Abstract
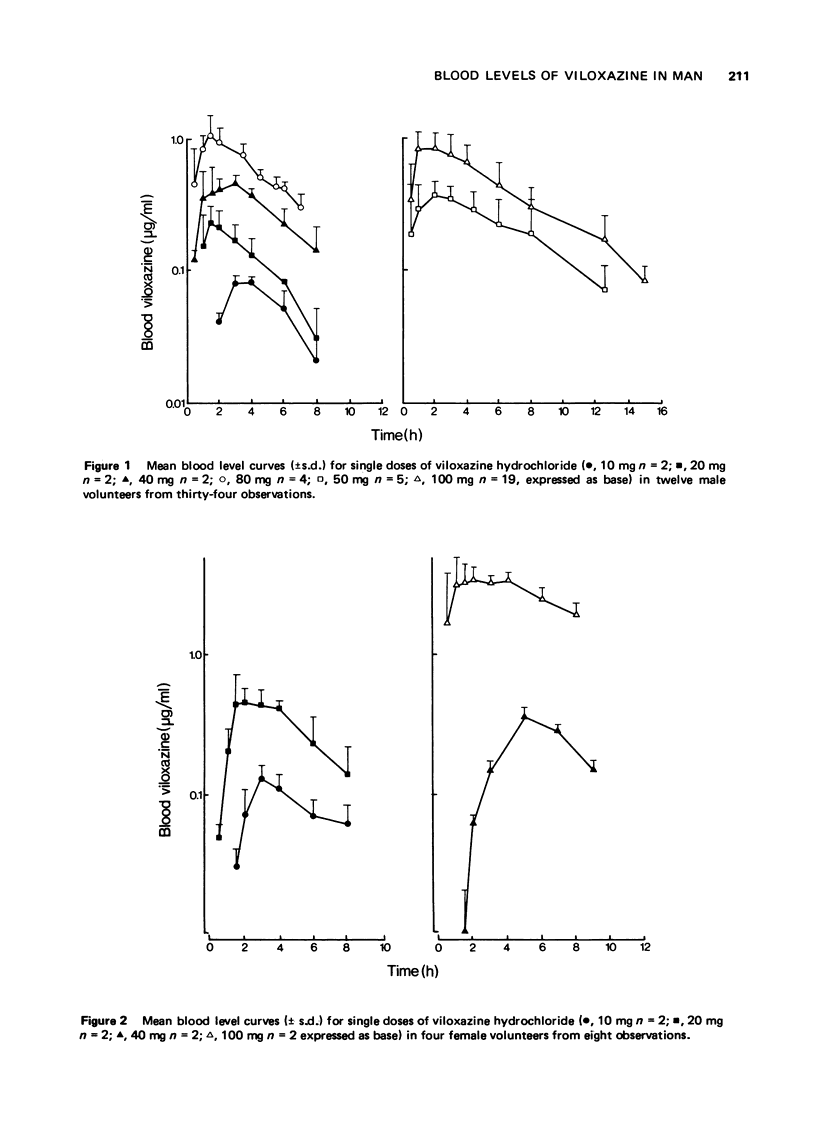
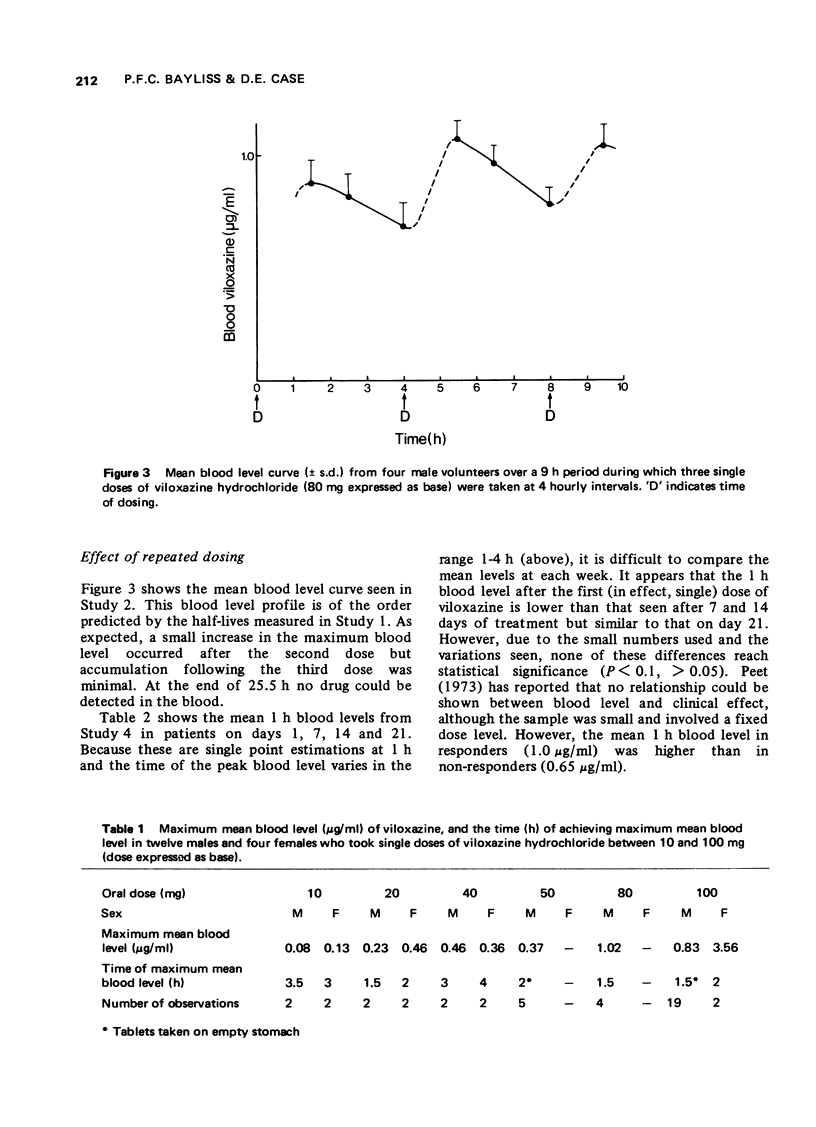
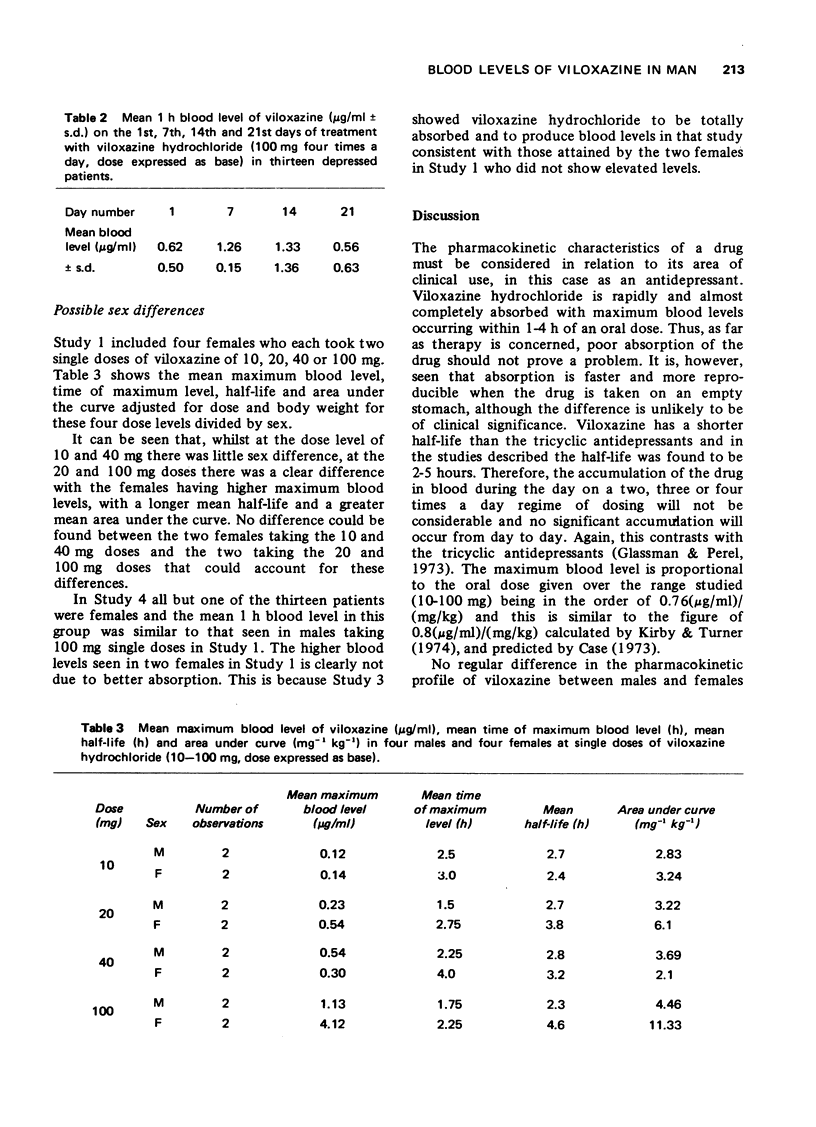
1 The pharmacokinetic characteristics of a new antidepressant, viloxazine hydrochloride, (ICI 58,834, Vivalan), have been investigated in four separate studies. 2 In Study 1, blood levels were measured over a period of 24 h after single doses of viloxazine hydrochloride from 10-100 mg (expressed as base). In Study 2, blood levels were measured over 24 h, during which three single doses of viloxazine hydrochloride (80 mg, expressed as base) were given 4 h apart. In Study 3, blood samples and urine and faeces were collected for 96 h after doses of 40 and 100 mg of [14C] viloxazine hydrochloride (40 muCi). In Study 4, 1 h blood levels were measured at weekly intervals during a comparative clinical trial in which viloxazine was given at a dose of 100 mg four times a day. 3 The half-life of the drug is in the range 2-5 h with maximum blood levels occurring in 1-4 h of the oral dose. Maximum blood levels are proportional to the oral dose given over the range studied (0.76(mug/ml)/(mg/kg)). The drug is very well absorbed orally, only 2% being found in faeces. Repeated dosing at 4 hourly intervals leads to slightly higher blood levels after the second, but not subsequent, doses. No accumulation was seen from week to week in depressed patients. No regular sex difference was seen in the pharmacokinetic characteristics of viloxazine hydrochloride but two females in one study did show a markedly higher maximum blood level and apparently longer half-life than the males. 4 It is concluded that viloxazine is rapidly and almost totally absorbed after an oral dose, and has a shorter half-life than the tricyclic antidepressants; therapy with it should be easily controllable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Case D. E. Gas-liquid chromatographic estimation in blood of ICI 58 834. J Pharm Pharmacol. 1973 Oct;25(10):800–802. doi: 10.1111/j.2042-7158.1973.tb09944.x. [DOI] [PubMed] [Google Scholar]
- Glassman A. H., Perel J. M. The clinical pharmacology of imipramine. Implications for therapeutics. Arch Gen Psychiatry. 1973 May;28(5):649–653. doi: 10.1001/archpsyc.1973.01750350029006. [DOI] [PubMed] [Google Scholar]
- Mallion K. B., Todd A. H., Turner R. W., Bainbridge J. G., Greenwood D. T., Madinaveitia J., Somerville A. R., Whittle B. A. 2-(2-ethoxyphenoxymethyl)tetrahydro-1,4-oxazine hydrochloride, a potential psychotropic agent. Nature. 1972 Jul 21;238(5360):157–158. doi: 10.1038/238157a0. [DOI] [PubMed] [Google Scholar]
- Tsegos I. K., Ekdawi M. Y. A double-blind controlled study of viloxazine and imipramine in depression. Curr Med Res Opin. 1974;2(8):455–460. doi: 10.1185/03007997409115242. [DOI] [PubMed] [Google Scholar]


