Invasive aspergillosis is a disease that affects immunodepressed individuals and has become one of the leading causes of death in many transplant centers (1). Owing to the difficulties encountered in diagnosis of the disease and to the high sensitivity of the PCR technique, PCR-based methods have been developed since 1993 to detect the presence of Aspergillus DNA in patients.
In our laboratory, we began to use the PCR technique to detect the DNA of Aspergillus fumigatus in the blood of experimentally infected rats. In our first studies, the negative results obtained for almost all the plasma samples from the infected rats led us to suspect that the anticoagulant used could be interfering in the PCR. Therefore, we decided to ascertain the effect of the most widely used anticoagulants on this method for diagnosing invasive aspergillosis.
DNA was obtained using the strain A. fumigatus 48238, isolated from a human case of invasive aspergillosis and obtained from a culture collection from Glaxo Smith Kline (Greenford, United Kingdom). The DNA of the samples was extracted according to the method used by Tokimatsu et al. (16).
In order to prepare the samples used in the reaction, blood was obtained from two male Spragüe-Dawley rats by cardiac puncture. This blood was quickly separated into four aliquots, of which the first three contained sodium citrate (Sarstedt, Granollers, Spain), tripotassium-EDTA (Sarstedt), and heparin (Rovi, Barcelona, Spain), respectively, in order to obtain plasma. The last aliquot remained free of anticoagulant so that serum could be obtained. The tubes were centrifuged (3,000 × g for 10 min), aliquots (100 μl) of each type of plasma or serum were taken, and 1 μl of a solution of genomic DNA (15 mg/ml) was added to each one, in such a way that each plasma and serum sample contained a 0.15-mg/ml concentration of DNA.
The oligonucleotides used were obtained from genes of sequence 18S of the rRNA belonging to Aspergillus (20). PCR was performed using a nested PCR technique in which the internal primers were Asp.5 (5′ GATAACGAACGAGACCTCGG 3′) and Asp.8 (5′ TGCCAACTCCCCTGAGCCAG 3′), which amplify a sequence of 384 bp, and the external primers were Asp.1 (5′ CGGCCCTTAAATAGCCCGGTC 3′) and Asp.7 (5′ CCTGAGCCAGTCCGAAGGCC 3′), which amplify a sequence of 357 bp. The reaction mixture and the conditions of both cycles were the same as those described previously (20).
In all cases, the initial concentration of DNA in the PCR mixture was 30 μg/ml (1.5 μg in 50 μl of the reaction mixture). The first PCR was performed starting with 10-μl samples of treated plasma, to reach a final volume of 50 μl. The second reaction was performed with 1 μl of the product obtained in the first reaction. The products of the nested PCR were subjected to electrophoresis in a 2% agarose gel that contained ethidium bromide. The concentrations of the different anticoagulants in blood, plasma, and the PCR mixtures are detailed in Table 1.
TABLE 1.
Concentrations of the different anticoagulants in blood and plasma samples, as well as the PCR mixture
| Sample type or mixture | Anticoagulant concna in:
|
||
|---|---|---|---|
| Sodium citrate | Tripotassium EDTA | Heparin | |
| Bloodb | 0.106 | 2 | 5 (500) |
| Plasma | 0.2 | 4 | 10 (1,000) |
| PCR mixture | 0.02 | 0.4 | 1 (100) |
The sodium citrate concentrations are molar concentrations, the tripotassium EDTA concentrations are in milligrams per milliliter, and the heparin concentrations are in milligrams per milliliter (values in parentheses are in international units per milliliter).
In the blood centrifuge, hematocrit values close to 47% were obtained.
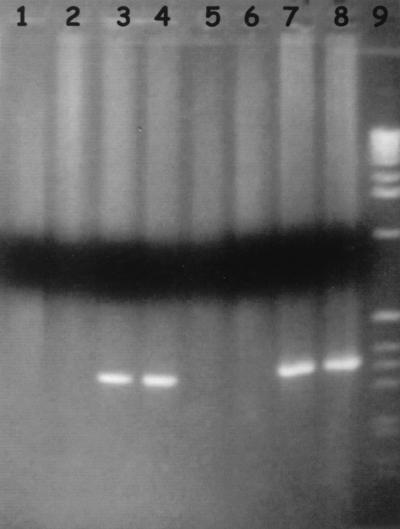
The results obtained are shown in Figure 1. The validity of PCR can be appreciated in serum and plasma with EDTA, whereas interference leading to negative results was observed in plasma with citrate and plasma with heparin.
FIG. 1.
Electrophoresis with a 2% agarose gel. Lanes: 1, plasma from rat A with citrate; 2, plasma from rat B with citrate; 3, plasma from rat A with EDTA; 4, plasma from rat B with EDTA; 5, plasma from rat A with heparin; 6, plasma from rat B with heparin; 7, serum from rat A; 8, serum from rat B; 9, size marker.
Several studies report on the inhibitory effect of different anticoagulants in different PCRs for viruses such as hepatitis C virus (5, 9, 12, 18), human immunodeficiency virus (3, 6, 7, 8, 13, 19), and hepatitis B virus (14); for protozoa such as Plasmodium falciparum (11); and for bacteria such as Streptococcus pneumoniae (4). However, only limited data are available for the detection of fungal DNA in plasma samples. Loeffler et al. (10) concluded that although plasma and whole blood spiked with Aspergillus conidia showed an identical lower detection limit, the sensitivity of PCR performed with plasma samples was lower than that of PCR performed with whole-blood samples.
On the other hand, there are studies that point out the absence of any inhibitory effect with some anticoagulants, such as the equivalence of results obtained in the amplification of human cytomegalovirus DNA from plasma samples with heparin and EDTA, respectively (15), or that of results obtained with plasma samples and serum (2). Similarly, some authors did not find differences between the results obtained in a PCR using heparinized plasma or serum to detect human immunodeficiency virus RNA (17). In the case of DNA amplification of S. pneumoniae from blood, Friedland et al. (4) observed inhibition of PCR in the presence of EDTA and citrate but not in the presence of sodium heparin.
In order to determine the reason for the inhibition caused by heparin in PCR assays, Yokota et al. (21) reached the conclusion that PCR is clearly affected when heparinized plasma is used as the DNA source, and that the degree of interference depends on three factors, as follows: (i) the type of Taq DNA polymerase used in the reaction, (ii) the number of leukocytes present in the blood (that is, the quantity of DNA at the beginning of the reaction), and (iii) the concentration of heparin in the samples.
In our PCR, we used Taq polymerase (Perkin Elmer, Bedford, Mass.), for which Yokota et al. (21) pointed out a maximum tolerable quantity of heparin of 0.1 U, starting from 100 ng of DNA in the reaction mixture. We had a concentration of 1,500 ng of DNA and 0.5 IU of heparin in the reaction mix. Thus, we can deduce that the quantity of heparin was sufficient to interfere with the reaction.
In conclusion, given the results obtained, we recommend not using plasma to carry out PCR in the diagnosis of invasive aspergillosis in order to ensure the absence of any inhibitory effect.
This work was partly supported by Health Ministry of Spain grant 00/0016-02 from the Fondo de Investigacion Sanitaria.
REFERENCES
- 1.Blanco, J. L., J. Guedeja-Marron, J. Caballero, and M. E. Garcia. 1998. Aspergilosis: mecanismos de patogenicidad implicados y aproximación al diagnóstico de laboratorio. Rev. Iberoam. Micol. 15:10-15. [PubMed] [Google Scholar]
- 2.Boom, R., C. Sol, J. Weel, Y. Gerrits, M. de Boer, and D. P. Wertheim. 1999. A highly sensitive assay for detection and quantitation of human cytomegalovirus DNA in serum and plasma by PCR and electrochemiluminescence. J. Clin. Microbiol. 37:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickover, R. E., S. A. Herman, K. Saddiq, D. Wafer, M. Dillon, and Y. J. Bryson. 1998. Optimization of specimen-handling procedures for accurate quantitation of levels of human immunodeficiency virus RNA in plasma by reverse transcriptase PCR. J. Clin. Microbiol. 36:1070-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedland, L. R., A. G. Menon, S. F. Reising, R. M. Ruddy, and D. J. Hassett. 1994. Development of a polymerase chain reaction assay to detect the presence of Streptococcus pneumoniae DNA. Diag. Microbiol. Infect. Dis. 20:187-193. [DOI] [PubMed] [Google Scholar]
- 5.Furuwatari, C., E. Hidaka, M. Akahane, M. Ishikawa, I. Ueno, K. Furihata, Y. Ogiso, and T. Katsuyama. 1998. Clinical evaluation of RT-PCR method for detection of HCV-RNA: second report availability of RT-PCR system using PCR internal control for detection of HCV-RNA and the influence of interfering substances on the detection. Japan. J. Clin. Pathol. 46:151-157. [PubMed] [Google Scholar]
- 6.Imai, H., O. Yamada, S. Morita, S. Suehiro, and T. Kurimura. 1992. Detection of HIV-1 RNA in heparinized plasma of HIV-1 seropositive individuals. J. Virol. Methods 36:181-184. [DOI] [PubMed] [Google Scholar]
- 7.Izopet, J., C. Poggi, E. Dussaix, J. M. Mansuy, L. Cubaynes, N. Profizi, A. Lafeuillade, B. Marchou, P. Massip, C. Sayada, and J. Puel. 1996. Assessment of a standardized reverse-transcriptase PCR assay for quantifying HIV-1 RNA in plasma and serum. J. Virol. Methods 60:119-129. [DOI] [PubMed] [Google Scholar]
- 8.Kirstein, L. M., J. W. Mellors, C. R. J. Rinaldo, J. B. Margolick, J. V. Giorgi, J. P. Phair, E. Dietz, P. Gupta, C. H. Sherlock, R. Hogg, J. S. Montaner, and A. Munoz. 1999. Effects of anticoagulant, processing delay, and assay method (branched DNA versus reverse transcriptase PCR) on measurement of human immunodeficiency virus type 1 RNA levels in plasma. J. Clin. Microbiol. 37:2428-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, H. J., T. Tanwandee, and F. B. Hollinger. 1997. Improved methods for quantification of human immunodeficiency virus type 1 RNA and hepatitis C virus RNA in blood using spin column technology and chemiluminescent assays of PCR products. J. Med. Virol. 51:56-63. [PubMed] [Google Scholar]
- 10.Loeffler, J., H. Hebart, U. Brauchle, U. Schumacher, and H. Einsele. 2000. Comparison between plasma and whole blood specimens for detection of Aspergillus DNA by PCR. J. Clin. Microbiol. 38:3830-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long, G. W., L. Fries, G. H. Watt, and S. L. Hoffman. 1995. Polymerase chain reaction amplification from Plasmodium falciparum on dried blood spots. Am. J. Trop. Med. Hyg. 52:344-346. [DOI] [PubMed] [Google Scholar]
- 12.Miyachi, H., A. Masukawa, T. Ohshima, H. Fusegawa, T. Hirose, C. Impraim, and Y. Ando. 1998. Monitoring of inhibitors of enzymatic amplification in polymerase chain reaction and evaluation of efficacy of RNA extraction for the detection of hepatitis C virus using the internal control. Clin. Chem. Lab. Med. 36:571-575. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura, S., S. Katamine, T. Yamamoto, S. K. Foung, T. Kurata, Y. Hirabayashi, K. Shimada, S. Hino, and T. Miyamoto. 1993. Amplification and detection of a single molecule of human immunodeficiency virus RNA. Virus Genes 7:325-338. [DOI] [PubMed] [Google Scholar]
- 14.Pardoe, I. U., and T. I. Michalak. 1995. Detection of hepatitis B and woodchuck hepatitis viral DNA in plasma and mononuclear cells from heparinized blood by the polymerase chain reaction. J. Virol. Methods 51:277-288. [DOI] [PubMed] [Google Scholar]
- 15.Storch, G. A., M. Gaudreault-Keener, and P. C. Welby. 1994. Comparison of heparin and EDTA transport tubes for detection of cytomegalovirus in leukocytes by shell vial assay, pp65 antigenemia assay, and PCR. J. Clin. Microbiol. 32:2581-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokimatsu, I., T. Tashiro, and M. Nasu. 1995. Early diagnosis and monitoring of human cytomegalovirus pneumonia in patients with adult T-cell leukemia by DNA amplification in serum. Chest 107:1024-1027. [DOI] [PubMed] [Google Scholar]
- 17.Vandamme, A. M., S. Van Dooren, W. Kok, P. Goubau, K. Fransen, T. Kievits, J. C. Schmit, E. de Clercq, and J. Desmyter. 1995. Detection of HIV-1 RNA in plasma and serum samples using the NASBA amplification system compared to RNA-PCR. J. Virol. Methods 52:121-132. [DOI] [PubMed] [Google Scholar]
- 18.Willems, M., H. Moshage, F. Nevens, J. Fevery, and S. H. Yap. 1993. Plasma collected from heparinized blood is not suitable for HCV-RNA detection by conventional RT-PCR assay. J. Virol. Methods 42:127-130. [DOI] [PubMed] [Google Scholar]
- 19.Witt, D. J., and M. Kemper. 1999. Techniques for the evaluation of nucleic acid amplification technology performance with specimens containing interfering substances: efficacy of boom methodology for extraction of HIV-1 RNA. J. Virol. Methods 79:97-111. [DOI] [PubMed] [Google Scholar]
- 20.Yamakami, Y., A. Hashimoto., I. Tokimatsu, and M. Nasu. 1996. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J. Clin. Microbiol. 34:2464-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota, M., N. Tatsumi, O. Nathalang, T. Yamada, and I. Tsuda. 1999. Effects of heparin on polymerase chain reaction for blood white cells. J. Clin. Lab. Anal. 13:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]