Abstract
Many viruses take advantage of receptor-mediated endocytosis in order to enter target cells. We have utilized influenza virus and Semliki Forest virus (SFV) to define a role for protein kinase C βII (PKCβII) in endocytic trafficking. We show that specific PKC inhibitors prevent influenza virus infection, suggesting a role for classical isoforms of PKC. We also examined virus entry in cells overexpressing dominant-negative forms of PKCα and -β. Cells expressing a phosphorylation-deficient form of PKCβII (T500V), but not an equivalent mutant form of PKCα, inhibited successful influenza virus entry—with the virus accumulating in late endosomes. SFV, however, believed to enter cells from the early endosome, was unaffected by PKCβII T500V expression. We also examined the trafficking of two cellular ligands, transferrin and epidermal growth factor (EGF). PKCβII T500V expression specifically blocked EGF receptor trafficking and degradation, without affecting transferrin receptor recycling. As with influenza virus, in PKCβII kinase-dead cells, EGF receptor was trapped in a late endosome compartment. Our findings suggest that PKCβII is an important regulator of a late endosomal sorting event needed for influenza virus entry and infection.
Endocytosis is a fundamental property of all eukaryotic cells that is used for uptake of nutrients and growth factors, as well as being exploited by invading microorganisms such as viruses (29, 32, 44). The process of cell surface endocytosis involves the internalization of ligands, often in a clathrin-dependent, receptor-mediated manner (42). Clathrin-coated vesicles are released from the plasma membrane by the action of the GTPase dynamin for entry into the endocytic pathway (43). Endocytic compartments are pleiomorphic structures that fuse with one another to promote ligand trafficking (16, 34). Two principal endocytic internalization pathways exist in the cell, which can be termed recycling or lysosome targeted (reviewed in reference 16). The regulation of such sorting and trafficking is determined by inherent signals on the internalized receptor and by signaling events within the cell.
Following rapid release of the clathrin coat, the internalized vesicle acquires properties that are defined temporally, and are thus termed early and late endosomes. The early endosome is an often-pleiomorphic tubulo-vesicular structure (50), the formation of which is regulated by the Rab5 GTPase (15). Early endosomes are major sorting stations, and internalized cargo can be delivered back to the plasma membrane (the recycling pathway) or can progress to the late endosome. Formation of late endosomes is dependent on the function of the Rab7 GTPase (5, 12). Compared to early endosomes, late endosomes have a mostly juxtanuclear distribution, are more spherical, and contain internal vesicles—leading to the term multivesicular bodies (MVBs) (37). They also differ from early endosomes in that they have a significantly lowered pH. Late endosomes subsequently progress to lysosomes, which are characterized by the presence of degradative proteases, delivered by communication of endosomes with the trans-Golgi network (22).
Many viruses take advantage of receptor-mediated endocytosis in order to gain entry into a target cell (27, 28) and are excellent model systems to study endocytic trafficking. Two viruses that have served as paradigms of virus entry are influenza virus and Semliki Forest virus (SFV). Influenza virus is an enveloped virus that is believed to enter host cells in a clathrin-dependent manner (31). For infection, the virus genome (consisting of individual viral ribonucleoproteins [vRNPs]) is released from the endosome following a low-pH-dependent fusion event mediated by the viral hemagglutinin (HA) glycoprotein (47, 53). The virus also sheds the matrix protein M1 in a low-pH-dependent manner (6), and the vRNPs enter the nucleus for replication (55). It is generally believed that the late endosome has the correct pH (approximately 5.5) required to trigger fusion (48, 56). SFV is another enveloped virus that is known to enter cells by clathrin-dependent endocytosis (10, 19). SFV is a cytoplasmically replicating virus that has a significantly higher pH threshold for fusion, with conformational changes in the spike (E1/E2) protein occurring at pH 6.2 (52). SFV is thought to enter cells via fusion through early endosomes (40). However, the specific endocytic compartments that either SFV or influenza virus passes through to reach a properly acidified vesicle have not yet been conclusively determined. Additionally, the numerous cellular factors required for the viral entry pathways have not been well characterized.
Two well-characterized cellular ligands that serve as models for recycling and lysosome-targeted endocytosis, respectively, are transferrin and epidermal growth factor (EGF). Both transferrin and EGF bind their respective receptors at the plasma membrane and are internalized into the cell via clathrin-coated pits (24). However, the two receptors are differentially sorted following internalization. Transferrin receptor follows the recycling pathway and, following release of bound iron in the mildly acidic early endosome, the transferrin receptor and its iron-free ligand are recycled to the plasma membrane ready for a new round of internalization (24). The EGF receptor (EGFR), on the other hand, is sorted into the lysosome-targeted pathway. Receptor internalization is stimulated following binding of EGF ligand (18). The EGFR traverses the low-pH environment of the late endosome, where it is sorted into the intraluminal elements of the MVB, before being finally degraded within the lysosome (14). Signaling from activated EGFR is believed to occur both from the cell surface and from within the endocytic pathway (7).
It is becoming appreciated that protein kinase activity plays an important role in endocytosis. Among such kinases, the protein kinase C (PKC) superfamily is responsible for diverse regulatory roles in many cellular processes (33, 36). The more than 11 PKC isoforms are divided into three subtypes, classical, novel, and atypical, based on their activation requirements and functional activity (33). PKC inhibition has been shown to affect endocytosis (13), but the activity of specific PKC isoforms, in general, has not been assigned to specific endocytic pathway components.
PKC has also been implicated in virus entry processes. The entry of several enveloped viruses, including rhabdoviruses, alphaviruses, poxviruses, and herpesviruses, has been proposed to require PKC based on the action of inhibitors such as H7 and staurosporine (8). More recently, it has been shown that the successful entry of adenovirus type 2 requires PKC. In the presence of calphostin C, an inhibitor of the classical and novel PKC isoforms, adenovirus is prevented from escaping endosomes and accumulates in cytoplasmic vesicles near the cell periphery (35). During entry, influenza virus traverses the endocytic pathway and, in addition, has the ability to activate PKC upon binding to host cell surface receptors (23). We have reported previously that bisindolymaleimide I, a broad-spectrum, highly specific PKC inhibitor, prevented influenza virus entry and subsequent infection (38). As this drug affects all PKC isoforms, we wished to determine which of these isoforms is required for successful influenza virus entry and infection.
Here, we show that PKCβII, specifically, is necessary for successful endocytic trafficking of the influenza virus, but not SFV. In cells lacking PKCβII activity, influenza virus accumulates in cytoplasmic vesicles, which we have identified as late endosomes through colocalization studies. This effect is not limited to influenza virus. Normal trafficking of EGF also required PKCβII activity, with the EGFR being trapped in an undegraded form in the absence of PKCβII function. In contrast, the trafficking of transferrin was unaffected. Based on these results, we suggest that PKCβII is a specific regulator of late endosome function required for influenza virus entry and infection.
MATERIALS AND METHODS
Viruses and cells
Influenza A/WSN/33 (H1N1) and A/X-31 (H3N2) virus stocks were grown in MDBK cells (WSN) or MDCK cells (X-31), and plaque titers were determined on MDCK cells. Unless stated otherwise, the WSN strain was used for all experiments. SFV strain M1 was provided by Margaret Kielian, Albert Einstein College of Medicine. Infections were performed essentially as previously described (38). Briefly, viral stocks were diluted in RPMI 1680 medium containing 0.2% bovine serum albumin and buffered to pH 6.8 with HEPES. Virus was adsorbed for 90 min at 4°C, and cells were then maintained in growth medium containing 2% serum at 37°C for either 60 min (at ∼100 to 200 PFU per cell) or 5 h (at ∼1 to 5 PFU per cell).
HeLa cells (American Type Culture Collection) were maintained in α-modified Eagle medium containing 10% calf serum, penicillin (100 U/ml), and streptomycin (10 μg/ml). Chemical inhibitors, Gö6976 and bafilomycin A (Alexis Biochemicals), were added during both the adsorption and incubation periods.
Antibodies.
Influenza virus nucleoprotein (NP) was detected using either the monoclonal antibody H10, L16-4R5 (American Type Culture Collection), or polyclonal antibody 15473 (Whittaker laboratory). The conformation-sensitive influenza virus HA and monoclonal antibodies N2 and A2 (9) were provided by Judith White (University of Virginia). SFV was detected with the E1-1 monoclonal antibody (provided by Margaret Kielian, Albert Einstein College of Medicine).
PKCβII was detected using the monoclonal anti-PKCβ antibody (Transduction Laboratories). PKCα was detected with the M2 monoclonal anti-FLAG antibody (Sigma). EGFR was detected using a monoclonal anti-EGFR antibody (Calbiochem). Transferrin receptor was detected using the monoclonal anti-TfnR antibody H68.4 (provided by Ira Mellman, Yale University). Cellular compartments were detected by monoclonal antibodies against early endosome antigen 1(EEA1) (Transduction Laboratories), cation-independent mannose 6-phosphate receptor (CI-M6PR) (Affinity BioReagents), CD63/Lamp3 (Chemicon), and Golgi apparatus Ab-1 (Oncogene Sciences).
Transfections.
Plasmid constructs of wild-type and phosphorylation-deficient PKCβII (T500V) were provided by Alexandra Newton, University of California, San Diego (11). Wild-type and phosphorylation-deficient (K368R) FLAG-tagged PKCα constructs were provided by Alex Toker, Harvard Medical School. Constructs of wild-type and dominant-negative HA-tagged PKCλ/ι (41) were provided by Jorge Moscat, Centro de Biología Molecular “Severo Ochoa,” Universidad Autónoma, Madrid, Spain. Unless described otherwise, transfections were performed using an Effectene transfection kit (Qiagen) according to manufacturer's protocols and routinely exceeded 70% efficiency. For transfection, HeLa, CHO, Mv1 Lu, or BHK cells were grown on coverslips in 24-well plates and transfected with 1 μg of DNA. Transfections were typically allowed to proceed for 16 to 18 h. Transfections in polarized MDCK cells were performed as described by Basler et al. (2).
Indirect immunofluorescence microscopy.
Preparation of cells for immunofluorescence microscopy was performed as described previously (54). Unless otherwise stated, secondary antibodies used were Oregon Green 514-labeled and Texas Red-labeled goat anti-mouse and goat anti-rabbit immunoglobulin G (Molecular Probes). For double-labeling experiments utilizing two monoclonal antibodies, we used Alexa 488- and Alexa 568-labeled isotype-specific secondary antibodies (Molecular Probes). Hoechst 33258 (Molecular Probes) was used at a concentration of 1 μg/ml. Cells were viewed using a Zeiss Axioskop 2 plus microscope with a 63× objective lens. Images were captured with a Zeiss Axiocam using Axiovision 3.0.6.1 software before being transferred into Adobe Photoshop 6.0. Confocal microscopy was performed using an Olympus FluoView confocal station. Oregon Green 514 and Alexa 488 were excited with the 488-nm line of an argon laser, and Texas Red and Alexa 568 were excited with the 568-nm line of a krypton laser. Cells were viewed with a 60× objective lens and images were captured with FluoView software (Olympus) before being transferred into Adobe Photoshop 6.0.
Endocytosis assays.
Transferrin uptake assays were performed using Alexa 594-labeled human transferrin (provided by Colin Parrish, Cornell University). HeLa cells were serum starved for 30 min, incubated with Alexa 594-transferrin (50 μg/ml) for 20 min at 4°C for binding, washed, and transferred to 37°C for 15 min. Cells were washed with 0.1 M glycine-0.1 M NaCl, pH 3.0, to remove any uninternalized ligand and were returned to 37°C for 15 min to monitor recycling. Transferrin receptor was detected using an anti-transferrin receptor antibody. EGF uptake and degradation were assayed essentially as described previously (41). HeLa cells were serum starved for 24 h and then incubated with human EGF (100 ng/ml; Amersham) for 60 min at 4°C. Cells were washed and transferred to 37°C for the indicated time. EGFR trafficking was monitored using an anti-EGFR antibody.
RESULTS
Specific inhibitors of classical PKCs prevent influenza virus entry and infection.
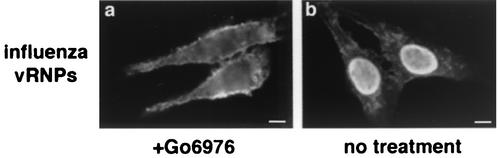
We have reported previously that bisindolymaleimide I, a highly specific inhibitor of all the PKC isoforms, prevents influenza virus entry and subsequent infection of the host cell (38). To identify specific PKC isoforms required for virus entry, we first examined more selectively acting PKC inhibitors. Calphostin C, an inhibitor of all classical and novel (but not atypical) PKC isoforms (20), effectively blocked influenza virus infection (data not shown), as did Gö6976, an inhibitor of classical PKCs (30) (Fig. 1). In the absence of drug, virus replication occurred normally, as demonstrated by the presence of influenza vRNPs in the nuclei of cells. In the presence of Gö6976, the nucleus was devoid of influenza vRNPs, with some signal present on the periphery of the cell—most likely due to particles arrested in the entry process.
FIG. 1.
PKC inhibitor Gö6976 prevents influenza virus entry. Immunofluorescence assay of inhibitor treated cells. HeLa cells were infected for 4 h at a low multiplicity of infection in the presence of 20 μM Gö6976 (a) or left untreated (b). Influenza infection was detected by the monoclonal NP antibody. Bars = 5 μm.
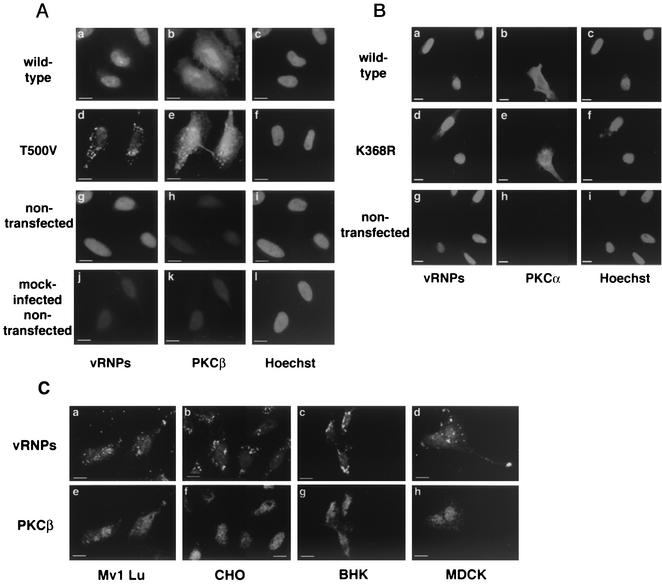
As Gö6976 is thought to selectively target the PKCα and -β isoforms (30), we next examined the role of individual PKCs by transient transfections of phosphorylation-deficient versions of PKCα and -β. We first examined the effects of wild-type and phosphorylation-deficient PKCβII constructs, whose activity has been described previously (11). Transfection with the phosphorylation-deficient PKCβII (T500V) clearly resulted in a loss of nuclear vRNP signal, indicating a block in virus entry. In the presence of PKCβII (T500V) the viral protein was visible in a punctate distribution in the cytoplasm (Fig. 2A, panel d). Interestingly, the expression pattern of the PKCβII T500V was different from that of the wild-type protein and partially overlapped that of the vRNPs (Fig. 2A, panel e). The overexpression of wild-type PKCβII showed no effect on viral entry and the presence of vRNPs in the nucleus (Fig. 2A, panel a). Mock-infected cells showed a small amount of nuclear signal based on some cross-reactivity due to the polyclonal NP antisera used for these experiments (Fig. 2A, panel j). These results with T500V were also observed when virus infection was monitored at either a 4- or 6-h time point (data not shown), indicating that the block was not a transient event in the entry process (although infectivity assays suggest that the virus may be able to escape the dominant-negative block at longer infection times). These data indicate that PKCβII is necessary for the successful endocytic trafficking of influenza virus.
FIG. 2.
Expression of phosphorylation-deficient PKCβII, but not PKCα, prevents influenza virus entry. (A) HeLa cells were transiently transfected with wild-type and phosphorylation-deficient T500V PKCβII before infection at a high multiplicity of infection for 60 min. (B) Mv1 Lu, CHO, BHK, and polarized MDCK cells were transiently transfected with PKCβII T500V plasmid before a high-multiplicity infection for 60 min. (C) HeLa cells were transiently transfected with wild-type or phosphorylation-deficient (K368R) PKCα plasmids before infection at a high multiplicity of infection for 60 min. Control cells were not transfected before infection. Influenza virus signal was detected via indirect immunofluorescence using a polyclonal NP antibody. Expression was detected using a monoclonal antibody against PKCβ or FLAG epitope. Bars = 5 μm.
To ensure the effects of PKCβII expression were not cell type dependent, the above-described experiments were repeated with Mv1 Lu, CHO, BHK, and polarized MDCK cells. In all cell lines, the expression of PKCβII T500V caused the vRNPs to localize in a punctate distribution throughout the cytoplasm (Fig. 2B, panels a to d). As in HeLa cells, the overexpression of wild-type PKCβII had no effect on virus infection, the cells exhibiting a strong nuclear vRNP signal (data not shown).
Because of the inhibitory effect of Gö6976 on PKCα and the known role of PKCα in phagocytosis (1, 4), we also examined cells transfected with K368R, a phosphorylation-deficient form of PKCα (A. Toker, personal communication). Expression of K368R or wild-type PKCα did not inhibit influenza virus entry, as shown by the strong nuclear vRNP signal visible in all transfected cells using our immunofluorescence assay (Fig. 2C). Overall, these data indicate that PKCβII, but not PKCα, is required for the endocytic trafficking of influenza virus.
Uptake of SFV is not dependent on PKCβII activity.
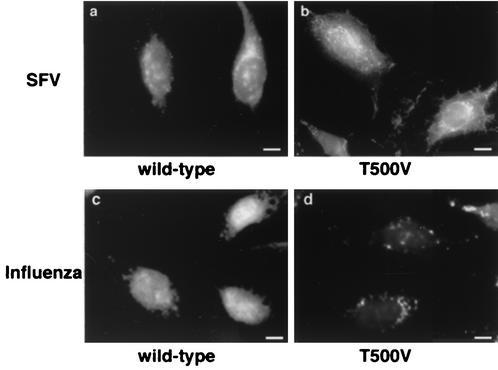
Because of the marked requirement for PKCβII in influenza virus infection, we wished to determine whether other viruses were similarly inhibited. We examined SFV, an enveloped virus that replicates in the cytoplasm and is pH sensitive for fusion. Because of its pH threshold for fusion (approximately pH 6.2), SFV is commonly believed to enter cells from early endosomes (40). When cells were transfected with PKCβII wild type or PKCβII T500V, we saw little or no difference in SFV infection (Fig. 3, panels a and b). Likewise, Gö6976 had no apparent effect on SFV infection when analyzed at the 5-h time point (data not shown). Because this assay relies on a relatively long time of SFV infection (5 h), it remains possible that the virus simply overwhelms any experimental effects of a molecular inhibitor. To confirm the functionality of our assay, we repeated the SFV infections in cells overexpressing a dominant-negative mutant of Eps15 (provided by Jennifer Lippincott-Schwartz, National Institutes of Health), previously shown to block clathrin-mediated endocytosis (3). Expression of dominant-negative Eps15 gives a complete block of SFV infection in our system (45), confirming that our assay is sufficiently sensitive to molecular inhibition. Similarly, influenza virus replication was clearly blocked in PKCβII T500V-expressing cells under similar infection conditions (Fig. 3, panel d). Overall, our data show that influenza virus and SFV have differential requirements for PKCβΙΙ during entry and indicate that PKCβII is not universally required by viruses that enter cells by endocytosis.
FIG. 3.
SFV infection is not inhibited by expression of PKCβII T500V. HeLa cells were transiently transfected with the PKCβII wild-type or T500V plasmid for 16 h before infection. Cells were infected with SFV (a and b) or influenza virus (c and d) at a low multiplicity of infection for 5 h. Virus signal was detected by the monoclonal anti-SFV E1-1 antibody or anti-influenza virus NP antibody. PKCβII expression was detected by a monoclonal antibody against PKCβ. Bars = 5 μm.
Influenza virus is accumulating in late endosomes in PKCβII T500V-transfected cells.
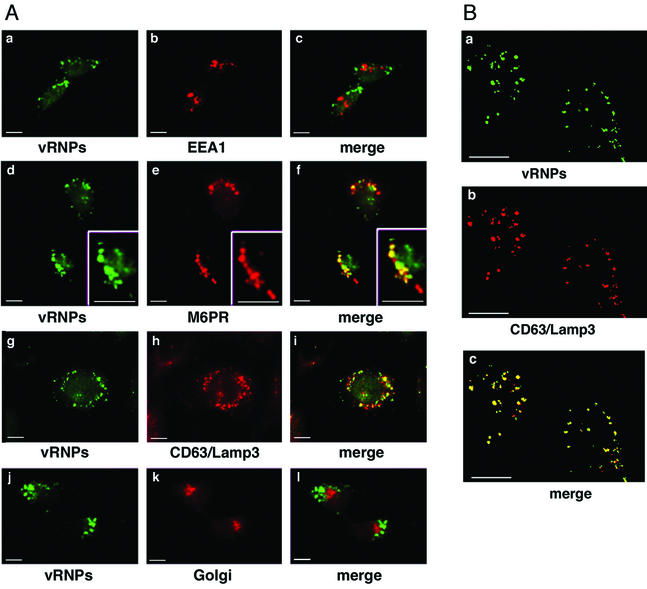
The immunofluorescence expression pattern of influenza vRNPs in cells transfected with PKCβII suggested that the virus was accumulating in an endocytic compartment. To determine the exact localization of the accumulated virus, we performed colocalization studies with markers of the endocytic and exocytic pathways (Fig. 4). We first labeled cells with antibodies for both vRNPs and EEA1, a marker of early endosomes (46). Upon merging both channels in a PKCβII T500-transfected cell, the cytoplasmic speckles of vRNP did not colocalize to any significant degree with EEA1 (Fig. 4A, panel c). We next examined possible colocalization with CI-M6PR, a well-established marker of late endosomes (22). In a merge of both channels from a T500V-transfected cell, the vRNPs colocalized well with the M6PR marker (Fig. 4A, panel f), indicating that the influenza virus is trapped within late endosomes in cells lacking active PKCβII. To confirm this localization, we examined the distribution of vRNPs in comparison with an independent late endosome marker, CD63/Lamp3 (21). In T500V expressing cells, the vRNPs also showed a high degree of colocalization with CD63/Lamp3 (Fig. 4A, panel i), confirming the site at which virus infection is arrested. As both M6PR and CD63/Lamp3 can transit between the endosome and the Golgi, we also performed colocalization studies with a Golgi apparatus marker control to ensure that the observed colocalization was specific to late endosomes. In T500V-expressing cells vRNPs showed no significant colocalization with our Golgi marker (Fig. 4, panel l).
FIG. 4.
Influenza vRNPs accumulate in late endosomes in cells lacking PKCβII activity. HeLa cells were transiently transfected with the PKCβII wild-type or T500V plasmids for 16 h before a high-multiplicity infection for 60 min. (A) Indirect immunofluorescence studies were used to analyze the expression pattern of influenza virus and cellular vesicles. Influenza virus localization was determined using a polyclonal NP antibody. EEA1 (a marker for early endosomes), M6PR and CD63/Lamp3 (markers for late endosomes), and the Golgi apparatus were localized using their respective monoclonal antibodies. (B) Confocal microscopy studies were used to analyze the expression pattern of influenza virus and late endocytic vesicles. Influenza localization was determined by the polyclonal NP antibody, and late endosomes were localized with a monoclonal antibody to CD63/Lamp3. Bars = 5 μm.
To further confirm our colocalization results of vRNPs and late endosomes, we also performed confocal microscopy (Fig. 4B). By confocal microscopy, a punctate distribution of vRNPs was observed, with no signal within the nucleus. In these cells, the distribution of late endosomes almost completely colocalized with the incoming vRNPs, as demonstrated by the pattern of yellow vesicles in the merged image (Fig. 4B, panel c). For both M6PR and CD63/Lamp3, a fraction of endocytic vesicles were positive only for M6PR and CD63/Lamp3 (indicating that not all late endosomes contain incoming viruses), and a tiny minority of vesicles were positive only for vRNPs, confirming the specificity of the observed colocalization. Overall, these data indicate that the lack of PKCβII activity results in the arrest of endocytic trafficking at the level of late endosomes and in the accumulation of influenza virus within this vesicle.
PKCβII T500V does not affect the pH status of late endosomes.
As influenza virus has a strict requirement for low pH during virus entry, it is possible that the effects of PKCβII T500V were caused by defective acidification of the endosome. To address this issue, we performed several assays to examine the pH status of the endosomes in T500V-expressing cells. First, we repeated the influenza virus entry assay and treated the cells with the ionophores nigericin and monensin, in medium that was externally buffered to pH 7.3 or 5.5. Our reasoning was that the ionophore would equilibrate the interior of the cell to the external pH and the presence of pH 5.5 buffer would rescue any possible acidification defect caused by PKCβII T500V. However the virus still remained trapped in late endosomes in all cases (Fig. 5A).
FIG. 5.
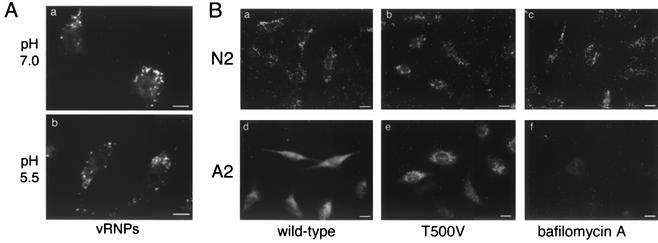
Expression of PKCβII T500V does not affect endosome acidification needed for virus entry. (A) HeLa cells were transiently transfected with the PKCβII wild-type or T500V plasmid for 16 h before a high-multiplicity infection for 60 min. Cells were then treated with 10 μM nigericin and monensin and incubated in medium buffered to either pH 7.0 or 5.5 with 20 mM HEPES. Cells were fixed at 60 min, and vRNPs were detected via indirect immunofluorescence using a polyclonal NP antibody. Bars = 5 μm. (B) HeLa cells were transiently transfected with the PKCβII wild-type or T500V plasmid for 16 h before a high-multiplicity infection with the X-31 strain of influenza virus. The viral HA glycoprotein was detected by indirect immunofluorescence with the conformation-specific monoclonal antibodies N2 (neutral) and A2 (acidic). Controls were not transfected but were treated with 25 nM bafilomycin A. Bars = 8 μm.
The influenza virus HA glycoprotein is crucial for fusion of the virus envelope with the endosomal membrane. The HA protein exits in two principal conformations—one at neutral pH and a second at acidic pH—in which the fusion peptide is exposed (51). It is possible to specifically detect either the neutral or low pH form of HA using conformation-specific antibodies (9). During an entry assay, influenza virus is visible on the cell surface and within early endocytic compartments using the neutral-pH-specific antibody N2. The acid-specific A2 antibody acts as a probe of endosomal pH, as it only recognizes HA below pH 6.0 (49). We therefore utilized the A2 and N2 antibodies as probes of endosomal pH in cells transfected with wild-type or T500V PKCβII. In both cases we observed no differences between cells transfected with wild-type or T500V PKCβII (Fig. 5B). Some differences in staining pattern were observed between the two antibodies. We believe this represents the quite different epitopes recognized by the antibodies. N2 recognizes HA in a neutral form and detects viruses in multiple stages of entry, i.e., bound to the cell surface and the coverslip, and within early endosomes and vesicles. Therefore, more N2 signal is seen compared to the A2 antibody, which only recognizes HA below approximately pH 6, i.e., in late endosomes. We also included bafilomycin A, a well-characterized inhibitor of the vacuolar H+ ATPase that prevents endosome acidification and influenza virus entry (17), as a positive control. Under these conditions, the A2 antibody was not able to detect HA (Fig. 5B, panel f), confirming the conformational specificity of the antibody labeling. These results provide further evidence that the defect in virus entry in T500V-expressing cells is not due to an alteration of endosomal pH, a result supported by the apparently normal uptake and signal intensity of Lysotracker Red, a pH-sensitive fluorescent probe, in T500V-expressing cells (data not shown).
PKCβII is also required for the normal trafficking of epidermal growth factor.
Because of the differential effects of T500V PKCβII on influenza and SFV, we wished to determine if the kinase was similarly important for uptake and trafficking of cellular ligands that follow different routes. EGF and transferrin are well-characterized markers of endocytic trafficking that follow distinct pathways through the cell. The transferrin receptor is taken into early endosomes and then shuttled into recycling vesicles and back to the plasma membrane. The EGFR, on the other hand, follows a pathway similar to that likely for influenza virus, as it is directed into late endosomes and subsequently into lysosomes, where it is degraded (24).
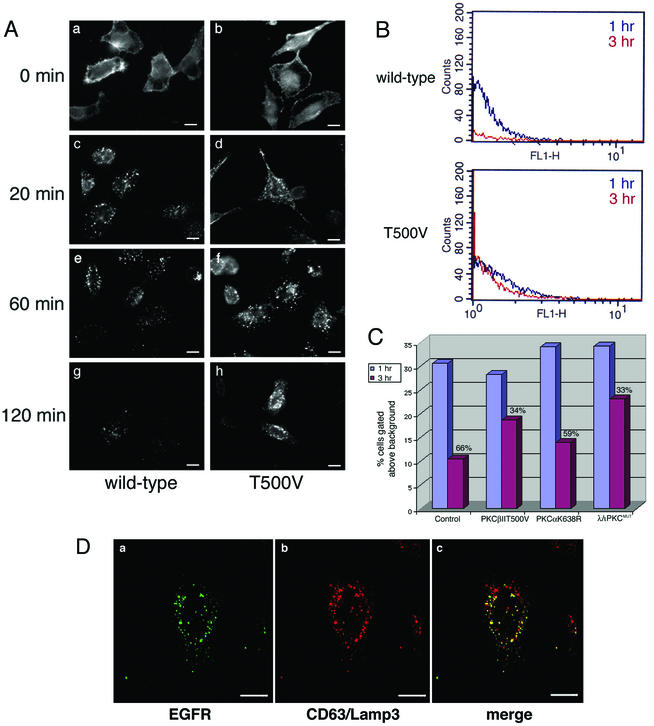
We first addressed the effects of PKCβII T500V on the uptake of EGF. The localization of the EGFR in transfected cells was monitored over a 2-h time course by indirect immunofluorescence assay (Fig. 6). At time zero, equivalent levels of EGFR were present on the cell surface of both wild-type and T500V-transfected cells. By 20 min, the receptor was internalized, with similar levels of internalized receptor present under both conditions. In wild-type PKCβII-transfected cells, the level of antibody labeling was markedly reduced at 60 min and was almost undetectable at 120 min, indicating efficient degradation of the receptor. However, in PKCβII T500V-tranfected cells the levels and localization of the EGFR remained essentially the same at both 60 and 120 min, indicating a block in receptor trafficking and degradation.
FIG. 6.
EGFR is less efficiently degraded in cells expressing PKCβII T500V. (A) HeLa cells were transiently transfected with the wild-type or kinase-dead (T500V) PKCβII plasmid for 16 h. Cells were then serum starved for 24 h at 37°C. EGF was bound at 4°C for 60 min, and cells were washed and then incubated at 37°C for the indicated times. EGFR was detected by indirect immunofluorescence using an anti-EGFR monoclonal antibody. Bars = 8 μm. (B) HeLa cells were transiently transfected with the PKCβII T500V plasmid for 16 h or were mock transfected. Cells were serum starved, and the EGF uptake assay was performed at 1 h (blue trace) and 3 h (red trace). Cells were prepared for flow cytometry using the monoclonal antibody against the EGFR. (C) HeLa cells were transiently transfected with the kinase-dead (T500V) PKCβII, kinase-dead PKCα (K368R), or dominant-negative PKCλ (λ/ι PKCMUT) plasmid for 16 h or were untransfected. Cells were serum starved, and the EGF uptake assay was performed as described for panel B. Cells were prepared for flow cytometry using the monoclonal antibody against the EGFR. The signal intensity was normalized at the 1 h time point, and the relative amount of EGFR degraded by the 3 h time point is shown as a percentage. (D) HeLa cells were transiently transfected with the PKCβII T500V plasmid for 16 h and then serum starved. EGF uptake was allowed to occur for 120 min. Confocal microscopy studies were used to analyze the expression pattern of the EGFR and late endocytic vesicles. EGFR localization was determined using an anti-EGFR antibody, and late endosomes were localized with an antibody to CD63/Lamp3. Bars = 5 μm.
To quantitate the apparent block in EGFR degradation for PKCβII T500V-expressing cells, we performed flow cytometry at the 1- and 3-h time points (Fig. 6B and C). For control untransfected cells a clear decrease in the EGFR signal was observed between the 1-h (blue profile) and 3-h (red profile) time points. Compared to the 1-h time point, we determined that 66% of the EGFR was degraded by 3 h (Fig. 6C). In contrast, in PKCβII T500V-expressing cells, the 1- and 3-h traces had very similar degradation profiles. In this case, only 34% of the EGFR was degraded over the time course. This residual degradation most likely represents the pool of untransfected cells in the total population. The data for PKCβII T500V-expressing cells were similar to results we obtained when expressing a dominant-negative form of PKCλ/ι (33% degraded), which has previously been shown to inhibit EGFR trafficking (41). However, cells expressing a kinase-dead form of PKCα (K638R) showed very similar levels of EGF degradation to control cells (59% versus 66%). These data confirm our immunofluorescence experiments with PKCβII, confirm previous reports of a role for PKCι/λ in EGFR trafficking, and show that PKCα does not seem to affect trafficking through early or late endosomes.
To determine the cellular compartment in which EGF trafficking was arrested in T500V-expressing cells, we performed confocal microscopy. We used two late endosome markers, M6PR (not shown) and CD63/Lamp3 (Fig. 6D). As with influenza virus, we saw a significant colocalization between EGFR and the late endosome marker, a distribution not seen with the wild-type-transfected cells (not shown), in which the receptor was extensively degraded. For both M6PR and CD63/Lamp3, a fraction of vesicles were positive only for the late endosome marker, showing that not all compartments contained EGFR and confirming the specificity of the observed colocalization. These colocalization studies demonstrate that the EGFR is being retained in late endosomes. This indicates that the EGFR was prevented from completing its uptake pathway by the lack of a functional PKCβII and that the kinase is specifically affecting the lysosome-targeted endocytic pathway.
PKCβII T500V does not affect transferrin receptor recycling.
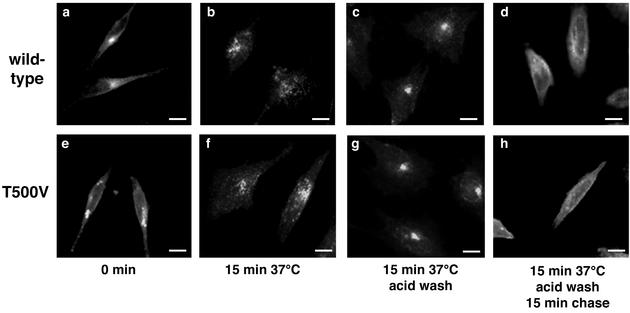
Finally, we addressed the effects of PKCβII T500V on transferrin receptor, a marker of the recycling pathway that does not reach the late endosome. The localization of the transferrin receptor within the cell was determined by immunofluorescence microscopy (Fig. 7). Expression of PKCβII T500V had no apparent effect on the ability of the transferrin receptor to be internalized or to recycle from internal vesicles back to the plasma membrane. These data indicate that PKCβII specifically affects lysosome-targeted endosome trafficking, without affecting the recycling pathway.
FIG. 7.
Transferrin receptor recycling is unaffected by the lack of PKCβII activity. HeLa cells were transiently transfected with the PKCβII wild-type or T500V plasmid for 16 h. Alexa 594-labeled transferrin (50 μg/ml) was then bound to serum-starved cells for 20 min at 4°C. Cells were then shifted to 37°C for 15 min, washed in low-pH glycine to remove any uninternalized ligand, and returned to 37°C for 15 min to monitor receptor recycling. Cells were analyzed by fluorescence microscopy with an antibody to transferrin receptor. Bars = 8 μm.
DISCUSSION
We show here that PKCβII is necessary both for influenza virus entry and infection and for EGFR trafficking and degradation. The lack of functional PKCβII results in the accumulation of both influenza vRNPs and EGFR in late endosomal compartments. Uptake of SFV and transferrin, which utilize the early endosome/recycling pathway, were not influenced by PKCβII activity. These data indicate a general role for PKC in endocytic trafficking, specifically, PKCβII activity at the level of late endosomes.
Beyond a requirement for dynamin at the initial stages of internalization (39), the endocytic pathway followed by influenza virus has not been clearly defined to date. In cells lacking PKCβII activity, the accumulation of both the virus and the EGFR in M6PR- and CD63/Lamp3-positive vesicles indicates that both substrates follow the same route of endocytic trafficking; i.e., the virus follows the lysosome-targeted endocytic pathway. Our data therefore represent the first functional information on the specific endocytic trafficking events and cellular factors used by influenza virus during its entry into target cells. We show that the virus enters late endosome compartments, from which the virus would be expected to undergo fusion and release of vRNPs ready for nuclear import. However, in T500V-expressing cells, release of vRNPs from the late endosome is defective. At this time, we cannot directly address the question of whether the virus has undergone fusion with the membrane of the late endosome; however, the reactivity of the conformation-specific A2 antibody suggests that PKCβII T500V does not affect the fusion event. It remains possible that PKCβΙΙ is controlling a cellular component of fusion or possibly a later step needed for nuclear transport and/or entry.
The outcome of defective PKCβII phosphorylation is most likely that the late endosome is not fully functional and cannot deliver the virus genome into the cytoplasm. The inhibition of entry, then, appears to be in virus uncoating or in the release of the virion from its endocytic vesicle.
The suggestion that influenza virus requires late endosome function beyond the need for acidification reinforces the idea that endocytic trafficking events during viral entry play a major part in the successful delivery of the incoming virus to the site of replication. Such effects have been elegantly demonstrated for SFV (26), where fusion of SFV at the cell surface led to the trapping of the virus within cortical actin in certain cell types. Likewise, we have failed to see successful infection of our cell culture lines when influenza virus was bound and cell surface fusion was induced by a pH drop (A.-M. Roy and G. Whittaker, unpublished results). Such data, along with those presented in this paper, show that influenza virus requires successful endosomal trafficking, and not just low pH, for infection. It is likely that analogous endocytic trafficking is crucial for other viruses, even if they do not have a direct pH requirement for fusion or uncoating, such as picornaviruses and retroviruses (25).
At present, we can only speculate on any specific activation of PKCβII by the virus, which might be used to up-regulate the endocytic pathway and ensure efficient virus infection. However, the previously described influenza virus-induced activation of both phospholipase C and PKC following binding of the virus to respiratory epithelial cells (23) makes this an attractive possibility. PKC plays a role in a wide range of intertwining signaling pathways, and because of this complexity, the study of PKC function is challenging. Here, by the use of a specific molecular tool targeted to PKC, we provide insight into both the influenza virus life cycle and the cellular mechanisms of endocytosis. Our data imply a distinct effect of PKCβII at the level of the late endosome/MVB, with the kinase acting on an aspect of the endocytic trafficking machinery that is required for both EGF degradation and influenza virus entry.
Acknowledgments
We thank Ruth Collins for helpful discussion throughout the course of this work and for critical reading of the manuscript, Liz Wills for technical support, and John Parker and Beate Sodeik for helpful discussions. We also thank Alexandra Newton, Alex Toker, Jorge Moscat, Colin Parrish, Ira Mellman, Steve Wharton, Jennifer Lippincott-Schwartz, Margaret Kielian, and Judy White for their kind contributions of reagents.
S.B.S. was supported by a training grant from the National Institutes of Health (T32AI07618). This work was supported by a Scientist Development Grant from the American Heart Association and by grant R01AI48678 from the National Institutes of Health (to G.R.W.).
REFERENCES
- 1.Allen, L. H., and A. Aderem. 1995. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J. Exp. Med. 182:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 4.Breton, A., and A. Descoteaux. 2000. Protein kinase C-alpha participates in FcγR-mediated phagocytosis in macrophages. Biochem. Biophys. Res. Commun. 276:472-476. [DOI] [PubMed] [Google Scholar]
- 5.Bucci, C., P. Thomsen, P. Nicoziani, J. McCarthy, and B. van Deurs. 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11:467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bui, M., G. Whittaker, and A. Helenius. 1996. Effect of M1 protein and low pH on nuclear transport of influenza virus ribonucleoproteins. J. Virol. 70:8391-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clague, M. J., and S. Urbe. 2001. The interface of receptor trafficking and signalling. J. Cell Sci. 114:3075-3081. [DOI] [PubMed] [Google Scholar]
- 8.Constantinescu, S. N., C. D. Cernescu, and L. M. Popescu. 1991. Effects of protein kinase C inhibitors on viral entry and infectivity. FEBS Lett. 292:31-33. [DOI] [PubMed] [Google Scholar]
- 9.Copeland, C. S., R. W. Doms, E. M. Bolzau, R. G. Webster, and A. Helenius. 1986. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J. Cell Biol. 103:1179-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeTulleo, L., and T. Kirchausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutil, E. M., and A. C. Newton. 2000. Dual role of pseudosubstrate in the coordinated regulation of protein kinase C by phosphorylation and diacylglycerol. J. Biol. Chem. 275:10697-10701. [DOI] [PubMed] [Google Scholar]
- 12.Feng, Y., B. Press, and A. Wandinger-Ness. 1995. Rab 7: an important regulator of late endocytic membrane traffic. J. Cell Biol. 131:1435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari, S. L., V. Behar, M. Chorev, M. Rosenblatt, and A. Bisello. 1999. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves β-arrestin 2. Real-time monitoring by fluorescence microscopy. J. Biol. Chem. 274:29968-29975. [DOI] [PubMed] [Google Scholar]
- 14.Futter, C. E., A. Pearse, L. J. Hewlett, and C. R. Hopkins. 1996. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 132:1011-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorvel, J. P., P. Chavrier, M. Zerial, and J. Gruenberg. 1991. rab5 controls early endosome fusion in vitro. Cell 64:915-925. [DOI] [PubMed] [Google Scholar]
- 16.Gruenberg, J. 2001. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell. Biol. 2:721-730. [DOI] [PubMed] [Google Scholar]
- 17.Guinea, R., and L. Carrasco. 1995. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J. Virol. 69:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haigler, H. T., J. A. McKanna, and S. Cohen. 1979. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J. Cell Biol. 81:382-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirchhausen, T. 1998. Vesicle formation: dynamic dynamin lives up to its name. Curr. Biol. 8:R792-R794. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, E., H. Nakano, M. Morimoto, and T. Tamaoki. 1989. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 159:548-553. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi, T., U. M. Vischer, C. Rosnoblet, C. Lebrand, M. Lindsay, R. G. Parton, E. K. O. Kruithof, and J. Gruenberg. 2000. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human epithelial cells. Mol. Biol. Cell 11:1829-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornfeld, S., and I. Mellman. 1989. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5:483-525. [DOI] [PubMed] [Google Scholar]
- 23.Kunzelmann, K., A. H. Beesley, N. J. King, G. Karupiah, J. A. Young, and D. I. Cook. 2000. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc. Natl. Acad. Sci. USA 97:10282-10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamaze, C., T. Baba, T. E. Redelmeier, and S. L. Schmid. 1993. Recruitment of epidermal growth factor and transferrin receptors into coated pits in vitro: differing biochemical requirements. Mol. Biol. Cell 4:715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsh, M. 1984. The entry of enveloped viruses into cells by endocytosis. Biochem. J. 218:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh, M., and R. Bron. 1997. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 110:95-103. [DOI] [PubMed] [Google Scholar]
- 27.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh, M., and A. Pelchen-Matthews. 1994. The endocytic pathway and virus entry, p. 215-240. In E. Wimmer (ed.), Cellular receptors for animal viruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Marsh, M., and A. Pelchen-Matthews. 2000. Endocytosis in viral replication. Traffic 1:525-532. [DOI] [PubMed] [Google Scholar]
- 30.Martiny-Baron, G., M. G. Kazanietz, H. Mischak, P. M. Blumberg, G. Kochs, H. Hug, D. Marme, and C. Schachtele. 1993. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268:9194-9197. [PubMed] [Google Scholar]
- 31.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1981. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 91:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 33.Mellor, H., and P. J. Parker. 1998. The extended protein kinase C family. Biochem. J. 332:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhergee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. Rev. 77:759-803. [DOI] [PubMed] [Google Scholar]
- 35.Nakano, M. Y., K. Boucke, M. Suomalainen, R. P. Stidwell, and U. G. Greber. 2000. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J. Virol. 74:7085-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton, A. C. 1996. Protein kinase C: ports of anchor in the cell. Curr. Biol. 6:806-809. [DOI] [PubMed] [Google Scholar]
- 37.Piper, R. C., and J. P. Luzio. 2001. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2:612-621. [DOI] [PubMed] [Google Scholar]
- 38.Root, C. R., E. G. Wills, L. L. McNair, and G. R. Whittaker. 2000. Entry of influenza viruses into cells is inhibited by a highly specific protein kinase C inhibitor. J. Gen. Virol. 81:2697-2705. [DOI] [PubMed] [Google Scholar]
- 39.Roy, A.-M. M., J. S. Parker, C. R. Parrish, and G. R. Whittaker. 2000. Early stages of influenza virus entry into Mv-1 lung cells: involvement of dynamin. Virology 267:17-28. [DOI] [PubMed] [Google Scholar]
- 40.Russell, D. G., and M. Marsh. 2001. Endocytosis in pathogen entry and replication, p. 247-280. In M. Marsh (ed.), Endocytosis. Oxford University Press, Oxford, United Kingdom.
- 41.Sanchez, P., G. de Carcer, I. V. Sandoval, J. Moscat, and M. Diaz-Meco. 1998. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol. Cell. Biol. 18:3069-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid, S. L. 1997. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 66:511-548. [DOI] [PubMed] [Google Scholar]
- 43.Sever, S., A. B. Muhlberg, and S. L. Schmid. 1999. Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature 398:481-486. [DOI] [PubMed] [Google Scholar]
- 44.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 45.Sieczkarski, S. B., and G. R. Whittaker. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 76:10455-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonsen, A., R. Lippe, S. Christoforidis, J. M. Gaullier, A. Brech, J. Callaghan, B. H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494-498. [DOI] [PubMed] [Google Scholar]
- 47.Skehel, J. J., T. Bizebard, P. A. Bullough, F. M. Hughson, M. Knossow, D. A. Steinhauer, S. A. Wharton, and D. C. Wiley. 1995. Membrane fusion by influenza virus. Cold Spring Harbor Symp. Quant. Biol. LX:573-580. [DOI] [PubMed] [Google Scholar]
- 48.Stegmann, T., H. W. M. Morselt, J. Scholma, and J. Wilschut. 1987. Fusion of influenza virus in an intracellular acidic compartment measured by fluorescence dequenching. Biochim. Biophys. Acta 904:165-170. [DOI] [PubMed] [Google Scholar]
- 49.Stegmann, T., J. M. White, and A. Helenius. 1990. Intermediates in influenza induced membrane fusion. EMBO J. 9:4231-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tooze, J., and M. Hollinshead. 1991. Tubular early endosomal networks in AtT20 and other cells. J. Cell Biol. 115:635-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White, J. 1990. Viral and cellular membrane fusion proteins. Annu. Rev. Physiol. 52:675-697. [DOI] [PubMed] [Google Scholar]
- 52.White, J., and A. Helenius. 1980. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc. Natl. Acad. Sci. USA 77:3273-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White, J., J. Kartenbeck, and A. Helenius. 1982. Membrane fusion activity of influenza virus. EMBO J. 1:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whittaker, G., I. Kemler, and A. Helenius. 1995. Hyperphosphorylation of mutant influenza virus matrix (M1) protein causes its retention in the nucleus. J. Virol. 69:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whittaker, G. R., M. Kann, and A. Helenius. 2000. Viral entry into the nucleus. Annu. Rev. Cell Dev. Biol. 16:627-651. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura, A., and S. Ohnishi. 1984. Uncoating of influenza virus in endosomes. J. Virol. 51:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]