Abstract
Schizosaccharomyces pombe cdc42+ regulates cell morphology and polarization of the actin cytoskeleton. Scd1p/Ral1p is the only described guanine nucleotide exchange factor (GEF) for Cdc42p in S. pombe. We have identified a new GEF, named Gef1p, specifically regulating Cdc42p. Gef1p binds to inactive Cdc42p but not to other Rho GTPases in two-hybrid assays. Overexpression of gef1+ increases specifically the GTP-bound Cdc42p, and Gef1p is capable of stimulating guanine nucleotide exchange of Cdc42p in vitro. Overexpression of gef1+ causes changes in cell morphology similar to those caused by overexpression of the constitutively active cdc42G12V allele. Gef1p localizes to the septum. gef1+ deletion is viable but causes a mild cell elongation and defects in bipolar growth and septum formation, suggesting a role for Gef1p in the control of cell polarity and cytokinesis. The double mutant gef1Δ scd1Δ is not viable, indicating that they share an essential function as Cdc42p activators. However, both deletion and overexpression of either gef1+ or scd1+ causes different morphological phenotypes, which suggest different functions. Genetic evidence revealed a link between Gef1p and the signaling pathway of Shk1/Orb2p and Orb6p. In contrast, no genetic interaction between Gef1p and Shk2p-Mkh1p pathway was observed.
INTRODUCTION
Schizosaccharomyces pombe rod-shaped cells grow by apical extension until mitosis and divide by medial fission. They undergo three main morphological transitions in a dynamic process tightly coupled to cell cycle progression. After cytokinesis, the newly divided cells initiate growth in a monopolar manner, elongating from the “old end” that existed before septation. This monopolar growth continues until an early G2 phase point known as new end take off or NETO. At this time, a transition to bipolar growth occurs by using the new end originated during cell division. Finally, when the cell reaches its maximal size, tip elongation ceases and mitosis occurs, followed by the formation of the septum and cell separation (Mitchison and Nurse, 1985).
The Cdc42p GTPase plays a critical role in the establishment of cell polarity in most eukaryotic organisms, regulating the rearrangements of the actin cytoskeleton in response to extracellular and intracellular signals (Johnson, 1999). Like all GTPases, Cdc42p cycles between an inactive (GDP-bound) and an active (GTP-bound) state. Guanine nucleotide exchange factors (GEFs) stimulate the exchange of GDP for GTP that activates Cdc42p. Saccharomyces cerevisiae Cdc42p is essential and Cdc24p, the sole Cdc42-GEF, is also essential. Activation of Cdc42p is required during all stages of the yeast life cycle that involve polarized growth (Pruyne and Bretscher, 2000).
S. pombe cdc42+ is essential. Cells lacking cdc42+ are round, small, and uninucleated (Miller and Johnson, 1994). So far, the only described GEF for Cdc42p is Scd1p, also called Ral1p (Fukui and Yamamoto, 1988; Chang et al., 1994). Scd1p is homolog to S. cerevisiae Cdc24p, and it is necessary for mating and to maintain an elongated cell shape. Scd1p is a Ras GTPase effector that forms part of a multiproteic complex: Ras1p-Scd1p-Scd2p-Cdc42p-Shk1p (Chang et al., 1999), similar to S. cerevisiae Bud1p-Cdc24p-Bem1p-Cdc42p-Ste20p complex. Scd1p also binds directly to Moe1p, a protein necessary for proper spindle formation in the nucleus (Chen et al., 1999). Interestingly, disruption of CDC24 is lethal, whereas scd1 deletion generates rounded cells with mating defects but is not lethal (Chang et al., 1994), suggesting that Scd1p is not the sole physiological GEF for Cdc42p in S. pombe. Moreover, recent data indicate that Cdc42p mainly localizes to the septum region (Merla and Johnson, 2000), whereas GFP-Scd1p can be detected mostly in the nucleus throughout the cell cycle (Chen et al., 1999).
Two S. pombe Cdc42p effectors have been described to date, Shk1p/Pak1p/Orb2p (Marcus et al., 1995; Ottilie et al., 1995) and Shk2p/Pak2p (Sells et al., 1998; Yang et al., 1998), both belonging to the family of p21-activated kinases (PAKs). The gene shk1+/pak1+/orb2+ is essential, whereas shk2+ is not (Sells et al., 1998). Shk1p is required for polarized growth and mating response (Marcus et al., 1995; Ottilie et al., 1995) and is necessary also to define “end” identity (Sawin et al., 1999). Thus, orb2-34 mutant cells at the restrictive temperature are round and never initiate cell growth at the new end. Shk2/Pak2p, activates the MAPK cascade: Mkh1p-Pek1p-Spm1p that regulates cell integrity and antagonizes chloride homeostasis in fission yeast (Merla and Johnson, 2001).
The molecular targets of Shk1p have not been described yet but genetic interactions suggest that Orb6p kinase might function downstream of this protein (Verde et al., 1998). Orb6p belongs to the Ndr kinase family, and it is closely related to S. cerevisiae Cbk1p, which is essential for morphogenesis and polarized growth (Bidlingmaier et al., 2001; Du and Novick, 2002). Orb6p is an essential protein required to maintain cell polarity during interphase and to promote actin reorganization in fission yeast (Verde et al., 1998). Interestingly, a role as mitotic inhibitor has been described for both Orb6p and Shk1p, suggesting a novel pathway in the coordination of cell growth and proliferation (Gilbreth et al., 1998; Verde et al., 1998).
In this work, we have identified gef1+ as a gene encoding a new S. pombe Cdc42-GEF. Gef1p specifically interacts with GDP-bound Cdc42p, and activates this GTPase in vivo and in vitro. Gef1p localizes to the septum and is involved in bipolar growth and septum formation. We also demonstrate that Gef1p and Scd1p share an essential function but play different roles in morphogenesis.
MATERIALS AND METHODS
Strains, Growth Conditions, and Genetic Methods
All the strains used in this work are described in Table 1. Yeast cells were usually grown in YES medium or minimal medium (EMM) supplemented with the necessary requirements. Incubations were carried out at 25, 28, 32, or 37°C. Growth was monitored by OD600 measurements. Standard S. pombe media and genetic manipulations were used (Moreno et al., 1991).
Table 1.
List of strains used in this study
| Strain | Genotype | Source | Reference |
|---|---|---|---|
| PPG102 | h− leu1-32 | S. Moreno | Moreno et al., 1991 |
| PPG103 | h− leu1-32 ura4-D18 | S. Moreno | Moreno et al., 1991 |
| PPG146 | h− cdc10-129 ura4-D18 | S. Moreno | Moreno et al., 1991 |
| PPG148 | h− cdc25-22 ura4-D18 | S. Moreno | Moreno et al., 1991 |
| PPG311 | h+ orb2-34 leu1-32 ade6 | P. Nurse | Verde et al., 1995 |
| PPG313 | h+ orb6-25 leu1-32 ade6 | P. Nurse | Verde et al., 1995 |
| PPG2611 | h+ ral1∷ura4 ura4-D18 leu1-32 ade6 | P. Nurse | Fukul and Yamamoto 1988 |
| PPG2433 | h+ mkh1∷ura4 ura4-D18 leu1-32 ade6 | D. Young | Sengar et al., 1997 |
| PPG104 | h+/h− leu1-32 ura4-D18 adeM216/leu1-32 ura4-D18 adeM210 | Our collection | |
| PPG2601 | h+ gef1∷ura4+ ura4-D18 leu1-32 | This work | |
| PPG2473 | h+ cdc10-129 gef1∷ura4 ura4-D18 leu1-32 | This work | |
| PPG2346 | h− EGFPgef1 leu1-32 ura4-D18 | This work | |
| PPG2516 | h+ cdc25-22 gef1∷kanMX6 ura4-D18 leu1-32 | This work | |
| PPG2549 | h+ cdc25-22 gef1∷kanMX6 EGFPgef1 leu1-32 ura4-D18 | This work | |
| PPG2364 | h+/h− gef1∷ura4+ scd1∷kanMX6 leu1-32 ura4-D18 adeM216/gef1∷ura4+ scd1+ leu1-32 ura4-D18 adeM210 | This work | |
| PPG2408 | h− shk2∷kanr leu1-32 ura4-D18 ade6 | This work | |
Plasmids and Recombinant DNA Methods
All DNA and RNA manipulations were carried out by established methods (Ausubel et al., 1995; Sambrock and Russell, 2001). S. pombe was transformed by the lithium acetate method (Ito et al., 1983). Determination of nucleotide sequences was done by automated sequencing. The nmt1 promoter containing-vectors pREP3X, pREP4X, pREP41X, pREP42X, pREP81X, and pREP82X (Forsburg, 1993) and pREP-KZ (Craven et al., 1998) were used for the overexpression experiments. The open reading frames of gef1+ or scd1+ were amplified by polymerase chain reaction (PCR) from a cDNA library and cloned in the BamHI site of the vectors by using the appropriate primers.
Gene Deletion and Tagging
To delete gef1+ from the S. pombe genome, the whole open reading frame was replaced with the ura4+ gene by the method described in Bähler et al. (1998). PCR primers were 90 nucleotides in length and high-performance liquid chromatography purified. The PCR fragment including gef1+ 5′- and 3′-flanking sequences and the ura4+ gene was used to transform the diploid strain PPG104. Stable ura+ transformants were selected and then screened by PCR and Southern blotting for the appropriate gene replacement. scd1+ and shk2+ were deleted using the same method (Bähler et al., 1998) and substituted by kanMX6 gene. cdc25-22 gef1Δ mutant (PPG2516) was constructed by deleting gef1+ in the cdc25-22 background. A PCR fragment, including gef1+ 5′ (1 kb) and 3′ (0.8-kb)–flanking sequences and the kanMX6 gene replacing the gef1+ open reading frame (ORF), was transformed into a cdc25-22 strain.
PPG2346 and PPG2549 strains, carrying a genomic version of gef1+ with the gene coding for enhanced green fluorescent protein (EGFP) fused to the 5′ end of the ORF, were generated by transforming PPG2601 and PPG2616 strains, lacking gef1+, with an the integrative plasmid pJK148 containing EGFP-gef1 and the gef1+ 5′ (1-kb)– and 3′ (0.8-kb)–flanking sequences. The plasmid was cut in the XbaI site of the gef1+ promoter.
Two-Hybrid Analysis
Protein interactions were analyzed using the two-hybrid system (Durfee et al., 1993). To avoid prenylation of the GTPases, rho1+, rho2+ rho3+, and rho4+ alleles had the C-terminal cysteine replaced for serine. cdc42+ alleles were amplified by PCR by using the appropriate primers without the sequence coding the four C-terminal amino acid residues. All the PCR products were sequenced and cloned into pAS2 as described previously (Arellano et al., 1999). They were used as bait against gef1+ cloned in the pACT2 plasmid. The S. cerevisiae Y190 (MATa gal4 gal80 his3 trp1-901 ura3-52 leu2-3, −112 URA3::GAL–::GAL (UAS)–r) strain was transformed with both plasmids and β-galactosidase activity was analyzed. Expression of all the proteins in S. cerevisiae was monitored by Western blot by using the anti-HA 12CA5 monoclonal antibody (mAb).
Immunoprecipitation
The entire coding sequences for Cdc42p was PCR amplified, sequenced, and fused in frame to the C terminus of the glutathione S-transferase (GST) sequence by using the NdeI-NotI sites in the pREP-KZ vector (Craven et al., 1998). Similarly, gef1 was amplified and fused in frame to the C terminus of three hemagglutinin (HA) epitope coding sequence by using the NdeI-BamHI sites in the pREP42-HA-N vector (Craven et al., 1998). These constructs were used to cotransform leu1-32 ura4D-18 S. pombe cells and expression of the proteins was induced by growing the cells in the absence of thiamine for 14 h.
Extracts from 2 × 108 cells expressing: GST-Cdc42p/HA-Gef1p, GST-Cdc42T17Np/HA-Gef1p, GST/HA-Gef1p, and GST-Cdc42/Gef1p were obtained as described previously (Arellano et al., 1997), by using 200 μl of lysis buffer (50 mM Tris pH 7.5, 2 mM EDTA, 137 mM NaCl, 0.5% NP-40, 10% glycerol, containing 100 μM p-aminophenyl methanesulfonyl fluoride, leupeptin, and aprotinin). The extracts were incubated with glutathione beads for 2 h at 4°C. The beads were washed four times with lysis buffer and then resuspended in sample buffer and subjected to 12% SDS-PAGE. The separated proteins were electrophoretically transferred to an Immobilon-P membrane (Millipore, Bedford, MA) and blotted to detect HA-Gef1p with 1:2000 diluted 12CA5 mAb as primary antibody and the enhanced chemiluminescence detection kit (Amersham Biosciences, Piscataway, NJ). Total HA-Gef1p levels were monitored in whole-cell extracts aliquots (90 μg of total protein) used directly for Western blot.
In Vivo Analysis of GEF Activity
The expression vectors pGEX-CRIB (PAK-Cdc42 binding domain) (Manser et al., 1998) and pGEX-C21RBD (Rhotekin binding domain) (Reid et al., 1996) were used to transform Escherichia coli. The fusion proteins were produced according to the manufacturer's instructions and immobilized on glutathione-Sepharose 4B beads (Amersham Biosciences). After incubation the beads were washed several times and the bound proteins were analyzed by SDS-PAGE and Coomassie staining.
The amount of GTP-bound Cdc42p or Rho1p proteins was analyzed using the Rho-GTP pull-down assay modified from Ren et al. (1999). Briefly, wild-type PPG103 strain transformed with either pREP42X or pREP42X-gef1 and gef1Δ PPG2601 strain were transformed with either pREP41X-HA-cdc42 or pREP3X-HA-rho1 and grown for 16 h in minimal medium without thiamine. Extracts from 108 cells were obtained as described previously (Arellano et al., 1997), by using 500 μl of lysis buffer (50 mM Tris, pH 7.5, 20 mM NaCl, 0.5% NP-40, 10% glycerol, 0.1 mM dithiothreitol, 1 mM NaF, 2 mM MgCl2, containing 100 μM p-aminophenyl methanesulfonyl fluoride, leupeptin, and aprotinin). GST-CRIB or GST-RBD fusion proteins (100 μg) coupled to glutathione-agarose beads was used to immunoprecipitate 3–5 mg of the cell lysates. The extracts were incubated with GST-CRIB or GST-RBD beads for 2 h, washed four times, and blotted against 1:2000 diluted 12CA5 mAb as primary antibody to detect HA-Cdc42p or HA-rho1, respectively, by using 1:5000 horseradish peroxidase-anti-mouse IgG mAb and the enhanced chemiluminescence detection kit (Amersham Biosciences). Total HA-Cdc42p or HA-Rho1p levels were monitored in whole-cell extracts (25 μg of total protein) that were used directly for Western blot and developed with 12CA5 mAb.
GDP/GTP Exchange Assay
gef1+, cdc42+ and rho1+ were PCR amplified and cloned into the NdeI-NotI sites of the pREP-KZ vector (Craven et al., 1998), fused in frame to the 3′ end of the GST. These constructs were used to transform S. pombe and expression of the proteins was induced by growing the cells in the absence of thiamine for 14 h. GST-tagged proteins were purified by affinity chromatography. Briefly, cell extracts from cultures were obtained as described previously (Arellano et al., 1999) and incubated with glutathione-Sepharose beads at 4°C for 1 h. The beads were washed four times with lysis buffer and resuspended in 100 μl of the same buffer. The quantities of GTPases, GST, and GST-Gef1p used were determined by Coomassie blue staining of SDS-PAGE by using bovine serum albumin as a standard.
The time courses for [3H]GDP/GTP exchange by GST-Cdc42p or GST-Rho1p were performed in the presence of GST or GST-Gef1p as described previously (Hart et al., 1991). Briefly, ∼0.5 μg of purified GST-Rho proteins on glutathione-Sepharose beads was first incubated for 25 min at 25°C with 10 μM [3H]GDP (5 μCi) in 20 mM Tris-HCl, pH 7.5, 0.5% NP-40, 20 mM NaCl, 0,1 mM dithiothreitol, 3 mM MgCl2; 1 mM ATP, and 50 μg/ml bovine serum albumin, to allow loading of the GTPase in a total volume of 50 μl. Then 10 μl of 100 mM GTP and 30 μl of either GST or GST-Gef1p (0.5 μg) were added. Duplicate 10-μl aliquots were removed at 3-min intervals and diluted with 1 ml of the reaction buffer (1 ml). After filtration through nitrocellulose filters, the amount of radioactivity bound to the protein was determined by scintillation counting.
Microscopy Techniques
For Calcofluor staining, exponentially growing S. pombe cells were harvested, washed once, and resuspended in water with calcofluor at final concentration of 0.1 mg/ml for 5 min at room temperature. After washing with water cells were observed in a DMRXA microscope (Leica, Wetzlar, Germany).
Immunofluorescence was performed as described previously (Hagan and Hyams, 1988). Cells were fixed in methanol for at least 15 min. Actin staining was performed with Alexa-Fluor 488-phalloidin (Molecular Probes, Eugene, OR) basically as described previously (Chang et al., 1996). Cells were observed using a DMRXA microscope.
Other Methods
Labeling and fractionation of cell wall polysaccharides was performed as described previously (Arellano et al., 1997)
RESULTS
Isolation of gef1+
Comparison of the consensus sequence for dbl-like (DH) domains, present in GEFs for the Rho family of GTPases with the genomic sequence of S. pombe revealed the presence of seven ORFs that encode putative Rho-GEFs. SPAC16E8.09 corresponds to scd1+. SPCC645.06C and SPCC645.07C, named rgf3+ and rgf1+, respectively, are similar to S. cerevisiae Rho1-GEFs ROM1 and ROM2 (Ozaki et al., 1996). Rgf3p and Rgf1p localize to the cell division site and Rgf1p also localizes to cell ends. Both genes show genetic interaction with septins, and are not essential (J.-Q. Wu and J.R. Pringle, personal communication; http://www.genedb.org/genedb/pombe/index.jsp). We have studied SPAC24H6.09 ORF, which we named gene gef1+. It encodes a protein of 753-amino acid residues with a predicted molecular mass of 84.5 kDa. Structural analysis of Gef1p showed that it contains the putative DH domain, common to all Rho-GEFs (amino acid residues 315–506) as well as an N-terminal region that is rich in serine/threonine and has no significant motifs. Interestingly, Gef1p does not contain a pleckstrin-homology domain adjacent to the DH domain, which is characteristic of most Rho-GEFs (reviewed in Zheng, 2001; Schmidt and Hall, 2002). Homology searching showed 19.7% identity between Gef1p and Scd1p and 16.7% between Gef1p and S. cerevisiae Cdc24p. It also showed significant similarity to the serine/threonine-rich region and the GEF domain of the unconventional myosin from Dictyostelium discoideum, MyoM, that is specific for Rac (Geissler et al., 2000) and to the GEF domain of Tiam1 (Habets et al., 1994), a mammalian Cdc42-GEF implicated in metastatic development of tumors (Stam et al., 1998).
Gef1p Interacts with GDP-bound Cdc42p and Promotes the GDP-GTP Exchange
We analyzed whether different S. pombe proteins belonging to the Rho family were able to interact with Gef1p. Point mutations that keep the GTPases in the GTP or the GDP-bound forms were introduced and the C-terminal cysteine was either deleted or changed to serine to prevent membrane association of the GTPases. All cdc42+, rho1+, rho2+, rho3+, and rho4+ mutant alleles were cloned in the pAS2 vector to use them in the two-hybrid system with Gef1p as bait. The possible interaction was assayed by β-galactosidase staining. As shown in Table 2, Gef1p specifically interacted with the GDP-bound form of Cdc42p (Cdc42D118A or Cdc42T17N) but not with GTP-bound Cdc42p (Cdc42G12V) nor with any of the other Rho proteins bound to GDP.
Table 2.
Two-hybrid analysis of the interactions between different Rho GTPases (pAS2) and GEF1p (pACT2) used as bait
| Gene (pAS2) | Gene (pACT2) | β-Galactosidase |
|---|---|---|
| None | gef1 | − |
| cdc42ΔCa | gef1 | +/− |
| cdc42ΔC | none | +/− |
| cdc42D118AΔC (GDP)b | gef1 | 219 |
| cdc42D118AΔC | none | − |
| cdc42T17NΔC (GDP) | gef1 | 219+ |
| cdc42T17NΔC | none | − |
| cdc42G12VΔC (GTP) | gef1 | − |
| rho1-T20NC199S (GDP) | gef1 | − |
| rho2-T22NC198S (GDP) | gef1 | +/− |
| rho2-T22NC198S | none | +/− |
| rho3-T29NC198S (GDP) | gef1 | − |
| rho4-T28NC198S (GDP) | gef1 | − |
+, blue color appeared in <30 min; +++, blue color appeared in 1–2 h; +/−, blue color appeared in >6 h.
In all the ΔC constructs the sequence coding for the four C-terminal amino acid residues was eliminated.
The nucleotide bound to the GTPase is indicated in parentheses.
To examine whether there was a direct interaction between Cdc42p and Gef1p, we performed coimmunoprecipitation experiments by using extracts from transformed cells expressing either GST-Cdc42p and HA-Gef1p or GST-Cdc42T17Np and HA-Gef1p (see MATERIALS AND METHODS). Cells expressing GST and HA-Gef1p or GST-Cdc42p and Gef1p were used as control. As shown in Figure 1A, the band corresponding to HA-Gef1p was detected in the GST-Cdc42p immunoprecipitates and was stronger in GST-Cdc42T17N immunoprecipitates. Some HA-Gef1p was also visualized in the lane corresponding to cells expressing GST. This nonspecific interaction was probably due to the overexpression of both proteins.
Figure 1.
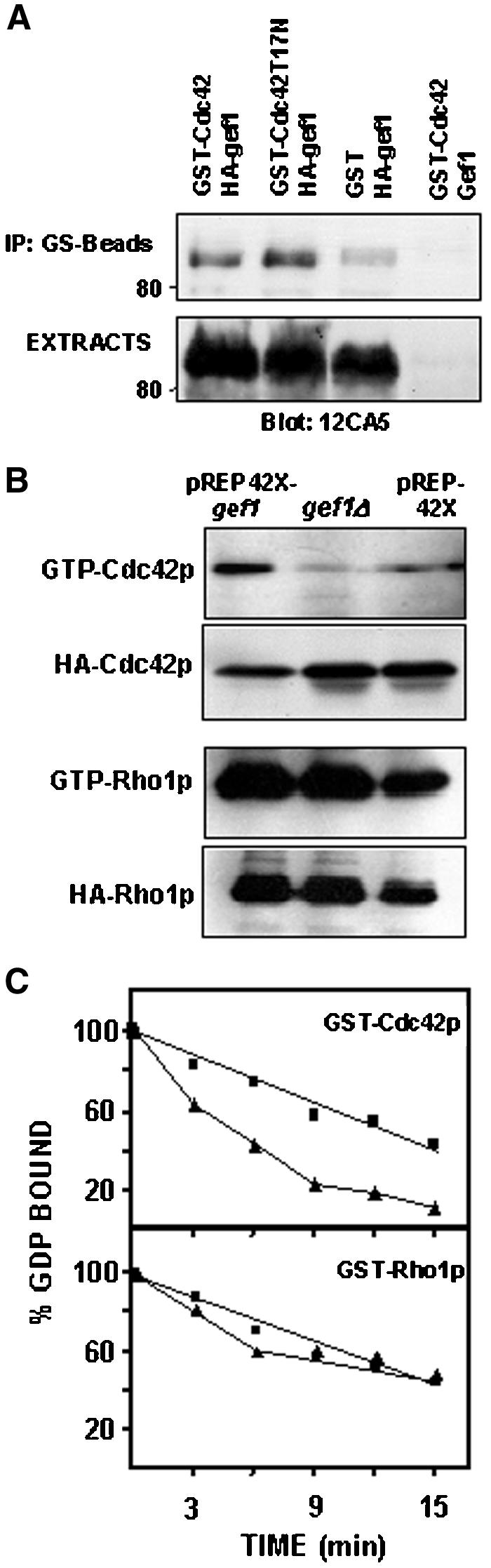
Gef1p is a specific Cdc42-GEF. (A) Coimmunoprecipitation of Gef1p and Cdc42p. Cell extracts from cells expressing: GST-Cdc42p and HA-Gef1; GST-Cdc42T17Np and HA-Gef1p; GST and HA-Gef1p, and GST-Cdc42p and Gef1p were immunoprecipitated with glutathione beads and blotted against 12CA5 monoclonal anti-HA antibody (top). Western blot with anti-HA antibody was performed on total cell extracts to visualize total HA-Gef1p levels (bottom). (B) Gef1p level modulates in vivo the amount of GTP-bound Cdc42p. Wild-type (PPG103) cells expressing pREP42X or pREP42X-gef1+ and gef1Δ (PPG2601) cells were transformed with either pREP41X-HA-cdc42 or pREP3X-HA-rho1. GTP-Cdc42p or GTP-Rho1p were pulled down from the cell extracts with GST-CRIB or GST-C21RBD, respectively, and blotted against 12CA5, anti-HA mAb. Total HACdc42p or HA-Rho1p was visualized by Western blot. (C) Stimulation of GDP exchange by Gef1p. Purified GST-Cdc42p or GST-Rho1p was preloaded with [3H]GDP, as described in MATERIALS AND METHODS, followed by addition of GST (▪) or GST-Gef1p (▴). Aliquots were removed at different times and the amount of radioactivity bound to the protein was determined. Data shown are representative of three independent experiments.
To further investigate the possible role of Gef1p as Cdc42p activator, we carried out a pull-down assay by using a GST fusion with the Cdc42 binding domain (CRIB) of mouse Pak2 that specifically interacts with GTP-bound Cdc42p. pREP41XHA-cdc42 was used to transform wild-type cells carrying either pREP42X or pREP42X-gef1+ and gef1Δ cells (PPG2061 strain). After induction of the nmt1 promoter for 16 h, the amount of Cdc42p bound to GTP was analyzed by precipitation with GST-CRIB that was previously obtained and purified from bacteria, and blotting with anti-HA antibody (Figure 1B). Western blot from whole-cell extracts (25 μg of protein) showed that the total amount of HA-Cdc42p was similar in wild-type and gef1Δ strains and slightly lower in cells transformed with pREP42X-gef1+ (Figure 1B). The amount of active Cdc42p increased considerably in the strain overexpressing Gef1p, even though the total amount of Cdc42p was lower, compared with the control strain. Moreover, only a minor amount of GTP-Cdc42p was detected in the strain lacking Gef1p. As a control, we also analyzed the amount of GTP-bound Rho1p in wild-type, gef1Δ, and cells overexpressing gef1+ (Figure 1B). These cells were transformed with the plasmid pREP3X-HA-rho1 and GTP-bound Rho1p was pulled down from the extracts by binding to GST-C21RBD (Rhotekin binding domain). No change in the level of Rho1p bound to GTP was observed in the gef1Δ strain or in cells overexpressing gef1+ (Figure 1B). These results demonstrate that Gef1p acts as a specific Cdc42p activator in S. pombe.
We also examined potential GEF activity of Gef1p toward Cdc42p in vitro. S. pombe cells were transformed with the plasmids pREP3X-GST, pREP3X-GST-Cdc42, pREP3X-GST-rho1, and pREP3X-GST-Gef1. The different GST-fusion proteins were purified from cells extracts by using glutathione-agarose beads. As shown in Figure 1C, purified GST-Gef1p was capable of stimulating the dissociation of [3H]GDP from purified GST-Cdc42p but not from GST-Rho1p. These results confirm the in vivo data and prove that Gef1p can function as an effective GEF for Cdc42p.
Overexpression of gef1+ Causes Morphological Alterations
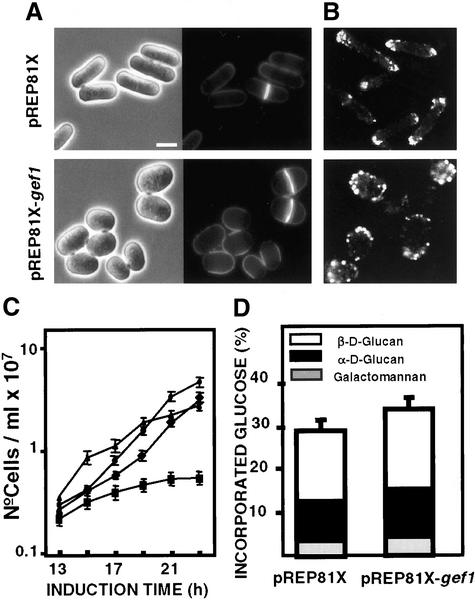
gef1+ was cloned into the pREP3X, 41X, and 81X plasmids under the control of the nmt1 promoter. Cells transformed with any of these plasmids and grown in the absence of thiamine became rounded after 12 h (Figure 2A). This phenotype was similar to that observed in cells overexpressing the cdc42G12V constitutively active allele (Miller and Johnson, 1994). Gef1p overproduction also altered the actin cytoskeleton. Actin patches seemed consistently brighter and depolarized around the cell, although more concentrated in one pole (Figure 2B). Overexpression of gef1+ under mild conditions did not cause growth defects, but overexpression from the strongest nmt1 promoter (pREP3X) completely stopped cell growth (Figure 2C).
Figure 2.
Phenotypes of Gef1p overexpression. (A) Micrographs of S. pombe wild-type cells (PPG102) transformed with pREP81X or pREP81X-gef1+ grown without thiamine during 14 h. Calcofluor staining of the same cells. (B) Fluorescence micrographs of these cells fixed and stained with rhodamine-phaloidin. The bar correspond to 4 μm. (C) Growth curves of wild-type cells transformed with pREP3X (●); pREP81X-gef1+ (▴); pREP41X-gef1+ (♦); pREP3X-gef1+ (▪), and grown without thiamine at 32°C. (D) Cell wall composition of cells transformed with pREP81X or pREP81X-gef1+. Cells were grown in the absence of thiamine for 12 h and then [14C]glucose was added 4 h before harvesting the cells. Values are the mean of three independent experiments with duplicate samples. SDs for the total carbohydrate values are shown.
Cells overexpressing gef1+ seemed brighter than wild-type cells when stained with Calcofluor (Figure 2A). Therefore, we analyzed the possible role of Gef1p as activator of S. pombe cell wall biosynthesis. The cell wall composition of cells transformed with pREP81X-gef1+ grown without thiamine for 16 h at 32°C was analyzed. As shown in Figure 2D, there was a mild but significant increase in the total amount of glucose incorporated into the cell wall compared with wild-type cells (28% in wild-type cells and 34% in pREP81X-gef1+), but no difference in the cell wall polymer composition was detected. These results indicate that there is a general increase in cell wall biosynthesis but not in a particular polysaccharide and suggest that Gef1p is not activating specifically Rho1p or Rho2p, the GTPases that regulate the biosynthesis of the (1-3)β-d-glucan and (1-3)α-d-glucan, respectively (Arellano et al., 1996; Calonge et al., 2000).
gef1+ Localizes to Septum
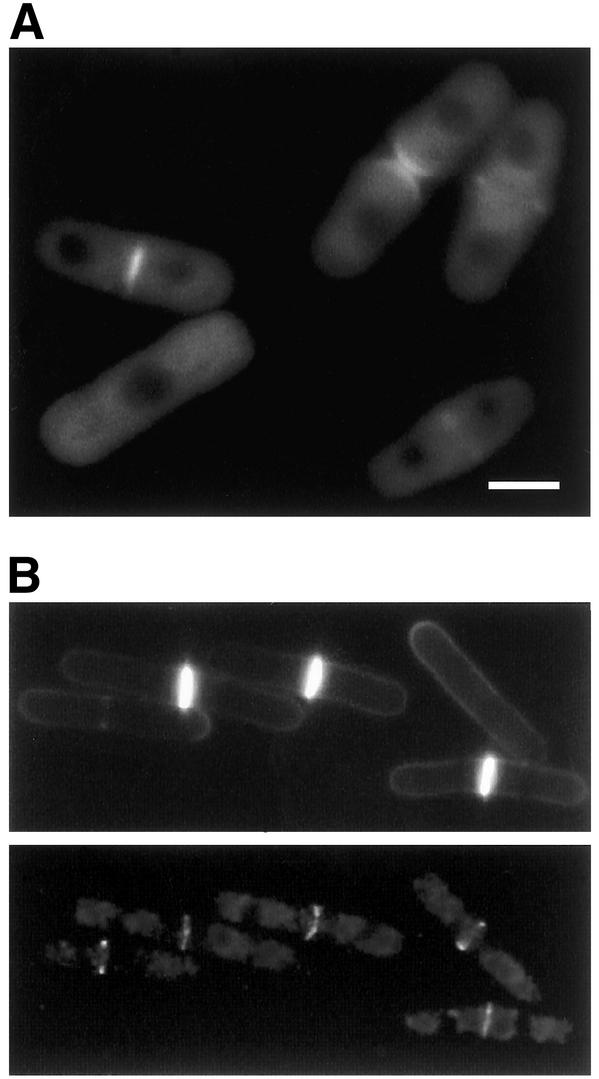
To gain further insight into the function of Gef1p, we determined its subcellular localization. The genomic locus of gef1+ was tagged with the gene encoding EGFP fused in frame to the 5′ end of gef1+ ORF. We demonstrated previously, by using the plasmid pREP81X-GFP-gef1, that the protein caused immediate rounding of the cells and therefore was functional. The staining pattern observed in cells carrying EGFP-gef1 was consistent with the localization of Gef1p mainly to the septum (Figure 3A). GFP-Gef1p remained in the septum until cell separation. Nonseptating cells exhibit diffuse staining throughout the cell except the nucleus (Figure 3A). To further analyze GFP-Gef1p localization, we constructed the double mutant cdc25-22 EGFP-gef1 and synchronized cells in G2 by cdc25-22 arrest at 37°C. Cells were grown at 25°C to log phase, and then changed to 37°C during 4 h, and returned to 25°C. Aliquots were taken at different times to observe GFP-Gef1p and septum by calcofluor staining. GFP-Gef1p appears in the medial region even before the septum was stained with calcofluor (Figure 3B). This localization suggests that Gef1p is involved in the whole process of septum formation.
Figure 3.
Gef1p localizes to the septum. (A) Fluorescent micrographs of PPG2346 cells carrying EGFP-gef1 in the chromosomal locus. Bar, 5 μm. (B) Calcofluor (top) and GFP-gef1 fluorescent micrographs (bottom) of cdc25-22 cells carrying EGFP-gef1 (PPG2549). Cells were grown at 25°C to OD600 0.15, shifted to 37°C during 4 h and then grown at 25°C for 90 min.
We also examined cells overexpressing GFP-Gef1p to see whether it was additionally localized in other weakly stained structures but we could not see any other cellular area where it locates (our unpublished data).
Northern analysis of gef1+ mRNA in synchronized cultures did not show any change in the transcription of gef1+ along the cell cycle (our unpublished data), indicating that there is not cycle regulation of this gene. Therefore, the protein might remain delocalized in nonseptating cells.
Gef1p Is Involved in Septum Formation
Scd1p/Ral1p, the only described guanine nucleotide exchange factor for Cdc42p (Chang et al., 1994), is necessary for mating and to maintain an elongated cell shape, and the lack of Scd1p worsen the defects of spindle formation in tubulin mutants (Li et al., 2000).
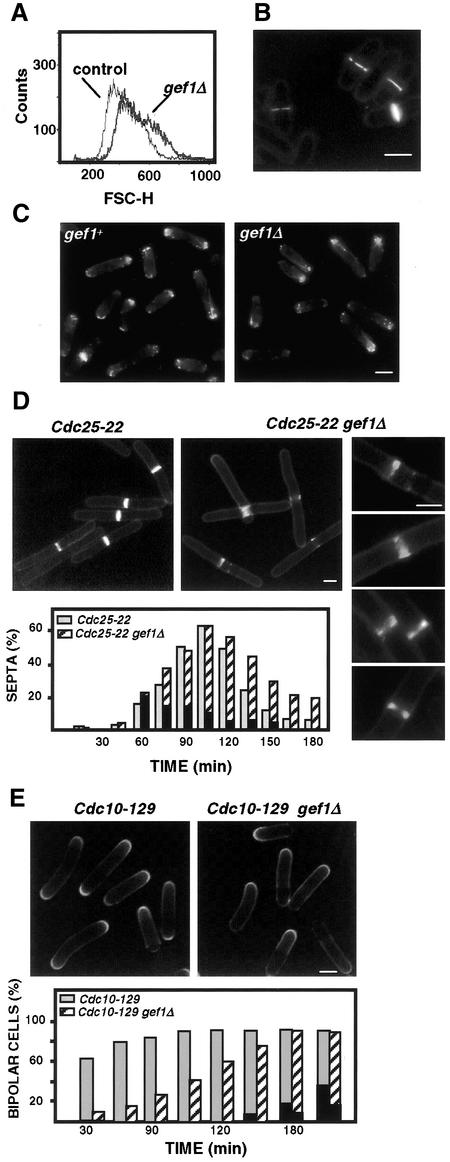
To further investigate the possible role of Gef1p in the regulation of cell morphology, the gene was disrupted by replacing the entire open reading frame with the ura4+ gene. A diploid strain containing one disrupted allele and one wild-type copy of gef1+, as confirmed by PCR and Southern analysis, originated tetrads with four viable spores. Therefore gef1+ is not essential for vegetative growth. gef1Δ cells did not show any growth defect at 25, 32, or 37°C. However, gef1Δ cells were slightly elongated with respect to wild-type cells. Fluorescence-activated cell sorting forward scatter analysis of cell size in an asynchronous population showed 17% increase in the mean channel (437 for wild-type cells and 526 for gef1Δ cells) (Figure 4A), suggesting a cell cycle delay. There was also an increase in the number of monopolar cells, growing at only one tip (64% in gef1Δ cells compared with 35% in wild type), and in the number of cells undergoing septation or cytokinesis (36% compared with 22% in wild type). Actin staining of gef1Δ cells showed most actin accumulated in a single pole (Figure 4C). Interestingly, around 20% of septating gef1Δ cells also showed irregular septa (Figure 4B), suggesting that Gef1p is not essential for septum formation, but it participates in this process. To confirm this, we constructed the double mutant cdc25-22 gef1Δ (see MATERIALS AND METHODS) and synchronized cells in G2 by cdc25-22 arrest at 37°C. Cells were grown at 25°C to log phase, and then changed to 37°C during 4 h, and returned to 25°C. Aliquots were taken at different times to count cells with septa. As shown in Figure 4D, upon shifting to permissive temperature, septation was initiated at the same time in cdc25-22 and cdc25-22 gef1Δ cells. However, most cells lacking Gef1p showed irregular septa at early times (60–75 min). In some cells seemed that septa were initiated at different points; other septa were not completed, or not very precisely defined (Figure 4D). Interestingly, septa became normal at later times (120 min). The first round of septation in cdc25-22 cells finished around 165 min, whereas in the cdc25-22 gef1Δ strain >20% of the cells were still septating after 180 min (Figure 4D). These results corroborated that septum formation proceeded more efficiently in the presence of Gef1p.
Figure 4.
Phenotypes caused by the lack of Gef1p. (A) FACS forward scatter analysis of control (PPG102) and gef1Δ (PPG2601) cells. (B) Calcofluor stained gef1Δ cells showing some defective septa. (C) Actin stained of control (PPG102) and gef1Δ (PPG2601) cells. (D) cdc25-22 (PPG148) and cdc25-22 gef1Δ (PPG2516) cells grown at 25°C to OD600 0.15, shifted to 37°C during 4 h and then grown at 25°C for 180 min. Aliquots of cells were harvested immediately before and every 15 min after the shift to 25°C. The graphic represents the percentage of septated cdc25-22 (gray bars) and cdc25-22 gef1Δ (hatched bars) cells at each time point, determined by calcofluor staining. Black bars represent percentage of defective septa. Micrographs showing cdc25-22 and cdc25-22 gef1Δ cells 75 min after the shift to 25°C. Details of cdc25-22 gef1Δ septa. (E) cdc10-129 (PPG146) and cdc10-129 gef1Δ (PPG2473) grown at 25°C to OD600 0.15, shifted to 37°C during 4 h, and then grown at 25°C for 180 min. Aliquots of cells were harvested immediately before and every 30 min after the shift to 25°C. The graphic represents the percentage of bipolar cdc10-129 (gray bars) and cdc10-129 gef1Δ (hatched bars) cells at each time point. Black bars represent percentage of septa. Micrographs show calcofluor stained cdc10-129 and cdc10-129 gef1Δ cells 60 min after the shift to 25°C. Bars, 5 μm.
To investigate the increase in monopolar growth of gef1Δ cells, we constructed the double mutant cdc10-129 gef1Δ and synchronized cells in G1 by arrest at 37°C. Sixty minutes after shifting to permissive temperature 91% of cdc10-129 cells displayed bipolar growth, whereas only 15% of cdc10-129 gef1Δ cells where bipolar (Figure 4E). At 150 min after shifting most cdc10-129 gef1Δ cells were bipolar but septation was delayed respect to cdc10-129 cells.
In summary, gef1Δ cells display several phenotypes that are consistent with a role in actin reorganization during activation of bipolar growth and septation, two main changes in polarized growth during S. pombe morphogenetic cycle.
Because cells lacking Scd1p/Ral1p function are sterile (Fukui and Yamamoto, 1988), we also analyzed the mating ability of gef1Δ cells. These mutant cells mated as wild-type cells, indicating that Gef1p is dispensable for mating.
gef1+ Shares an Essential Function with scd1+ but Plays a Different Morphogenetic Role
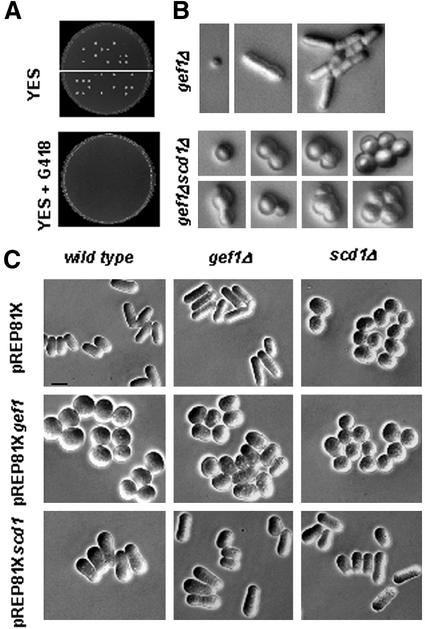
Cells lacking Cdc42p are not viable (Miller and Johnson, 1994). However, neither scd1Δ nor gef1Δ cells have obvious growth defects. We therefore investigated whether the role of Gef1p in the regulation of Cdc42p was overlapping the function of Scd1p. We disrupted scd1+ in a homozygous gef1Δ diploid strain by the method described in Bähler et al. (1998). The diploid carrying one of the scd1+ copies replaced by KanMX6 was induced to sporulate. Only a maximum of two spores in each tetrad were able to germinate and grow normally, and these spores were never resistant to kanamycin as they would have been if they were carrying scd1Δ (Figure 5A). The nonviable spores increased in size, with no polarized growth, and underwent one, two, or at most three cell divisions before arresting as rounded cells (Figure 5B). Therefore, the double mutant gef1Δscd1Δ is not viable, suggesting that Scd1p and Gef1p share an essential role as Cdc42p activators. However, these proteins also play different roles in the cell, because the phenotype of the single scd1 deletion is very different from that of gef1. Although scd1Δ cells are round and sterile, gef1Δ cells are slightly elongated, have a mild defect in bipolar growth and septum formation, and mate normally. Additionally, overexpression of gef1+ caused rounded and larger cells whereas overexpression of scd1+ generated a different morphological phenotype with cells mostly rounded in one of the poles. Overexpression of gef1+ in scd1Δ cells was not able to suppress the morphology defect caused by the lack of Scd1p; the cells were round but did not increase in size (Figure 5C). On the other hand, overexpression of scd1+ in a gef1Δ background caused a similar phenotype than that caused in wild-type cells (Figure 5C). These results corroborate that Scd1p and Gef1p share an essential function for S. pombe growth, but play different roles in the control of polarity and morphogenesis.
Figure 5.
gef1+ and scd1+ share an essential function but have different roles in growth polarity. (A) Tetrad analysis of the diploid strain PPG2364, homozygous gef1Δ and carrying one of the scd1+ copies replaced by KanMX6. Spores were grown on YES plates and replicated on YES+G418 plates. (B) Micrographs of gef1Δ and gef1Δ scd1Δ germinating spores. (C) Micrographs of S. pombe wild-type (PPG102), gef1Δ (PPG2601), or scd1Δ cells (PPG2611) transformed with either pREP81X, pREP81X-scd1, or pREP81X-gef1. Cells were grown in minimal medium without thiamine at 32°C for 20 h. Bar, 5 μm.
Genetic Evidence That Gef1p Functionally Interacts with Shk1/Pak1/Orb2p and Orb6p
Two kinases from the PAK family, Shk1p/Pak1p/Orb2p (Marcus et al., 1995; Ottilie et al., 1995) and Shk2p/Pak2p (Sells et al., 1998; Yang et al., 1998), are the only Cdc42p effectors described so far in S. pombe.
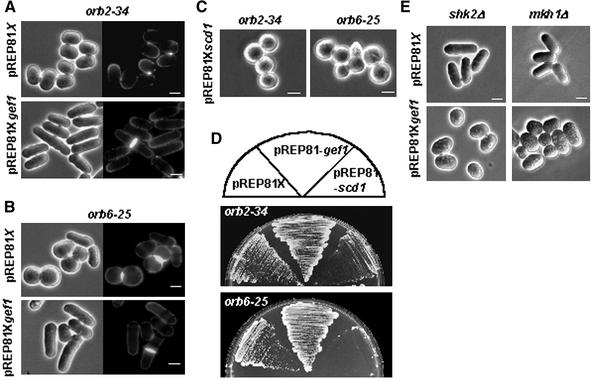
To define the possible functional relationship between Gef1p and the known Cdc42p effectors, we first examined the consequences of overexpressing gef1+ in orb2-34 cells, which have a nonlethal thermosensitive mutation in the essential shk1+ gene. When grown at 37°C, orb2-34 cells have a round morphology and grow slowly in a monopolar manner. Interestingly, overexpression of gef1+ in orb2-34 cells grown at 37°C, was able to suppress their rounded phenotype, cells became similar to wild type and were able to activate the second growth pole (Figure 6A).
Figure 6.
Morphology of S. pombe orb2-34 or orb6-25 strains overexpressing either gef1+ or scd1+. Micrographs of S. pombe orb2-34 (A) and orb6-25 cells (B) transformed with either pREP81X or pREP81X-gef1+. Cells were grown in minimal medium without thiamine at 25°C for 11 h and then incubated at 36°C for additional 5 h. (C) Cell morphology of S. pombe orb2-34 and orb6-25 cells transformed with pREP81X-scd1. Cells were grown in minimal medium without thiamine at 25°C for 11 h and then incubated at 36°C for additional 5 h. (D) orb2-34 and orb6-25 cells were transformed with either pREP81X; pREP81X-scd1 or pREP81X-gef1. Cells were grown in plates without thiamine at 37°C for 3 d. (E) Micrographs of S. pombe shk2Δ (PPG2408) or mkh1Δ (PPG2433) cells transformed with either pREP81X or pREP81X-gef1+. Cells were grown in minimal medium without thiamine for 16 h at 32°C. Bar, 5 μm.
Genetic interaction experiments suggested that Orb6p kinase might function downstream of Shk1p (Verde et al., 1998). To further corroborate Gef1p regulating Shk1p signaling pathway, it was overexpressed in orb6-25 mutant cells (Figure 6B). These cells also have a round morphology at 37°C and gef1+ overexpression suppressed their round phenotype. Moreover, overproduction of Gef1p not only corrected the morphologic phenotypes of either orb2-34 or orb6-25 but also improved growth of these strains at 37°C (Figure 6D). In contrast, scd1+ overexpression neither corrected the phenotypes nor allowed growth at 37°C. Conversely, it aggravated the defects of these mutants (Figure 6, C and D).
To determine whether Gef1p was also interfacing the Shk2p-Mkh1p-Pek1p-Spm1p signaling pathway, we analyzed the phenotypes of gef1+ overexpression in either shk2Δ or mkh1Δ cells. The excess of Gef1p caused the same morphological phenotype in cells lacking Shk2p or Mkh1p than in wild-type cells (Figure 6E), indicating that these molecules are not required for Gef1p morphological effect.
Taken together, these results indicate that Gef1p is involved in the Cdc42p-Shk1p-Orb6p signaling required for a correct polarized growth but not the signaling to Shk2 and Mkh1p-Pek1p-Spm1p cascade, and Scd1p is not able to replace Gef1p in this role.
DISCUSSION
Rho GTPases are implicated in many cellular processes such as cytoskeletal rearrangements and cell growth. Activation of these GTPases is under the direct control of GEFs, belonging to the Dbl family of proteins. Six Rho-GEFs have been identified in S. cerevisiae and seven in S. pombe. We have studied herein the Rho-GEF, termed Gef1, which similarly to other Rho-GEFs, contains a DH but lacks the pleckstrin-homology domain that is required for most Rho-GEFs to localize (Zheng, 2001). Gef1p interacted specifically with Cdc42p in its GDP-bound state but not with other Rho proteins, and efficiently catalyzed guanine nucleotide exchange of Cdc42p in vivo and in vitro. Consistent with these results was our finding that gef1+ overexpression causes a phenotype similar to that of the constitutively active allele Cdc42G12V (Miller and Johnson, 1994), giving rise to depolarized rounded cells.
Interestingly, gef1Δ cells grow similarly to wild-type cells, indicating that Gef1p is dispensable. However, cells have some delay or defect in two main transitions of the morphogenetic cycle: bipolar growth and cytokinesis, suggesting that the actin reorganization required for those transitions is affected. Besides, gef1Δ cells are slightly elongated, indicating a delay in cell cycle. In S. cerevisiae, a morphogenetic checkpoint delays cell cycle progression in G2 when the actin cytoskeleton is perturbed. This pause allows time for cells to complete bud formation before mitosis (McMillan et al., 1998). Wild-type S. pombe cells also arrest before mitosis after actin depolymerization. However, this arrest has been considered a manifestation of the cell size checkpoint in fission yeast (Rupes et al., 2001).
The fact that Gef1p is localized to the septum is consistent with the participation of this Cdc42-GEF in septum formation. Indeed, in synchronized cdc25-22 gef1Δ we observed that most gef1Δ cells show defects in early septation. Later on, those defects were corrected but the end of septation was delayed. Thus, it seems that septum formation proceeds more efficiently when Gef1p is present. The lack of Gef1p might affect the function of Cdc42p in the formation of the actomyosin ring and that might cause a delay of the septation (Le Goff et al., 1999).
The double deletion of gef1+ and scd1+ was not viable, suggesting that the activity of this novel Cdc42-GEF may be essential in the absence of Scd1p. It has been proposed that Cdc42p regulates at least two pathways, one mediating cellular morphology and the other mediating cell growth (Merla and Johnson, 2001). Both Scd1p and Gef1p might participate in Cdc42p activation required for cell growth. Possibly, Scd1 and Gef1p are the only Cdc42 activators and therefore are complementary and share that essential role. On the other hand, they do not seem to have overlapping roles in the establishment and maintenance of growth polarity. Similarly, it has been described recently that the two main Ras1p signaling pathways in S. pombe are regulated by two different GEFs, Ste6p and Efc25p, that are not interchangeable (Papadaki et al., 2002).
The main Cdc42p effector that controls cellular morphology and the establishment of cell polarity is Shk1/Orb2p (Ottilie et al., 1995). Apical growth of scd1Δ cells is impaired, and it has been established that Scd1p activates the signaling pathway Ras-Scd1-Cdc42-Shk1 for apical growth (Chang et al., 1999). Downstream in this signaling pathway must be Orb6p (Verde et al., 1998). Conversely, Gef1p does not seem to act on apical growth because gef1Δ cells are polarized. However, genetic experiments demonstrated that it may act on the same signaling pathway as Scd1p, because overexpression of Gef1p suppresses the orb2-34 and orb6-25 phenotypes. An immediate explanation would be that Gef1p plays a secondary role in the activation of Cdc42p and therefore gef1Δ cells do not have a drastic morphological defect, as scd1Δ cells. Cdc42p may be limiting and essential for growth and it may need to be activated mainly by Scd1p but if not, by Gef1p. It is also possible that Gef1p signals to the same effectors as Scd1p but is mainly required for the formation of the septum before cytokinesis. The specificity of Cdc42p signaling would be achieved by the different spatial localization of the two GEFs. Overexpression of Gef1p might increase the ectopic activation of Cdc42p, which causes bigger and rounded cells in a wild-type background. Similarly, the increase in active Cdc42p might correct the orb phenotypes that are due to defective Cdc42-effector kinases. However, overexpression of Scd1p is unable to correct orb2-34 and orb6-25 phenotypes; quite the opposite, it aggravates those phenotypes, indicating that orb suppression by Gef1p is not due to a nonspecific Cdc42p activation.
In summary, we have provided evidence that Gef1p is a new Cdc42-GEF. Our results suggest that Gef1p participates in the Cdc42p regulation of bipolar growth and septum formation and indicate that Gef1p is somehow modulating the Cdc42p-Shk1p-Orb6p signaling required for correct polarized growth but that it does not affect signaling to the mating cascade or signaling to Shk2p and the Mkh1p-Pek1p-Spm1p cascade. Together, these results represent some important progress in understanding the regulation of Cdc42p in S. pombe, performed by at least two GEFs, Scd1p and Gef1p, which cannot be substituted for one another in morphogenetic role. This seems to be a different mechanism than in S. cerevisiae, where Cdc42p is regulated by a unique GEF, Cdc24p, which controls both apical growth and septation (Gulli and Peter, 2001). Perhaps those mechanisms reflect the different growth patterns in budding and fission yeast. S. pombe uses two GEFs, Scd1p for apical growth and Gef1p for septation because these processes occur in different places, whereas in S. cerevisiae apical growth and septation occur in the same place.
ACKNOWLEDGMENTS
We thank J. Hayles and the members of the morphogenesis group in Paul Nurse's laboratory for useful discussion; B. Santos, J.C. Ribas, M. Arellano, and A. Duran for help with the manuscript; and D. Posner for editing. We also thank X. Bustelo and P. Crespo for the pGEX-CRIB and pGEX-RBD fusion plasmids, respectively. We thank B. Santos and T.M. Calonge for technical help. A.A. acknowledges support from a Initiation to Research fellowship granted by Consejo Superior de Investigaciones Científicas. This work was supported by grants BIO98-0814-C02-01 and BIO2001-1531 from the Comision Interministerial de Ciencia y Tecnología, Spain.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–07–0400. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–07–0400.
REFERENCES
- Arellano M, Durán A, Pérez P. Rho1 GTPase activates the (1-3)β-d-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- Arellano M, Duran A, Perez P. Localization of the Schizosaccharomyces pombe rho1 GTPase and its involvement in the organization of the actin cytoskeleton. J Cell Sci. 1997;110:2547–2555. doi: 10.1242/jcs.110.20.2547. [DOI] [PubMed] [Google Scholar]
- Arellano M, Valdivieso MH, Calonge TM, Coll PM, Durán A, Pérez P. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J Cell Sci. 1999;112:3569–3578. doi: 10.1242/jcs.112.20.3569. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1995. [Google Scholar]
- Bähler J, Wu J-Q, Longtine MS, Shah NG, McKenzie III A, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bidlingmaier S, Weiss EL, Seidel C, Drubin DG, Snyder M. The Cbk1p pathway is important for polarized cell growth, and cell separation in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:2449–2462. doi: 10.1128/MCB.21.7.2449-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonge TM, Nakano K, Arellano M, Arai R, Katayama S, Toda T, Mabuchi I, Perez P. Schizosaccharomyces pombe Rho2 GTPase regulates the cell wall α-glucan biosynthesis, through the protein kinase Pck2p. Mol Biol Cell. 2000;11:4393–4401. doi: 10.1091/mbc.11.12.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Barr M, Wang Y, Jung V, Xu HP, Wigler MH. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994;79:131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chang E, Bartholomeusz G, Pimental R, Che J, Lai H, Wang L, Yang P, Marcus S. Direct binding and in vivo regulation of the fission yeast p21-activated kinase shk1 by the SH3 domain protein scd2. Mol Cell Biol. 1999;19:8066–8074. doi: 10.1128/mcb.19.12.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Woollard A, Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile acting ring. J Cell Sci. 1996;109:131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Chen CR, Li YC, Chen J, Hou MC, Papadaki P, Chang EC. Moe1, a conserved protein in Schizosaccharomyces pombe, interacts with a Ras effector, Scd1, to affect proper spindle formation. Proc Natl Acad Sci USA. 1999;96:517–522. doi: 10.1073/pnas.96.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RA, Griffiths DJ, Sheldrick KS, Randall RE, Hagan IM, Carr AM. Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene. 1998;221:59–68. doi: 10.1016/s0378-1119(98)00434-x. [DOI] [PubMed] [Google Scholar]
- Du LL, Novick P. Pag1p, a Novel Protein Associated with Protein Kinase Cbk1p, Is Required for Cell Morphogenesis, and Proliferation in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:503–514. doi: 10.1091/mbc.01-07-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn AE, Lee W-H, Elledge SJ. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Forsburg SL. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, Yamamoto M. Isolation and characterization of Schizosaccharomyces pombe mutants phenotypically similar to ras1. Mol Gen Genet. 1988;215:26–31. doi: 10.1007/BF00331298. [DOI] [PubMed] [Google Scholar]
- Geissler H, Ullmann R, Soldati T. The tail domain of myosin M catalyzes nucleotide exchange on Rac1 GTPases, and can induce actin-driven surface protrusions. Traffic. 2000;1:399–410. doi: 10.1034/j.1600-0854.2000.010505.x. [DOI] [PubMed] [Google Scholar]
- Gilbreth M, Yang P, Bartholomeusz G, Pimental RA, Kansra S, Gadiraju R, Marcus S. Negative regulation of mitosis in fission yeast by the Shk1-interacting protein Skb1 and its human homolog, Skb1Hs. Proc Natl Acad Sci USA. 1998;95:14781–14786. doi: 10.1073/pnas.95.25.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli MP, Peter M. Temporal, and spatial regulation of Rho-type guanine-nucleotide exchange factors. the yeast perspective. Genes Dev. 2001;4:365–379. doi: 10.1101/gad.876901. [DOI] [PubMed] [Google Scholar]
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77:537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Eva A, Evans T, Aaronson SA, Cerione RA. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991;354:311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff X, Utzig S, Simanis V. Controlling septation in fission yeast: finding the middle, and timing it right. Curr Genet. 1999;35:571–584. doi: 10.1007/s002940050455. [DOI] [PubMed] [Google Scholar]
- Li YC, Chen CR, Chang EC. Fission yeast Ras1 effector Scd1 interacts with the spindle, and affects its proper formation. Genetics. 2000;156:995–1004. doi: 10.1093/genetics/156.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Marcus S, Polverino A, Chang E, Robbins D, Cobb MH, Wigler M. Shk1, a homolog of the Saccharomyces cerevisiae Ste20 and mammalian p65PAK protein kinases, is a component of a Ras/Cdc42 signaling module in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1995;92:6180–6184. doi: 10.1073/pnas.92.13.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan JN, Sia RA, Lew DJ. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J Cell Biol. 1998;142:1487–1499. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla A, Johnson DI. The Cdc42p GTPase is targeted to the site of cell división in the fisión yeast Schizosaccharomyces pombe. Int J Cell Biol. 2000;79:469–477. doi: 10.1078/0171-9335-00073. [DOI] [PubMed] [Google Scholar]
- Merla A, Johnson DI. The Schizosaccharomyces pombe Cdc42p GTPase signals through Pak2p, and the Mkh1p-Pek1p-Spm1p MAPK pathway. Curr Genet. 2001;39:205–209. doi: 10.1007/s002940100210. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Johnson DI. Cdc42p GTPase is involved in controlling polarized growth in Schizosaccharomyces pombe. Mol Cell Biol. 1994;14:1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizasaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Ottilie S, Miller PJ, Johnson DI, Creasy CL, Sells MA, Bagrodia S, Forsburg S, Chernoff J. Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO J. 1995;14:5908–5919. doi: 10.1002/j.1460-2075.1995.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Tanaka K, Imamura H, Hihara T, Kameyama T, Nonaka H, Hirano H, Matsuura Y, Takai Y. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 1996;15:2196–2207. [PMC free article] [PubMed] [Google Scholar]
- Papadaki P, Pizon V, Onken B, Chang EC. Two ras pathways in fission yeast are differentially regulated by two ras Guanine nucleotide exchange factors. Mol Cell Biol. 2002;22:4598–4606. doi: 10.1128/MCB.22.13.4598-4606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J Cell Sci. 2000;113:365–375. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupes I, Webb BA, Mak A, Young PG. G2/M arrest caused by actin disruption is a manifestation of the cell size checkpoint in fission yeast. Mol Biol Cell. 2001;12:3892–3903. doi: 10.1091/mbc.12.12.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrock J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- Sawin KE, Hajibagheri MA, Nurse P. Mis-specification of cortical identity in a fission yeast PAK mutant. Curr Biol. 1999;9:1335–1338. doi: 10.1016/s0960-9822(00)80058-5. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases. turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Sells MA, Barratt JT, Caviston J, Ottilie S, Leberer E, Chernoff J. Characterization of Pak2p, a pleckstrin homology domain-containing, p21-activated protein kinase from fission yeast. J Biol Chem. 1998;273:18490–18498. doi: 10.1074/jbc.273.29.18490. [DOI] [PubMed] [Google Scholar]
- Stam JC, Michiels F, van der Kammen RA, Moolenaar WH, Collard JG. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc Natl Acad Sci USA. 1998;95:7526–7531. doi: 10.1073/pnas.95.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Kansra S, Pimental RA, Gilbreth M, Marcus S. Cloning and characterization of shk2, a gene encoding a novel p21-activated protein kinase from fission yeast. J Biol Chem. 1998;273:18481–18489. doi: 10.1074/jbc.273.29.18481. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]