However, mutation of these leucine residues did not affect p27 binding to cyclin E-Cdk 2, and thus, the stability of p27NES cannot be attributed to impaired cyclin E-Cdk2-mediated p27 degradation.
Nuclear export of the p27NES protein was impaired but not abolished. This may reflect the incomplete inhibition of CRM1-NES binding by these two leucine mutations, the involvement of other motifs on p27 in CRM1 binding, or the existence of another non-CRM1-dependent export mechanism for p27.
Surprisingly, LMB did not prevent the interaction between p27 and CRM1 in vitro or in vivo in LMB-treated cells. In addition, LMB did not inhibit p27 nuclear export from digitonin-permeabilized cells nor did it impair p27 export in vivo in heterokaryons. In contrast, the binding of CRM1 to cyclin D1 (Alt et al., 2001) and nuclear export of another CRM1 cargo, p53 (Stommel et al., 1999) were notably impaired by LMB drug concentrations that did not affect p27. LMB modifies the exportin CRM1 at C529 (Kudo et al., 1999) and is thought to inhibit protein export by impairing CRM1-substrate NES interaction. The lack of effect of LMB on cellular p27-CRM1 binding and on p27 nuclear export are consistent with p27 binding to CRM1 at sites other than or in addition to the LMB-sensitive motif at C529. Our observation that classical HIV1 Rev NES peptide, which reduced cyclin D1/CRM1 interactions, did not impair p27 binding to CRM1 in vitro suggests that p27 binds to a site distinct from the classical NES-binding motif on CRM1. Transport factors have been shown to bind different cargo using slightly different binding sites (Conti and Kuriyan, 2000).
The effect of LMB on p27 export may be modulated by the binding of other proteins to the p27-CRM1 complex. The ability of LMB to interfere with CRM1 binding to Rev can be modulated by the binding of other proteins (Askjaer et al., 1998). Recently, Tomoda et al. (2002) demonstrated that p27 nuclear export was LMB sensitive in the presence of overexpressed p38Jab1. When the p38Jab1 NES was mutated, effectively removing the protein from the p27-CRM1 complex, p27 export became LMB insensitive. Thus, the formation of an export-competent p27-CRM1 complex in vivo may involve other proteins that modulate the sensitivity of p27 nuclear export to LMB. This process may show important cell type- and species-specific differences (Swanson et al., 2000; Rodier et al., 2001). More intensive investigation of the specific site(s) of CRM1-p27 interaction and of the composition of p27-CRM1 complexes is warranted. Nonetheless, our data raise the concern that LMB-insensitive nuclear export may not always be CRM1 independent.
LMB treatment of quiescent cells prevents their subsequent progression through G1 into S phase (M. Connor and J.M. Slingerland, unpublished data). Because p27 export from G0 nuclei is less efficient than from nuclei in early G1, the timing of LMB addition may be important when interpreting the effects of LMB on p27 export. Using p27 immunofluorescence, others have shown that LMB blocks the transient cytoplasmic accumulation of p27 that occurs when cells are released from quiescence (Rodier et al., 2001; Ishida et al., 2002). Since treatment with LMB in quiescence blocks G0 to G1 progression, the early G1 activation of p27 export would be compromised. Thus, the lack of cytoplasmic p27 after LMB treatment observed by others may reflect failure to exit quiescence and be an indirect effect of LMB on p27 localization.
Recent data using T187A knock-in and Skp2-/- mice suggest that more than one mechanism regulates p27 proteolysis, and that p27 proteolysis is T187 independent in early G1 (Hara et al., 2001; Malek et al., 2001). We observed that the interaction between p27 and CRM1 begins at a time in the cell cycle when both Skp2 protein levels and cyclin E-Cdk2 activities are low. Furthermore, neither nuclear export of p27 nor its binding to CRM1 are dependent on phosphorylation at T187. The present data and that of others allow the allowing model of two distinct mechanisms regulating p27 proteolysis. Mitogen-dependent phosphorylation of p27 in early/mid-G1 may lead to a reduction in p27-cyclin E-Cdk2 binding, thereby exposing the p27 NES located within the cyclin-binding domain. This would facilitate p27-CRM1 interaction and the formation of an export competent protein complex, including RanGTP. Phosphorylation at serine 10 (S10) may be a prerequisite for subsequent events that mediate CRM1 binding and nuclear export. Both our own data and that of others indicate that S10 phosphorylation is important for CRM1 binding and is required for p27 export (Rodier et al., 2001; Ishida et al., 2002). Binding of nucleoporins, such as Nup50, may facilitate translocation of p27 to the cytoplasm (Guan et al., 2000), where it is ubiquitylated and degraded (Hara et al., 2001). This early phase of export-linked p27 proteolysis appears to precede Skp2 up-regulation and cyclin E-cdk2 activation and is independent of phosphorylation on T187 by cyclin E-Cdk 2, consistent with other recent reports (Hara et al., 2001; Malek et al., 2001; Ishida et al., 2002). This initial mechanism of p27 degradation in early G1 would allow an incremental activation of cyclin E-Cdk2. This would be followed by rapid progressive kinase activation, as activated cyclinE-Cdk2 mediates subsequent T187 phosphorylation-dependent ubiquitylation of p27 by SCFSKP2 and degradation in late G1 and S phase.
If an initial mechanism of titrating down p27 via nuclear export-mediated degradation is required for the efficient activation of cyclin E-Cdk2-dependent p27 proteolysis, interference with export-mediated p27 proteolysis could significantly alter the kinetics of G1 to S phase progression. The relevance of the subcellular localization of p27 to T187 phosphorylation-dependent proteolysis remains unclear. However, it has been reported that Xic1 ubiquitylation occurs in oocyte nuclei (Swanson et al., 2000) and that nuclear p27 in Rat1 fibroblasts is efficiently degraded (Rodier et al., 2001). This may also be the case for p27 in epithelial cells, because both nuclear and cytoplasmic p27 levels are increased after LLnL treatment.
There appear to be multiple phosphorylation sites on p27 (Ishida et al., 2000; Donovan et al., 2001) whose role in regulating p27 function and degradation remain unknown. Additional work is necessary to elucidate what phosphorylation events follow that of S10 in early G1 and how p27 phosphorylation may regulate its nuclear export. Although we estimate that ∼20% of cellular p27 is present in CRM1 complexes in early to mid-G1, it remains unclear what proportion of the total cellular p27 is degraded after CRM1-mediated export. This may show both cell type variability and change during malignant tumor progression as a function of checkpoint losses that increase cyclin E-Cdk2 activity.
ACKNOWLEDGMENTS
We thank Geoffrey Wahl, Jayne Stommel, Ian Mattaj, Maarten Fornerod, Ralph Kehlenbach, Larry Gerace, and Alan Diehl for the reagents provided. We thank Barbara Wolff-Winiski of Novartis and Minoru Yoshida for their kind gifts of LMB. This work was supported by postdoctoral Fellowships from the Sunnybrook Trust and the U.S. Army Department of Defense Breast Cancer Research Program to M.C., and by a grant from the Canadian Breast Cancer Research Initiative (to J.M.S.). J.M.S. is supported by career awards from the Burrough's Wellcome Fund and the U.S. Army Department of Defense Breast Cancer Research Program.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–06–0319. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–06–0319.
REFERENCES
- Adachi M, Fukuda M, Nishida E. Nuclear export of MAP kinase (ERK) involves a MAPK kinase (MEK)-dependent active transport mechanism. J Cell Biol. 2000;148:849–856. doi: 10.1083/jcb.148.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam SA, Sterne-Marr R, Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. doi: 10.1016/0076-6879(92)19013-v. [DOI] [PubMed] [Google Scholar]
- Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2001;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P, Bachi A, Wilm M, Bischoff FR, Weeks DL, Ogniewski V, Ohno M, Niehrs C, Kjems J, Mattaj IW, Fornerod M. RanGTP regulated interactions of CRM1 with nucleoporins and a shuttling DEADbox helicase. Mol Cell Biol. 1999;19:6276–6285. doi: 10.1128/mcb.19.9.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P, Jensen TH, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. JBiolChem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Dasso M. The role of Ran in nuclear function. Curr Opin Cell Biol. 2000;12:302–307. doi: 10.1016/s0955-0674(00)00093-4. [DOI] [PubMed] [Google Scholar]
- Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, Nabel EG. A growth factor-dependent nuclear kinase phosphorylates p27Kip1 and regulates cell cycle progression. EMBO J. 2002;21:3390–3401. doi: 10.1093/emboj/cdf343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR. Protein sequence requirements for function of the human T-cell virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou S, Donovan JC, Flanagan WM, Milic A, Bhattacharya N, Slingerland JM. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc Natl Acad Sci USA. 2000;97:9042–9046. doi: 10.1073/pnas.160016897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. The p21(Cip1) and p27(Kip1) CDK “inhibitors” are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E, Kuriyan J. Crystallographic analysis of the specific yet versatile recognition of distinct nuclear localization signals by karyopherin α. Struct Fold Des. 2000;8:329–338. doi: 10.1016/s0969-2126(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Donovan JC, Milic A, Slingerland JM. Constitutive MEK/MAPK activation leads to p27Kip1 deregulation, and antiestrogen resistance in human breast cancer cells. J Biol Chem. 2001;276:40888–40895. doi: 10.1074/jbc.M106448200. [DOI] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- Guan T, Kehlenbach RH, Schirmer EC, Kehlenbach A, Fan F, Clurman BE, Arnheim N, Gerace L. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol Cell Biol. 2000;20:5619–5630. doi: 10.1128/mcb.20.15.5619-5630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S, Nakayama KI. Degradation of p27Kip1 at the G0–G1 transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem. 2001;276:48937–48943. doi: 10.1074/jbc.M107274200. [DOI] [PubMed] [Google Scholar]
- Hengst L, Dulic V, Slingerland JM, Lees E, Reed SI. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NFκB/IκBα complexes. Proc Natl Acad Sci USA. 2000;97:1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Hara T, Kamura T, Yoshida M, Nakayama K, Nakayama KI. Phosphorylation of p27Kip 1 on serine 10 is required for its binding to CRM1 and nuclear export. J Biol Chem. 2002;277:14355–14358. doi: 10.1074/jbc.C100762200. [DOI] [PubMed] [Google Scholar]
- Ishida N, Kitagawa M, Hatakeyama S, Nakayama K. Phosphorylation at Serine 10, a major phosphorylation site of p27Kip1, increases its protein stability. J Biol Chem. 2000;275:25146–25154. doi: 10.1074/jbc.M001144200. [DOI] [PubMed] [Google Scholar]
- Jin P, Hardy S, Morgan DO. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J Cell Biol. 1998;141:875–885. doi: 10.1083/jcb.141.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlenbach RH, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J Cell Biol. 1998;141:863–874. doi: 10.1083/jcb.141.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Matsumori N, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Macara IG. Nuclear transport: randy couples. Curr Biol. 1999;9:R436–R439. doi: 10.1016/s0960-9822(99)80275-9. [DOI] [PubMed] [Google Scholar]
- Malek NP, Sundberg H, McGrew S, Nakayama K, Kyriakidis TR, Roberts JM. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature. 2001;413:323–327. doi: 10.1038/35095083. [DOI] [PubMed] [Google Scholar]
- Mancuso VA, Hope TJ, Zhu L, Derse D, Phillips T, Parslow TG. Posttranscriptional effector domains in the rev proteins of feline immunodeficiency virus and equine infectious anemia virus. J Virol. 1998;68:1998–2001. doi: 10.1128/jvi.68.3.1998-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj IW, Engimeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- Melchior F, Gerace L. Two-way trafficking with Ran. Trends Cell Biol. 1998;8:175–179. doi: 10.1016/s0962-8924(98)01252-5. [DOI] [PubMed] [Google Scholar]
- Melchior F. Nuclear protein import in a permeabilized cell assay. Methods Mol Biol. 1998;88:265–273. doi: 10.1385/0-89603-487-9:265. [DOI] [PubMed] [Google Scholar]
- Melchior F, Paschal B, Evans J, Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard SS, Yan JS, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, Blobel G. The GTP binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Muller D, Thieke K, Burgin A, Dickmanns A, Eilers M. Cyclin E mediated elimination of p27 requires its interaction with the nuclear pore-associated protein mNPAP60. EMBO J. 2000;19:2168–2180. doi: 10.1093/emboj/19.10.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Ohta T, Michel JJ, Schottelius AJ, Xiong Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol Cell. 1999;3:535–541. doi: 10.1016/s1097-2765(00)80482-7. [DOI] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Hunter T. The differential localization of human cyclins A and B is due to a cytoplasmic retention signal in cyclin B. EMBO J. 1994;13:3772–3781. doi: 10.1002/j.1460-2075.1994.tb06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Reynisdottir I, Massague J. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- Rodier G, Montagnoli A, DiMarticullio L, Coulombe P, Draetta G, Pagano M, Meloche S. p27 cytoplasmic localization is regulated by phosphorylation on Ser10, and is not a prerequisite for its proteolysis. EMBO J. 2001;20:6672–6682. doi: 10.1093/emboj/20.23.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacer S, Dasso M. The ran decathlon: multiple roles of Ran. J Cell Sci. 2000;113:1111–1118. doi: 10.1242/jcs.113.7.1111. [DOI] [PubMed] [Google Scholar]
- Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Slingerland JM, Hengst L, Pan CH, Alexander D, Stampfer MR, Reed SI. A novel inhibitor of cyclin-Cdk activity detected in Transforming Growth Factor β-arrested epithelial cells. Mol Cell Biol. 1994;14:3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Characterization and targeted disruption of murine Nup50, a p27(Kip1)interacting component of the nuclear pore complex. Mol Cell Biol. 2000;20:563156–563142. doi: 10.1128/mcb.20.15.5631-5642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI. A CDK-independent function of mammalian Cks1. targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- Swanson C, Ross J, Jackson PK. Nuclear accumulation of cyclin E/Cdk2 triggers a concentration-dependent switch for the destruction of p27Xic1. Proc Natl Acad Sci USA. 2000;97:7796–7801. doi: 10.1073/pnas.97.14.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda K, Kubota Y, Arata Y, Mori S, Maeda M, Tanaka T, Yoshida M, Yoneda-Kato N, Kato JY. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277:2302–2310. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–165. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bardes ES, Moore JD, Brennan J, Powers MA, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Hirano K, Hirano M, Nishimura J, Kanaide H. Minimal requirements for the nuclear localization of p27Kip1, a cyclin-dependent kinase inhibitor. Biochem Biophys Res Commun. 2000;274:37–42. doi: 10.1006/bbrc.2000.3098. [DOI] [PubMed] [Google Scholar]