Abstract
Hyphal growth is prevalent during most Candida albicans infections. Current cell division models, which are based on cytological analyses of C. albicans, predict that hyphal branching is intimately linked with vacuolar inheritance in this fungus. Here we report the molecular validation of this model, showing that a specific mutation that disrupts vacuolar inheritance also affects hyphal division. The armadillo repeat-containing protein Vac8p plays an important role in vacuolar inheritance in Saccharomyces cerevisiae. The VAC8 gene was identified in the C. albicans genome sequence and was resequenced. Homozygous C. albicans vac8Δ deletion mutants were generated, and their phenotypes were examined. Mutant vac8Δ cells contained fragmented vacuoles, and minimal vacuolar material was inherited by daughter cells in hyphal or budding forms. Normal rates of growth and hyphal extension were observed for the mutant hyphae on solid serum-containing medium. However, branching frequencies were significantly increased in the mutant hyphae. These observations are consistent with a causal relationship between vacuolar inheritance and the cell division cycle in the subapical compartments of C. albicans hyphae. The data support the hypothesis that cytoplasmic volume, rather than cell size, is critical for progression through G1.
Vacuolar inheritance is thought to play an important role in cell cycle control and cellular division in the pathogenic fungus Candida albicans (1). C. albicans is polymorphic and diploid and has no exploitable sexual cycle (9, 21, 25). In nutrient-rich conditions this fungus exhibits bipolar budding, but under starvation conditions it undergoes morphological transitions to divide as pseudohyphae or hyphae (27). The degree of nutrient limitation regulates cell division in the growing hypha: branching frequencies decrease as the severity of the nutrient limitation increases (1). This is thought to represent a foraging response that allows the fungus to move efficiently to areas of nutrient sufficiency (18), and it has been suggested that this ability promotes fungal invasion of host tissue (19).
During hyphal development in C. albicans, the germ tube grows linearly throughout the first cell cycle, at which point a septum is laid down, thereby dividing the hypha into two distinct compartments. The two compartments are equal in length, and each contains a single nucleus, but the two compartments differ in total cytosolic volume. The apical compartment inherits the majority of the cytoplasm, leaving the subapical compartment highly vacuolated and with little cytoplasmic volume (17). This asymmetrical cell division has a profound impact on subsequent cell divisions. The apical compartment continues to grow at a constant rate and to divide as before. However, the subapical compartment exhibits a growth delay and does not enter a second cell cycle to form a branch until the vacuole volume decreases and the cytosolic volume increases. We have shown previously that these subapical compartments are held in G1 until critical cytosolic volume is achieved and they can progress through Start (1). However, a causal relationship between vacuolar inheritance and hyphal branching has not been confirmed.
Vacuolar inheritance in Saccharomyces cerevisiae is a spatially and temporally ordered event (48, 49, 52). In late G1 the vacuole orients itself toward the bud site. The onset of S phase results in the formation of a so called “segregation structure” that consists of a stream of small vesicles flowing from the now fragmented maternal vacuole into the bud (50). Reversible dynamic flow of vacuolar material occurs between mother and daughter. Translocation of vacuolar vesicles is dependent upon the actin cytoskeleton and myosin (Myo2p), which acts as the motor for this dynamic process (20, 22). The process of vacuolar division lasts for approximately 20 min, culminating in M phase, and it results in the accumulation of small vesicles in the daughter cell (13). These subsequently fuse to form a mature vacuole. This phenomenon of vacuole fusion has been studied extensively in vitro and can be divided into three stages: priming, docking, and fusion (53, 54).
S. cerevisiae mutants defective in vacuole inheritance or segregation have been isolated and characterized (14, 15, 38, 45, 51). Mutants defective in vacuole partitioning, known as vac mutants, have been categorized into three groups according to their vacuole morphology (3, 7, 52) and further subdivided into vacuole protein-sorting (vps) mutants (14, 32, 33, 38, 45). Class I mutants have normal vacuole morphology but do not exhibit polarization toward the bud site. Hence, the resultant daughter cells contain little or no maternal vacuolar material. Class II mutants have fewer vacuolar lobes and contain a node on the vacuolar membrane. Large single vacuoles that sometimes display a single contiguous open “figure eight” structure between mother and daughter cells are found in class III mutants (52).
The close relationship between vacuole segregation and protein transport pathways has inevitably resulted in mutants defective in both processes. The class D vps mutants, which missort vacuolar proteins from the Golgi to the cell surface and also display aberrant vacuole segregation, are examples of this. These are categorized as class II mutants according to their vacuole morphology. This overlap makes it difficult to separate vacuole segregation from protein transport and to distinguish between cause and effect. Mutant screens, however, have led to the isolation of a series of non-vps mutants. One of these mutants is vac8 (10, 29, 42, 46).
In S. cerevisiae, vac8 mutants exhibit a multilobed or fragmented vacuole phenotype and are defective in vacuolar inheritance and in cytoplasm-to-vacuole protein targeting (Cvt pathway) (37). However, proteins are transported normally from the Golgi to the vacuole (Vps pathway). As such, they are defined as class I, non-vps mutants. The Vac8p protein contains 11 armadillo (Arm) repeats and is most closely related to the Drosophila armadillo protein (34), β-catenin (24), and plakoglobin (12). Tandem arrays of Arm repeats, each about 42 amino acids in length, are proposed to form scaffold-like structures that act to support multiprotein complexes. Vac8p is localized to the vacuolar membrane and is required for multiple vacuole-related processes. Both myristoylation and palmitoylation of Vac8p are required for complete localization. Palmitoylation plays a role in vacuolar inheritance (10, 29, 46) and is critical for subsequent vacuole fusion in the daughter cell (42, 46). Vacuolar inheritance is dependent upon the formation of a complex between the molecular motor Myo2p, the Myo2p-specific binding protein Vac17p, and Vac8p (41). Vac17p provides the specific link between the motor protein and the vacuole. Its expression correlates with the cell cycle, and hence Vac17p plays a critical role in the temporal regulation of vacuole inheritance.
In this study, we tested whether a causal relationship exists between vacuolar inheritance and hyphal branching in C. albicans. Our approach was to disturb vacuolar inheritance by disrupting the VAC8 locus and then to examine vacuolar inheritance and hyphal growth in these mutants. Our data confirm the importance of vacuolar segregation in the growth and division of the hyphal form of this human pathogen.
MATERIALS AND METHODS
Strains and media.
Standard yeast media and microbiological techniques were used (39). For liquid growth assays, cells were pregrown in yeast extract-peptone-dextrose (YPD) (39) overnight at 30°C and then diluted to an optical density at 600 nm (OD600) of 0.1 in YPD, YPD containing 1.5 M NaCl, or YPD containing 2.5 M glycerol. Equivalent medium containing 2% (wt/vol) agar was used for the plate assays, and cell cultures were diluted as described by Palmer et al. (28). C. albicans strains are listed in Table 1.
TABLE 1.
C. albicans strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| CAF2-1 | URA3/Δura3::λ imm434 | 14 |
| CAI-4 | Δura3::λ imm434/Δura3::λ imm434 | 14 |
| CAVAC8-1 | Δura3::λ imm434/Δura3::λ imm434, Δvac8::hisG-URA3-hisG/VAC8 | This study |
| CAVAC8-2 | Δura3::λ imm434/Δura3::λ imm434, Δvac8::hisG/VAC8 | This study |
| CAVAC8-3 | Δura3::λ imm434/Δura3::λ imm434, Δvac8::hisG/Δvac8::hisG-URA3-hisG | This study |
| CAVAC8-4 | Δura3::λ imm434/Δura3::λ imm434, Δvac8::hisG/Δvac8::hisG | This study |
| CAVAC8-5 | Δura3::λ imm434/Δura3::λ imm434, Δvac8::hisG/Δvac8::hisG, CIp-URA3-VAC8 | This study |
| CAVAC8-6 | Δura3::λ imm434/Δura3::λ imm434, Δvac8::hisG/Δvac8::hisG, CIp10-URA3 | This study |
DNA manipulation.
Based on partial genome sequence data available at the time, the primers 5′-GGATCCTCAAATTGACTTCATTACTTG and 5′-ATAGAGTTCAAAGAAATGCTACTGGGG were used to PCR amplify 1.1 kb of the VAC8 open reading frame (ORF) from the C. albicans genome. This product was cloned into pGEMT to create pGEMT-VAC8 and sequenced using ABI Prism Dye Terminator cycle sequencing kits (Perkin-Elmer) and an ABI 377 automated DNA sequencer. Subsequently, this sequence was used to identify the VAC8 locus in contig4-2360 from the Stanford C. albicans genomic database. The complete C. albicans VAC8 ORF, including 1 kb of 5′ sequence, was then PCR amplified using the primers 5′-CAAGAAGCTTTCAAAATGGG (HindIII site underlined) and 5′-CCCAGCTAGCACTGTTGGTCC (NheI site underlined), cloned into pGEMT, and resequenced. The HindIII-NheI VAC8 fragment was then cloned into a version of CIp10 (26) that contains the S. cerevisiae CYC1 terminator sequence (2) to create CIp-URA3-VAC8 (CAVAC8-5).
Strain construction.
To generate the vac8Δ::hisG-URA3-hisG disruption cassette, a short linker region (28 bp) containing unique HindIII and BglII sites was cloned between the Pf1mI and EconI sites of pGEMT-VAC8. Then the hisG-URA3-hisG cassette was released from pMB-7 (11) by digestion with HindIII and BglII and cloned between the HindIII and BglII sites in pGEMT-VAC8. This vac8Δ::hisG-URA3-hisG cassette was released from pGEMT with ApaI and SacI and transformed into C. albicans strain CAI-4 with selection for Ura+ colonies (11), thereby deleting codons 339 to 459 of the 585-codon VAC8 ORF. This generated the VAC8/vac8::hisG-URA3-hisG heterozygote CAVAC8-1 (Table 1). Ura3− VAC8/vac8::hisG segregants were then selected on YPD containing 5-fluoroorotic acid (11), thereby generating strain CAVAC8-2. The second VAC8 allele was disrupted with the vac8::hisG-URA3-hisG cassette to generate the vac8::hisG/vac8::hisG-URA3-hisG homozygote CAVAC8-3. Ura3− segregants were then selected as described before, generating the null mutant CAVAC8-4. The VAC8 gene was reintroduced into CAVAC8-4 on the plasmid CIp-VAC8. This plasmid was linearized with StuI and transformed into CAVAC8-4 to create CAVAC8-5. At each stage of the process, the genotype of strains was confirmed by diagnostic PCR and Southern blotting (36). Diagnostic PCR was carried out with a three-primer mix—VAC8 ATG (5′ ATGGGTGCCTGCTGTAGTTGTTTAGG 3′), VAC8 DEL (5′ AAGCGGAAATTTCCAGTTGAACTG 3′), and HisG (5′ ACGCGCAGGATATCAATCGGCATGTTTTC 3′)—to differentiate between wild-type and disrupted alleles.
Western blotting.
Protein extracts were prepared from cell cultures growing exponentially (OD600 = 0.5 to 0.7) in YPD at 30°C. Cells were washed twice in ice-cold sterile water, and protein extracts were prepared as described previously (46). Equal protein loadings from each sample were run on NuPAGE 4 to 12% Tris-N,N-methylenebisacrylamide gels in MOPS (morpholinepropanesulfonic acid) buffer and transferred onto polyvinylidene difluoride membranes according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Membranes were incubated with antibodies against S. cerevisiae Vac8p (46) and then with sheep anti-rabbit antibody conjugated to HRPO (Sigma) and developed using enhanced chemiluminescence (Amersham Pharmacia Biotech UK Ltd., Buckinghamshire, England).
Vacuole staining.
C. albicans yeast cells were stained with FM4-64 (Molecular Probes Europe BV), as described previously (44). Briefly, yeast cultures were grown in YPD to an OD600 of about 0.1, harvested, and resuspended in 0.1 ml of medium containing 40 μM FM4-64. Samples were incubated at 30°C for 40 min and then washed in YPD. Cells to be grown in the yeast form were then resuspended in 1 ml of fresh YPD and incubated for 2.5 h at 30°C before analysis of the inheritance of vacuoles. To visualize newly synthesized as well as inherited vacuoles, the chase period was reduced to 90 min. To visualize hyphal cells, samples were incubated with FM4-64 as described and then resuspended in 1% serum at 37°C for 2.5 h (or 1.5 h, where specified). Vacuoles were stained with CDCFDA [5- (and 6-) carboxy-2′,7′-dichlorofluorescein diacetate] (Molecular Probes Europe BV), as described previously (30). Cells were grown to early log phase, harvested, and resuspended in 1 ml of YCM (1% yeast extract, 1% bactopeptone, 2% dextrose) (35) containing 50 μM CDCFDA. Cells were incubated at 30°C for 30 min, collected by centrifugation, and viewed.
Growth analyses.
To analyze the growth of budding cells, C. albicans strains were grown overnight at 30°C in YPD, and these cells were used to inoculate YPD, YPD containing 2.5 M glycerol, and YPD containing 1.5 M NaCl at a starting OD of 0.1. Growth was monitored at 30°C and 37°C using the A600. For each condition, triplicate cultures were analyzed and the whole experiment was repeated three times. Only reproducible differences in doubling time are reported. To examine hyphal growth and branching, approximately 104 yeast cells from an overnight C. albicans culture were inoculated onto a poly-l-lysine (Sigma) slide. The slides were incubated overnight in 1% serum at 37°C, washed in H2O, and stained with calcofluor white, as described previously (16). Hyphal growth and branching were measured (1).
Microscopy.
Phase-contrast and fluorescence microscopy were performed using an Axioplan 2 microscope (Carl Zeiss Ltd., United Kingdom) with filter sets XF 66, XF 67, and XF 77 (Omega Optical Inc., Brattleboro, VT). Images were generated using a Hamamatsu charge-coupled-device camera.
RESULTS
Isolation and analysis of C. albicans VAC8.
The overall aim of this study was to test whether there is a causal relationship between vacuolar inheritance and hyphal branching in C. albicans. To achieve this we needed to generate a C. albicans mutant in which vacuole inheritance was disrupted. We targeted the VAC8 locus, because of its key role in vacuolar inheritance in S. cerevisiae (10, 29, 46).
Initially we identified the homologue of S. cerevisiae VAC8 on the basis of partial C. albicans genome sequence information. Subsequently, the complete VAC8 sequence became available within the Stanford C. albicans genome sequence database. The VAC8 locus was PCR amplified and resequenced, revealing a 100% match with the genome sequence (orf19.745; IPF9747.1). The predicted amino acid sequence of C. albicans Vac8p is 69% identical and 81% similar to that of S. cerevisiae Vac8p (Fig. 1). The most conserved regions are the Arm repeats, which are believed to be important for Vac8p function.
FIG. 1.
Comparison of the predicted C. albicans and S. cerevisiae Vac8 proteins. Arm repeats are indicated between vertical lines. The conserved amino-proximal cysteine residues important for palmitoylation (42, 47) and the conserved amino-terminal glycine residue involved in myristoylation (46) are indicated by boldface type.
C. albicans VAC8 gene disruption.
Both alleles of the C. albicans VAC8 locus were disrupted sequentially using a vac8::hisG-URA3-hisG cassette that deleted amino acids 339 to 459 and blocked the expression of the 585-amino-acid protein. The disruption of the locus was confirmed by Southern blotting (not shown) and diagnostic PCR (Fig. 2A). Furthermore, Western blotting with antibodies raised against S. cerevisiae Vac8p (46) indicated that no Vac8p was detectable in the deletion mutant (Fig. 2B). C. albicans vac8Δ cells were viable, suggesting that VAC8, like its homologue in S. cerevisiae, is not an essential gene (46).
FIG. 2.
Confirmation of VAC8 gene deletion. (A) Transformants from each round of disruption were screened by PCR using a mix of three primers: VAC8 ATG, VAC8 DEL, and HisG. VAC8 ATG and VAC8 DEL produce a band of 1.2 kb in the wild-type locus, and the product of VAC8 ATG and HisG is 1.3 kb in a disrupted locus. (B) Western blot of protein extracts from CAF-2, CAVAC8-1, CAVAC8-3, and CAVAC8-5. Equal protein extracts from each strain were loaded into a gel and transferred to a polyvinylidene difluoride membrane, as described in Materials and Methods. The blot was probed with ScVac8p antiserum.
Effect of disruption of VAC8 upon vacuolar inheritance of C. albicans.
Our rationale for targeting Vac8p was that, based on the function of its homologue in S. cerevisiae (10, 29, 46), it was likely to play an important role in the inheritance, rather than the synthesis, of vacuoles in C. albicans. To test this we compared the vacuoles in wild-type and vac8Δ cells.
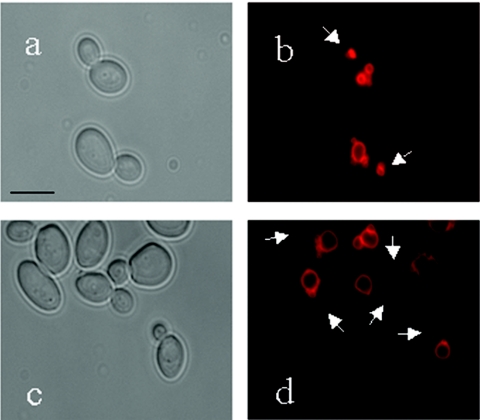
Vacuoles were visualized in budding cells by using a stable derivative of fluorescein diacetate (CDCFDA). The number of vacuoles per cell was determined for all cells within a field of view (Table 2). Wild-type (CAF2-1) and vac8Δ cells (CAVAC8-3) were compared. All wild-type buds contained visible vacuoles, but only 50% of the buds on vac8Δ cells contained visible vacuoles. Furthermore, more than four vacuoles were visible in most vac8Δ mother cells, and these were more fragmented than the vacuoles of wild-type mother cells. To gain greater insight into the morphology of the vacuoles in vac8Δ cells, we stained them with the lipophilic styryl dye FM4-64. The detectable vacuolar material was greatly reduced if not absent in vac8Δ daughter cells, compared with mother cells (Fig. 3). Furthermore, these vacuoles appeared to be more fragmented or multilobed than wild-type vacuoles. Hence, the inactivation of VAC8 caused defects in the vacuolar inheritance of budding C. albicans cells similar to those seen in S. cerevisiae vac8Δ mutants.
TABLE 2.
Effect of vac8Δ on vacuolar inheritance in budding cells
| Cell characteristic | % of cellsa
|
|
|---|---|---|
| Wild type | vac8Δ | |
| Buds with vacuoles | 100 | 53 |
| Mother cell with 1-3 vacuoles | 56 | 19 |
| Mother cell with ≥4 vacuoles | 44 | 81 |
Wild-type and CAVAC8-3 cells were stained with CDCFDA. The absence or presence of a vacuole(s) was recorded as a percentage of the total number of cells (n = 50 [each]).
FIG. 3.
Effect of vac8Δ upon vacuolar inheritance in budding cells. Cells were stained with FM4-64 and then visualized after a 2.5-h chase period. Microscopy of budding cells is as follows: phase-contrast image of CAF2-1 cells (a), FM4-64-stained CAF2-1 cells (b), phase-contrast image of CAVAC8-3 cells (c), and FM4-64-stained CAVAC8-3 cells (d). Arrows indicate vacuolar material inherited by daughter cells. Bar, 10 μm.
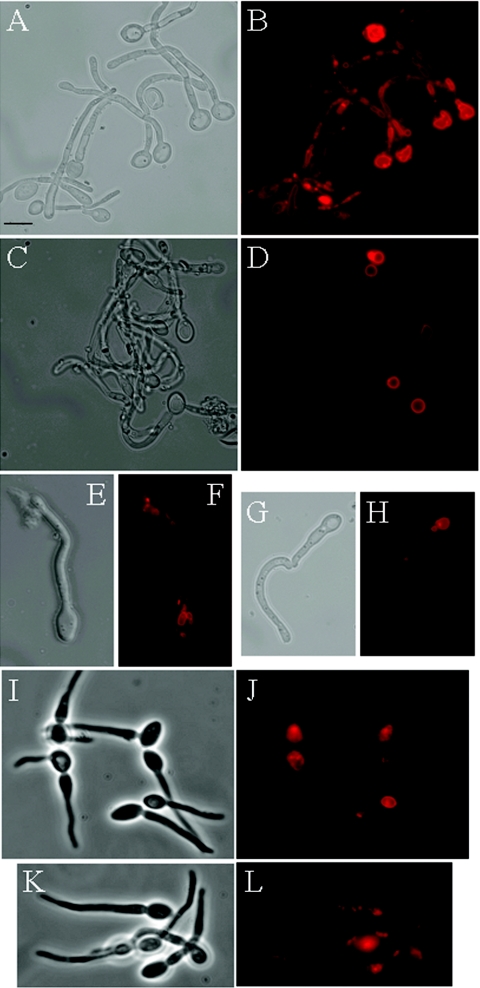
Vacuolar inheritance was also examined in hyphal cells (Fig. 4). VAC8 and vac8Δ cells were stained with FM4-64 and then induced to form hyphae in 1% serum at 37°C for 2.5 h. Vacuolar staining was evident throughout the length of the wild-type hyphae. The vacuolar staining in vac8Δ hyphae was significantly reduced in comparison with the wild-type hyphae. Furthermore, for vac8Δ cells, most staining was observed in the mother cell, and minimal staining was observed in subapical compartments. Normal vacuole staining was visualized in both yeast and hyphal forms in the reintegrant strain CAVAC8-5 (Fig. 4L). We conclude that, as predicted, the inactivation of VAC8 caused defects in vacuolar inheritance in both the yeast and hyphal growth forms of C. albicans.
FIG. 4.
Effect of vac8 deletion upon vacuolar inheritance in hyphal cells. Hyphal cells were stained with FM4-64 and then visualized after a chase period. (A to H) To visualize inherited vacuoles, a chase period of 2.5 h was used. Shown are a phase-contrast image (A) and FM4-64-stained (B) CAF2-1 hyphal cells. (C, E, and G) Phase-contrast image of CAVAC8-3 cells. (D, F, and H) FM4-64-stained CAVAC8-3 cells. Vacuolar inheritance was restored in CAVAC8-5 cells (not shown). (I to L) A shorter chase period (90 min) was used to visualize newly synthesized as well as inherited vacuoles. Shown are a phase-contrast image (I) and FM4-64-stained (J) CAVAC8-3 cells and a phase-contrast image (K) and FM4-64-stained (L) CAVAC8-5 cells. Bar, 10 μm.
Effects of disruption of VAC8 upon growth of C. albicans in the yeast form.
Our next objective was to determine whether C. albicans cell division was affected by the vac8 deletion. This was analyzed in both yeast and hyphal cells using Ura3+ cells, because ura3 mutants are known to display subtle hyphal growth defects (4, 8, 40).
No significant differences in the growth rates of wild-type and vac8Δ cells were detecttheed on YPD plates at 30°C (not shown). However, vacuolar dysfunction is known to cause sensitivity to osmotic stress and/or increased temperature (5, 28, 46). Therefore, we tested the sensitivity of vac8Δ cells to osmotic stress and elevated temperatures during growth in the yeast form. There were no detectable differences in the growth of wild-type (CAF2-1) and vac8Δ (CAVAC8-3) cells on YPD plates containing 1.5 M NaCl or 2.5 M glycerol at 30°C or 37°C (not shown). However, subtle differences were reproducibly observed in liquid cultures. The doubling time of vac8Δ cells was 1.25-fold longer than that of wild-type cells in YPD containing 1.5 M NaCl at 30°C. A twofold difference in growth rate was observed in this medium at 37°C. Similar differences in growth rate were observed when osmostress was applied with 2.5 M glycerol. At 30°C the doubling time of vac8Δ cells was 1.25-times longer than that of wild-type cells, and at 37°C this difference was increased to 1.5-fold. Therefore, the aberrant vacuolar inheritance of budding vac8Δ cells correlated with a slight increase in sensitivity to osmotic stress and temperature.
Effects of the VAC8 deletion upon hyphal growth.
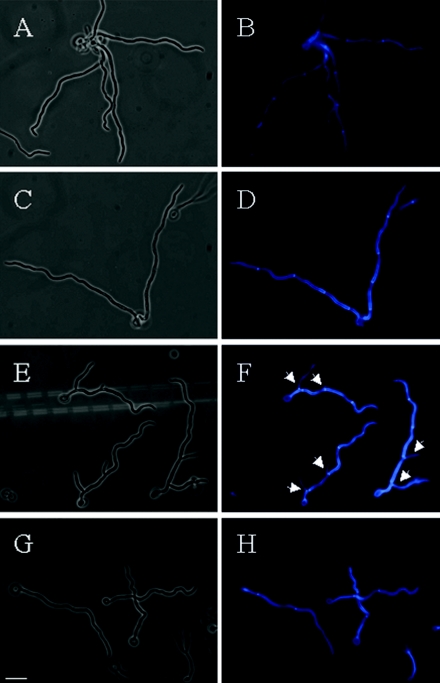
To quantify the effects of the VAC8 deletion upon hyphal development, wild-type (CAF2-1), heterozygous VAC8/vac8Δ (CAVAC8-1), homozygous vac8Δ/vac8Δ (CAVAC8-3), and reintegrant vac8Δ/vac8Δ/VAC8 (CAVAC8-5) cells were immobilized on poly-l-lysine slides and incubated in 1% serum at 37°C for 14 h. Hyphal development was stimulated with 1% serum because we had observed the strongest correlation between vacuolation and hyphal division under these conditions (1). The cells were stained with calcofluor white, which interchelates with chitin, to visualize cell walls and septa (Fig. 5). All hyphal cells within the field of view were counted. There was no significant difference in the number of compartments in VAC8 and vac8Δ hyphae over the duration of the experiment (Fig. 6A). However, there was a significant increase in the proportion of branched hyphae for vac8Δ cells compared with wild-type, heterozygous, and reintegrant cells (Fig. 6B). Furthermore, there was a significant increase in the number of branches emanating from the primary hyphae of the vac8Δ strain compared with the control strains (Fig. 6C). Significantly more branches were evident in CAVAC-3 hyphae than in CAF-2, CAVAC8-1, or CAVAC8-5 hyphae (Fig. 5 and 6). Similar data were generated in three independent experiments. Therefore, the inactivation of VAC8 caused a significant increase in hyphal branching. This was consistent with a role for vacuolar inheritance in the regulation of hyphal cell division.
FIG. 5.
Branching phenotype of hyphal VAC8 and vac8Δ cells. Individual cells were examined microscopically following calcofluor white staining. Phase-contrast images are shown on the left; calcofluor white-stained cells are shown on the right. (A and B) CAF2-1 hyphal cells. (C and D) CAVAC8-3 cells. (E and F) CAVAC8-3 cells. (G and H) CAVAC8-5 cells. Bar, 20 μm.
FIG. 6.
Quantification of hyphal growth and division of VAC8 and vac8 cells. The phenotypes of wild-type (CAF-2), VAC8/vac8 (CAVAC8-1), vac8/vac8 (CAVAC8-5), and vac8/vac8/VAC8 (CAVAC8-5) cells were quantified using the image types illustrated in Fig. 5. (A) Total length of hyphae determined by total number of compartments. Data are for length of the primary hypha only (n = 100). (B) Percent branched and unbranched hyphae. Only true branches were counted (n = 100). Bud-shaped or pseudohyphally shaped filaments were discounted. (C) Degree of branching was determined by counting the number of true branches emanating from the primary hypha (n = 300). Branching from secondary hyphae was not included in this analysis.
DISCUSSION
In true, unconstricted C. albicans hyphae, there is a correlation between cell cycle progression and vacuolar status (1). The more vacuolated the subapical cell, the greater the delay in cell division before a new branch is formed. We have shown previously that, under nutrient-limiting conditions that induce long, sparsely branched hyphae (for example, in media containing a low serum concentration), the apical compartment inherits most of the cytoplasmic material while subapical compartments inherit mostly vacuolar material. Hence, subapical cells are highly vacuolated, and they are also arrested in G1, prior to the point in the cell cycle at which a new branch would emerge from these subapical compartments (1). The addition of nutrients leads to a decrease in vacuole volume and an increase in cytoplasmic content, which correlates with increased hyphal branching. Hence, we proposed previously that there is a causal relationship between vacuolar status and cell cycle progression in subapical compartments (1). In this study, we set out to test this hypothesis. Our approach was to disturb vacuolar inheritance by inactivating VAC8 and then to examine the effects upon hyphal growth.
The vacuolar phenotype of C. albicans vac8Δ cells was similar to that visualized in S. cerevisiae (10, 29, 46). In S. cerevisiae, the vacuoles of vac8 null mutants display a fragmented or multilobed appearance and a reduced degree of vacuolar inheritance. The C-terminal truncation of S. cerevisiae Vac8p inhibits vacuolar inheritance, and the severity of this phenotype correlates with the extent of truncation (46). A deletion in S. cerevisiae VAC8 that is analogous to the deletion we created in C. albicans VAC8 causes a dramatic inhibition of vacuolar inheritance in S. cerevisiae (Fig. 3; Table 2). The Arm repeats within the Vac8p protein (Fig. 1) are believed to be important for the interaction of the vacuole with actin (20). Movement along actin filaments directs the segregation structure toward the daughter cell. The localization of Vac8p on the surface of the vacuole membrane is essential to its interaction with the Myo2p-specific receptor Vac17p. This specific three-way interaction provides spatial and temporal control over vacuolar inheritance. Although vacuole inheritance is not completely abolished in the C. albicans vac8Δ cells, it is greatly reduced, implying that Vac8p plays a role in C. albicans similar to that of its S. cerevisiae homologue.
C. albicans vac8Δ mutants still undergo morphogenesis to form true hyphae (Fig. 4). The fact that vac8Δ hyphae grow with normal extension rates suggests that Vac8p inactivation does not inhibit intracellular protein trafficking and vesicular delivery of cell wall synthetic material to the growing tip.
The vac8Δ hyphae were more branched under low-serum conditions than were wild-type cells (Fig. 5). An increased number of secondary hyphae emerged from the subapical compartments of the vac8Δ primary hypha (Fig. 5C). In addition, these cells exhibited tertiary and quaternary branching, unlike the control VAC8 cells (data not shown). We conclude that disturbing vacuolar inheritance causes a defect in hyphal branching patterns in C. albicans (Fig. 6). Aberrant vacuolar inheritance releases subapical cells from the delay in cell cycle progression that is normally observed in hyphae under nutrient-limiting conditions.
Bruckmann and coworkers isolated and characterized the C.albicans VPS34 gene, which encodes a phosphatidylinositol 3-kinase (5, 6). C. albicans vps34Δ cells have an enlarged vacuole (5, 6), but any adverse affects of Vps34p disruption on the recycling of cell surface receptors and signal transduction are currently unknown. On the basis of our model (Fig. 6), which relates cell vacuolation to cellular division, one would expect vps34Δ cells to display a delay in hyphal branching. Interestingly, C. albicans vps34Δ cells do exhibit delayed hyphal formation in liquid serum medium and an inability to switch on solid Spider medium and solid serum-containing medium (5). We note that a mechanistic link between endocytosis and the Rim101 pathway exists through Snf7 (23) and that Rim101 signaling regulates morphogenesis in response to ambient pH (31). However, other endocytosis mutants do not affect Rim101 signaling (23), and therefore it seems likely that, in general, the hyphal defects of vacuole mutants are not mediated by effects on the Rim101 pathway.
Another C. albicans vacuolar protein-sorting mutant (vps11) has been studied extensively by Palmer et al. (28). This mutant also shows defective hyphal formation in addition to increased sensitivity to temperature, salt, and osmotic stresses. The vps11 null mutant exhibits diffuse vacuolar staining indicative of a vacuolar biogenesis defect. C. albicans vps11Δ cells contain small amounts of vacuolar material, and they are unable to form germ tubes as efficiently as wild-type cells. It is not known whether this phenotype is due to the physical lack of a vacuole, to the loss of multiple transport steps in vacuolar protein delivery, to the loss of Kex2p activity, or simply to a temperature-related growth defect. As this mutant exhibits multiple phenotypes, it is difficult to attribute the defect in hyphal formation to vacuolar biogenesis.
An essential gene that results in altered budding growth (ABG1) has recently been described by Veses and coworkers (43). In hyphal cells, a null mutant displays small, fragmented vacuoles, which is concurrent with an increased branching frequency. This observation is entirely consistent with our working model in which decreased vacuolar content causes an increase in hyphal cell division.
In this study, we examined the effects of mutations in a gene involved in vacuolar inheritance rather than in vacuolar biogenesis. Indeed, C. albicans vac8Δ cells displayed normal growth rates in the absence of osmotic stress. Therefore, we were able to distinguish the effects of disrupting VAC8 on growth rate from those on cell division. Our data strongly support the hypothesis that cytoplasmic volume, rather than total cell volume, is a critical determinant for entry into S phase (1). Highly vacuolated subapical cells do not enter S phase until the cytoplasmic volume increases sufficiently to traverse Start (Fig. 7). This model might be generally applicable to the eukaryotic cell division cycle.
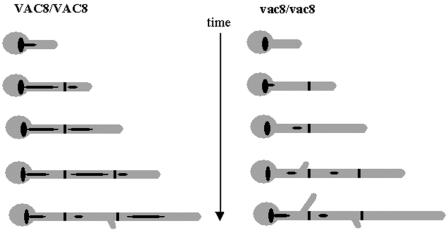
FIG. 7.
Model of a growing hypha. The septa are depicted as thick black lines, and the vacuoles are black ellipsoid shapes. As the growing hypha extends and divides, the subapical compartment inherits the majority of the vacuole and the apical cell inherits little. As a direct result of this, the apical cell can continue growing without delay and enter the next stage in the cell cycle. The subapical cell, however, does not have sufficient cell density to pass through Start. Hence, there is a delay in branching that correlates with the degree of vacuolation.
Acknowledgments
We thank Lois Weisman (Department of Biochemistry, University of Iowa, Iowa City, Iowa) for providing S. cerevisiae vac8 strains and for communicating data prior to publication. We are also grateful to Norbert Schnell (AstraZeneca) for providing early access to the C. albicans VAC8 sequence.
This work was supported by the U.K. Biotechnology and Biological Sciences Research Council (PAC02657), the Wellcome Trust (055015), and the European Commission.
REFERENCES
- 1.Barelle C. J., E. A. Bohula, S. J. Kron, D. Wessels, D. R. Soll, A. Schäfer, A. J. P. Brown, and N. A. R Gow. 2003. Asynchronous cell cycle and asymmetric vacuolar inheritance in true hyphae of Candida albicans. Eukaryot. Cell 2:398-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barelle, C. J., C. L. Manson, D. M. MacCallum, F. C. Odds, N. A. R. Gow, and A. J. P. Brown. 2004. GFP as a quantitative reporter of gene regulation in Candida albicans. Yeast 21:333-340. [DOI] [PubMed] [Google Scholar]
- 3.Bonangelino, C. J., N. L. Catlett, and L. S. Weisman. 1997. Vac7p, a novel vacuolar protein, is required for normal vacuolar inheritance and morphology. Mol. Cell. Biol. 17:6847-6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand, A., D. M. MacCallum, A. J. Brown, N. A. Gow, and F. C. Odds. 2004. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot. Cell 3:900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruckmann, A., W. Kunkel, A. Hartl, R. Wetzker, and R. Eck. 2000. A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth and virulence. Microbiology 146:2755-2764. [DOI] [PubMed] [Google Scholar]
- 6.Bruckmann, A., W. Kunkel, K. Augsten, R. Wetzker, and R. Eck. 2001. The deletion of CAVPS34 in the human pathogenic yeast Candida albicans causes defects in vesicle-mediated protein sorting and nuclear segregation. Yeast 18:343-353. [DOI] [PubMed] [Google Scholar]
- 7.Catlett, N. L., and L. S. Weisman. 2000. Divide and multiply: organelle partitioning in yeast. Curr. Opin. Cell Biol. 12:509-516. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, S., M. H. Nguyen, Z. Zhang, H. Jia, M. Handfield, and C. J. Clancy. 2003. Evaluation of the roles of four Candida albicans genes in virulence by using gene disruption strains that express URA3 from the native locus. Infect. Immun. 71:6101-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Backer, M. D., P. T. Magee, and J. Pla. 2000. Recent developments in molecular genetics of Candida albicans. Annu. Rev. Microbiol. 54:463-498. [DOI] [PubMed] [Google Scholar]
- 10.Flekenstein, D., M. Rhode, D. J. Klionsky, and M. Rudiger. 1998. Yel013p (Vac8p), an armadillo repeat protein related to plakoglobin and importin alpha, is associated with the yeast vacuole membrane. J. Cell Sci. 111:3109-3118. [DOI] [PubMed] [Google Scholar]
- 11.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franke, W. W., M. D. Goldschmidt, R. Zimblemann, H. M. Mueller, D. L. Schiller, and P. Cowin. 1989. Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc. Natl. Acad. Sci. USA 86:4027-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes de Mesquita, D. S., R. ten Hoopen, and C. L. Woldringh. 1991. Vacuolar segregation to the bud of Saccharomyces cerevisiae: an analysis of morphology and timing in the cell cycle. J. Gen. Microbiol. 137:2447-2454. [DOI] [PubMed] [Google Scholar]
- 14.Gomes de Mesquita, D. S., B. van den Haazel, J. Bouwman, and C. L. Woldringh. 1996. Characterization of new vacuolar segregation mutants, isolated by screening for loss of proteinase B self-activation. Eur. J. Cell Biol. 71:237-247. [PubMed] [Google Scholar]
- 15.Gomes de Mesquita, D. S, J. Shaw, J. A. Grimbergen, M. A. Buys, L. Dewi, and C. L. Woldringh. 1997. Vacuole segregation in the Saccharomyces cerevisiae vac2-1 mutant: structural and biochemical quantification of the segregation defect and formation of new vacuoles. Yeast 13:999-1008. [DOI] [PubMed] [Google Scholar]
- 16.Gow, N. A. R., and G. W. Gooday. 1982. Growth kinetics and morphology of colonies of the filamentous form of Candida albicans. J. Gen. Microbiol. 128:2187-2198. [DOI] [PubMed] [Google Scholar]
- 17.Gow, N. A. R., and G. W. Gooday. 1982. Vacuolation, branch production and linear growth of germ tubes of Candida albicans. J. Gen. Microbiol. 128:2195-2198. [DOI] [PubMed] [Google Scholar]
- 18.Gow, N. A. R. 1997. Germ tube growth of Candida albicans. Curr. Top. Med. Mycol. 8:43-55. [PubMed] [Google Scholar]
- 19.Gow, N. A. R., A. J. P. Brown, and F. C. Odds. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5:366-371. [DOI] [PubMed] [Google Scholar]
- 20.Hill, K. L., N. L. Catlett, and L. S. Weisman. 1996. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J. Cell Biol. 135:1535-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 22.Johnston, G. G., J. A. Pendergast, and R. A. Singer. 1991. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 3:539-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kullas, A. L., L. Mingchun, and D. A. Davis. 2004. Snf7, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans. Eukaryot. Cell 3:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macrea, P., C. Turck, and B. Gumbiner. 1991. A homologue of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254:1359-1361. [DOI] [PubMed] [Google Scholar]
- 25.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 26.Murad, A. M. A., P. R. Lee, I. D. Broadbent, C. J. Barelle, and A. J. P. Brown. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325-327. [DOI] [PubMed] [Google Scholar]
- 27.Odds, F. C. 1988. Candida and candidosis. Ballière Tindall, London, England.
- 28.Palmer, G. E., A. Cashmore, and J. Sturtevant. 2003. Candida albicans VPS11 is required for vacuole biogenesis and germ tube formation. Eukaryot. Cell 2:411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan, X., and D. D. Goldfarb. 1998. YEB3/VAC8 encodes a myristylated armadillo protein of the Saccharomyces cerevisiae vacuolar membrane that functions in vacuolar fusion and inheritance. J. Cell Sci. 111:2137-2147. [DOI] [PubMed] [Google Scholar]
- 30.Pringle, J. R., R. A. Preston, A. E. M. Adams, T. Stearns, D. G. Drubin, B. K. Haarer, and E. W. Jones. 1989. Fluorescence microscopy methods for yeast. Methods Cell Biol. 31:357-435. [DOI] [PubMed] [Google Scholar]
- 31.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-regulated transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raymond, C. K., P. J. O'Hara, G. Eichinger, J. H. Rothman, and T. H. Stevens. 1990. Molecular analysis of the yeast VPS3 gene and the role of its product in vacuolar protein sorting and vacuolar segregation during the cell cycle. J. Cell Biol. 111:877-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raymond, C. K., I. Howald-Stevenson, C. A. Vater, and T. H. Stevens. 1992. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3:1389-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riggleman, B. E., E. Wieschaus, and P. Schedl. 1989. Molecular analysis of the Armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with the Drosophila segment polarity gene. Genes Dev. 3:96-113. [DOI] [PubMed] [Google Scholar]
- 35.Rogers, D., and H. Bussey. 1978. Fidelity of conjugation in Saccharomyces cerevisiae. Mol. Gen. Genet. 162:173-182. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Scott, S. V., D. C. Nice III, J. J. Nau, L. S. Weisman, Y. Kamada, I. Keizer-Gunnick, T. Funakoshi, M. Veenhuis, Y. Ohsumi, and D. J. Klionsky. 2000. Apg13p and Vac8p are part of a complex of phophosproteins that are required for cytoplasm to vacuole targeting. J. Biol. Chem. 275:25840-25849. [DOI] [PubMed] [Google Scholar]
- 38.Shaw, J. M., and W. Wickner. 1991. vac-2: a yeast mutant which distinguishes vacuole segregation from Golgi-to-vacuole protein targeting. EMBO J. 10:1741-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 40.Sundstrom, P., J. E. Cutler, and J. F. Staab. 2002. Reevaluation of the role of HWP1 in systemic candidiasis by use of Candida albicans strains with selectable marker URA3 targeted to the ENO1 locus. Infect. Immun. 70:3281-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang, F., E. J. Kauffman, J. L. Novak, J. J. Nau, N. L. Catlett, and L. S. Weisman. 2003. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature 422:87-92. [DOI] [PubMed] [Google Scholar]
- 42.Veit, M., R. Laage, L. Dietrich, L. Wang, and C. Ungermann. 2001. Vac8p release from the SNARE complex and its palmitylation are coupled and essential for vacuole fusion. EMBO J. 20:3145-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veses, V., M. Casanova, A. Murgui, A. Dominguez, N. A. R. Gow, and J. P. Martinez. 2005. ABG1, a novel and essential Candida albicans gene encoding a vacuolar protein involved in cytokinesis and hyphal branching. Eukaryot. Cell 4:1088-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vida, T. A., and S. D. Emr. 1995. A new vital stain for visualising vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Y.-X., H. Zhao, T. M. Harding, D. S. Gomes de Mesquita, C. L. Woldringh, D. J. Klionsky, A. L. Munn, and L. S. Weisman. 1996. Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol. Biol. Cell 7:1375-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Y.-X., N. L. Catlett, and L. S. Weisman. 1998. Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from cytoplasm to vacuole. J. Cell Biol. 140:1063-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, Y.-X., E. J. Kauffman, J. E. Duex, and L. S. Weisman. 2001. Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem. 276:35133-35140. [DOI] [PubMed] [Google Scholar]
- 48.Warren, G., and W. Wickner. 1996. Organelle inheritance. Cell 84:395-400. [DOI] [PubMed] [Google Scholar]
- 49.Weisman, L. S., R. Bacallao, and W. Wickner. 1987. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J. Cell Biol. 105:1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisman, L. S., and W. Wickner. 1988. Intervacuolar exchange in the yeast zygote: a new pathway in organelle communication. Science 241:589-591. [DOI] [PubMed] [Google Scholar]
- 51.Weisman, L. S., S. D. Emr, and W. Wickner. 1990. Mutants of Saccharomyces cerevisiae that block intervacuole vesicular traffic and vacuole division and segregation. Proc. Natl. Acad. Sci. USA 87:1076-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weisman, L. S. 2003. Yeast vacuole dynamics and inheritance. Annu. Rev. Genet. 37:435-460. [DOI] [PubMed] [Google Scholar]
- 53.Wickner, W., and A. Haas. 2000. Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem. 69:247-275. [DOI] [PubMed] [Google Scholar]
- 54.Wickner, W. 2002. Yeast vacuoles and membrane fusion pathways. EMBO J. 21:1241-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]