Abstract
Intracranial infection of C57BL/6 mice with mouse hepatitis virus (MHV) results in an acute encephalomyelitis followed by a demyelinating disease similar in pathology to the human disease multiple sclerosis (MS). CD4+ T cells are important in amplifying demyelination by attracting macrophages into the central nervous system (CNS) following viral infection; however, the mechanisms governing the entry of these cells into the CNS are poorly understood. The role of chemokine receptor CCR5 in trafficking of virus-specific CD4+ T cells into the CNS of MHV-infected mice was investigated. CD4+ T cells from immunized CCR5+/+ and CCR5−/− mice were expanded in the presence of the immunodominant epitope present in the MHV transmembrane (M) protein encompassing amino acids 133 to 147 (M133-147). Adoptive transfer of CCR5+/+-derived CD4+ T cells to MHV-infected RAG1−/− mice resulted in CD4+-T-cell entry into the CNS and clearance of virus from the brain. These mice also displayed robust demyelination correlating with macrophage accumulation within the CNS. Conversely, CD4+ T cells from CCR5−/− mice displayed an impaired ability to traffic into the CNS of MHV-infected RAG1−/− recipients, which correlated with increased viral titers, diminished macrophage accumulation, and limited demyelination. Analysis of chemokine receptor mRNA expression by M133-147-expanded CCR5−/−-derived CD4+ T cells revealed reduced expression of CCR1, CCR2, and CXCR3, indicating that CCR5 signaling is important in increased expression of these receptors, which aid in trafficking of CD4+ T cells into the CNS. Collectively these results demonstrate that CCR5 signaling is important to migration of CD4+ T cells to the CNS following MHV infection.
T-cell infiltration into the central nervous system (CNS), in response to viral infection, is important in host defense by eliminating infectious virus through either secretion of antiviral cytokines or killing of virus-infected cells (35-37, 51). For example, activated virus-specific T cells can release antiviral cytokines such as interleukin-12 and gamma interferon (IFN-γ), which have been shown to exert a protective effect following infection of the CNS with various neurotropic viruses, including measles virus, Theiler's virus, herpes simplex virus type I, and lymphocytic choriomeningitis virus (8, 17, 18, 32, 50). The antiviral mechanisms by which IFN-γ acts vary but may include inhibiting viral replication through induction of antiviral products as well as increasing antigen presentation by increased expression of major histocompatibility complex class I and II molecules on infected cells (1, 7, 11, 41). Other mechanisms by which T cells participate in host defense following viral infection of the CNS include the CD8+-T-cell-mediated release of perforin as well as engagement of the Fas-FasL pathway and activation of CD4+ T cells through CD40 ligand expression (2, 30, 34). In addition, recent studies indicate that T cells are also important in contributing to the neuropathology of virus-induced CNS diseases through a variety of different mechanisms, including release of cytokines that may exert a damaging or toxic effect on resident glial cells (7, 16, 29). Given the importance of T cells in both host defense and disease, it is imperative to better understand the cellular and molecular events governing T-cell migration and accumulation within the CNS following viral infection. In recent years, there has been increasing evidence that points to an important role for chemokine receptors in both host defense and disease by allowing T cells to traffic to sites of viral infection where they may contribute to host defense and/or pathology (3, 5, 10, 21, 23, 24, 26, 40).
We have used a model of virus-induced neurologic disease to better understand the contributions of chemokines and chemokine receptors in host defense and disease development within the context of the CNS. Intracerebral infection of susceptible mice with neuroadapted strains of mouse hepatitis virus (MHV), a positive-strand RNA virus, results in an acute encephalomyelitis followed by a chronic demyelinating disease similar to the pathology of the human demyelinating disease multiple sclerosis (MS) (13, 20). The collective evidence indicates that the immune response to MHV infection is critical in host defense as well as in the development of demyelination (21, 28, 38, 39, 47, 53). Both CD4+ and CD8+ T lymphocytes are important in clearance of infectious virus from the brain during acute disease (21, 28, 53, 55). Infection of either CD4−/− or CD8−/− mice has been shown to result in delayed clearance of virus from the brain and increased mortality (21). In addition, depletion of T-cell subsets increased the viral burden in the brain and inhibited protection from death (46, 52). The mechanisms by which virus-specific T cells contribute to clearance of virus from the CNS include cytotoxic-T-lymphocyte activity and secretion of antiviral cytokines such as IFN-γ (22, 36). In addition, recent studies point to a role for CD4+ and CD8+ T lymphocytes in contributing to macrophage activation and infiltration into the CNS that leads to myelin destruction (21, 38, 39, 53). Activated T cells can influence macrophage accumulation within the CNS through the release of cytokines such as IFN-γ, which has recently been demonstrated to influence the course of disease within the CNS of MHV-infected mice (38, 39). Therefore, understanding how T cells traffic into the CNS in response to MHV infection remains an important question with respect to both host defense and disease development.
Recent studies from our laboratory have clearly demonstrated an important role for both chemokines and their receptors in contributing to leukocyte infiltration into the CNS following MHV infection (5, 10, 21, 23, 24, 26). CCR5 is a member of the CC chemokine receptor family that has previously been shown to regulate both T-lymphocyte and macrophage infiltration into the CNS of MHV-infected mice (10). Chemokines that are capable of binding to CCR5 include CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES), all of which have been shown to be expressed within the CNS following MHV infection (10, 19, 57). Moreover, we have determined that antibody-mediated depletion of CCL5 results in impaired T-cell and macrophage infiltration into the CNS of MHV-infected mice and diminished demyelination, suggesting that CCR5 signaling may be important in disease pathogenesis (21). In order to further increase our understanding of how CCR5 regulates host defense and disease pathogenesis, viral antigen-specific CD4+ T cells were generated from both CCR5+/+ and CCR5−/− mice and adoptively transferred into MHV-infected RAG1−/− mice. The results presented clearly demonstrate that CCR5 signaling is important in allowing CD4+ T cells to traffic into the CNS and eliminate virus as well as in amplifying the severity of demyelination by increasing macrophage accumulation within the CNS.
MATERIALS AND METHODS
Virus and mice.
MHV strain J2.2V-1 was kindly provided by John Fleming, University of Wisconsin, Madison. Age-matched (5 to 7 weeks) CCR5+/+ and CCR5−/− mice (eighth generation backcrossed to C57BL/6, H-2b background; kindly provided by W. Kuziel, University of Texas, Austin) were used for all experiments (10). PCR analysis of DNA obtained from tail preparations confirmed that CCR5 was not expressed in CCR5−/− mice. Following anesthetization by inhalation of methoxyflurane (Pitman-Moore Inc., Washington Crossing, N.J.), mice were injected intracranially (i.c.) with 1,000 PFU of MHV suspended in 30 μl of sterile saline. Control (sham-infected) animals were injected with 30 μl of sterile saline alone. Animals were sacrificed at defined time points, and brains and spinal cords were removed for analysis in studies described below. One half of each brain at each time point was used for plaque assay on the DBT astrocytoma cell line to determine viral burden (12, 19). The remaining halves were either used for histologic analysis, stored at −80°C for RNA isolation, or used for fluorescence-activated cell sorter analysis (21).
Generation of M133-147-specific CD4+ T cells.
CCR5+/+ and CCR5−/− mice were immunized intraperitoneally with 2 × 105 PFU of MHV, and animals were sacrificed at day 8 postimmunization. Spleens were harvested, and a population of cells was obtained through use of a magnetically labeled antibody specific for the CD8 antigen followed by passage over a magnetic column (Miltenyi Biotec, Auburn, Calif.). Cells separated with the CD8 antibody are considered CD4-enriched T cells, which was confirmed by flow cytometric analysis. The CD4-enriched population of cells were incubated in high-glucose Dulbecco modified Eagle medium containing 5 × 10−5 M 2-mercaptoethanol and 10% fetal bovine serum at a concentration of 5 × 106 cells/ml, and a 5 μM concentration of the CD4 immunodominant peptide corresponding to the viral transmembrane (M) protein spanning amino acids 133 to 147 (M133-147) was added (54). Following 2 days of culture in the presence of peptide, T-stim (BD Biosciences, Bedford, Mass.) was added to the culture according to the manufacturer's specifications and the cells were incubated for an additional 4 days. Live cells were separated by using Lympholyte-M (Cedarlane Laboratories Limited, Hornby, Ontario, Canada).
Intracellular cytokine staining.
Intracellular staining for IFN-γ was performed on peptide-expanded CD4+ T cells by using a previously described procedure (5, 53). In brief, following a 6-h incubation at 37°C in medium containing GolgiStop (Cytofix/Cytoperm kit; PharMingen, San Diego, Calif.), cells were washed and blocked with phosphate-buffered saline containing 10% fetal bovine serum and a 1:200 dilution of CD 16/32 (PharMingen). Cells were then stained for surface antigens with either fluorescein isothiocyanate (FITC)-conjugated CD4 (PharMingen) or rat immunoglobulin G2b for 45 min at 4°C. Cells were fixed and permeabilized by using the Cytofix/Cytoperm kit and stained for intracellular IFN-γ by using phycoerythrin (PE)-conjugated anti-IFN-γ (1:50; XMG1.2; PharMingen) for 45 min at 4°C. Cells were analyzed on a FACStar (Becton Dickinson, Mountain View, Calif.). Data are presented as the percentage of positive cells within the gated population.
Adoptive transfer.
M133-147-expanded CD4+-enriched T cells were adoptively transferred (2.5 × 106 cells suspended in 100 μl sterile of Hanks balanced salt solution [HBSS]) via intravenous (i.v.) injection into the retro-orbital sinuses of RAG1−/− mice 3 days following i.c. infection with 1,000 PFU of MHV (5). Mice were sacrificed at 9 days posttransfer (12 days postinfection [p.i.]), and brains and spinal cords were removed. One half of each brain was used for flow analysis, and the remaining halves were used to determine viral titers. Spinal cords were removed and stained with luxol fast blue (LFB) to assess the severity of demyelination. Control animals included MHV-infected (i.c.) RAG1−/− mice receiving i.v. sterile HBSS.
Mononuclear cell isolation and flow cytometry.
Cells were obtained from brains of experimental groups of RAG1−/− mice at 12 days p.i., and a single-cell suspension was obtained by using a previously described method (10). Antibodies used for immunophenotyping cells in these studies included FITC-conjugated rat anti-mouse CD4 (PharMingen), F4/80 (Serotec), and PE-conjugated rat anti-mouse CD45 (PharMingen) (10). In all cases, an isotype-matched FITC-conjugated antibody was used. Cells were incubated with antibodies for 1 h at 4°C, washed, and fixed in 1% paraformaldehyde. Following fixation, cells were analyzed on a FACStar. The data presented represent the number of positive cells present within the gated population (10, 21, 24).
RNase protection assay.
Total RNA was obtained by using Trizol reagent (Invitrogen Corporation, Carlsbad, Calif.), from CD4+ T cells expanded to the M133-147 epitope as well as from brains of RAG1−/− mice receiving CD4+ T cells from either CCR5+/+ or CCR5−/− mice. Chemokine receptor mRNA transcripts were analyzed by using a custom chemokine receptor multitemplate probe set (PharMingen). Chemokine transcripts were analyzed by using the mCK-5 multitemplate probe set (PharMingen). RNase protection assay analysis was performed with 15 μg of total RNA by using a previously described protocol (10, 19, 21). A probe for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was included to verify consistency in RNA loading and assay performance. For quantification of signal intensity, autoradiographs were scanned and individual chemokine or chemokine receptor transcript signals were normalized as the ratio of band intensity to that of the GAPDH control (10, 19, 21, 24). Analysis was performed using NIH Image 1.61 software.
Histology.
Spinal cords were removed at 12 days p.i. and fixed by immersion overnight in 10% normal buffered formalin for paraffin embedding. The severity of demyelination was determined by LFB staining of spinal cords and analyzed with a light microscope. LFB-stained spinal cord sections were coded and read blindly by two investigators. Demyelination was scored as follows: 0, no demyelination; 1, mild inflammation accompanied by loss of myelin integrity; 2, moderate inflammation with increasing myelin damage; 3, numerous inflammatory lesions accompanied by significant increase in myelin stripping; and 4, intense areas of inflammation accompanied by numerous phagocytic cells engulfing myelin debris. An average of five spinal cords were scored per group, with a minimum of six 10-μm sections per mouse investigated. Scores were averaged and are presented as means ± standard errors of the means (SEMs) (21).
Statistical analysis.
Statistically significant differences between groups of mice were determined by Student's t test, and P values of <0.05 were considered significant.
RESULTS
Generation and characterization of M133-147-expanded CD4+ T cells.
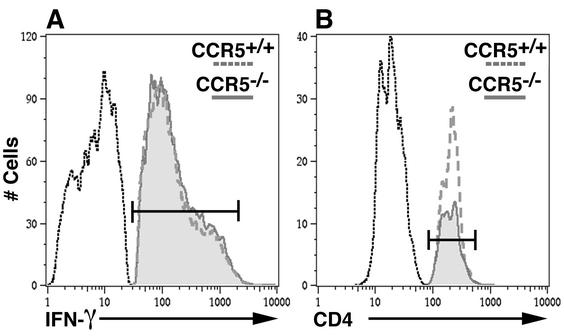
Age- and sex-matched CCR5+/+ and CCR5−/− mice were immunized with 2 × 105 PFU of MHV, and spleen cells were isolated at 8 days p.i. Enriched populations of CD4+ T cells were expanded for 6 days in the presence of peptide corresponding to the immunodominant CD4 epitope present within the viral transmembrane (M) protein spanning amino acids 133 to 147 (M133-147) (54). Such treatment resulted in a >99% pure population of CD4+ T cells as determined by flow cytometric analysis. In order to determine if expanded populations of CD4+ T cells from CCR5+/+ and CCR5−/− mice produced equivalent amounts of IFN-γ, cells were exposed to M133-147 peptide and IFN-γ production was determined by intracellular cytokine staining (Fig. 1A). Similar percentages of IFN-γ-producing CD4+ T cells from CCR5+/+ (79.3% ± 0.6%) and CCR5−/− (79.4% ± 0.8%) mice (means and SEMs) from two separate experiments with six mice per group were observed, and there was no difference in the level of IFN-γ production between these two populations as measured by the mean fluorescence intensity (205 ± 8.7 and 200 ± 5.6, respectively). These data indicate that CCR5 signaling is not required for either M133-147-specific expansion or IFN-γ production by M133-147-specific CD4+ T cells.
FIG. 1.
Flow cytometric analysis of M133-147-expanded CD4+ T cells. (A) IFN-γ expression by M133-147-expanded CD4+ T cells from CCR5+/+ and CCR5−/− mice. CD4+ T cells from either CCR5+/+ or CCR5−/− mice produced equivalent levels of IFN-γ following pulse with the M133-147 peptide. The data presented are representative of those from two separate experiments with a total of six mice per group. (B) Flow analysis of CD4+-T-cell entry into the CNS at day 9 following adoptive transfer. M133-147-expanded CD4+ T cells from CCR5+/+ mice exhibited an enhanced ability to enter the CNS of MHV-infected RAG1−/− mice compared to M133-147-expanded CD4+ T cells obtained from CCR5−/− mice. The data presented are representative of those from three individual mice from each group.
CCR5 expression and CD4+-T-cell migration into the CNS.
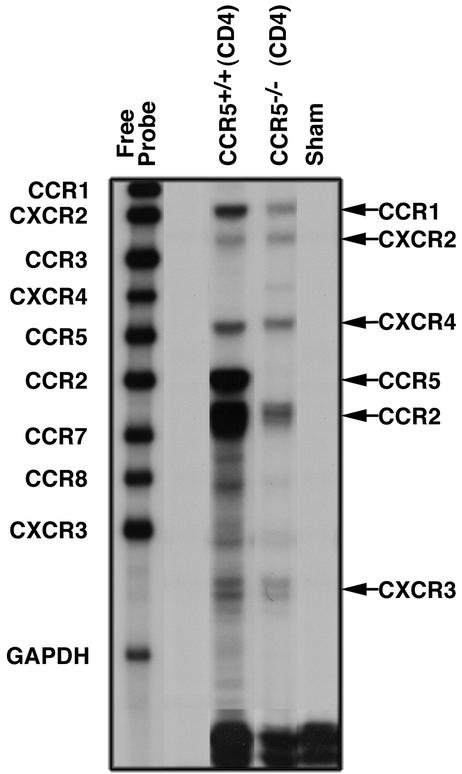
In order to assess the contribution of CCR5 expression to T-cell migration and entry into the CNS of MHV-infected mice, M133-147-expanded CD4+ T cells from either CCR5+/+ or CCR5−/− mice were adoptively transferred into RAG1−/− mice that were infected i.c. with MHV 3 days earlier (5). Recipient mice were injected i.v. with a total of 2.5 × 106 expanded CD4+ T cells (approximately 2 × 106 M133-147-specific cells) and sacrificed 9 days following transfer (12 days p.i.), and brains were collected to determine the viral titer by plaque assay as well as CD4+-T-cell accumulation (Table 1). There was a significant decrease (P < 0.003) in numbers of CCR5−/−-derived CD4+ T cells present in the CNS of RAG1−/− mice (5.4 × 104 ± 3.3 × 103 cells) compared to recipients of CCR5+/+-derived CD4+ T cells (2.2 × 105 ± 1.4 × 104 cells) (Fig. 1B; Table 1). The diminished ability of CCR5−/− donor CD4+ T cells to migrate into the CNS of MHV-infected RAG1−/− recipients correlated with a marked increase in viral titers (4.3 ± 0.1 PFU/g of brain tissue [log10]; n = 7) compared to those in recipients of CCR5+/+-derived CD4+ T cells, which cleared virus below level of detection (<2.0 PFU/g brain of tissue [log10]; n = 6) (Table 1). MHV-infected RAG1−/− mice receiving only HBSS were unable to eliminate virus, which is consistent with earlier studies (Table 1) (5). Despite the >4-fold decrease in CCR5−/−-derived CD4+ T cells present in the CNS of recipient RAG1−/− mice compared to recipients of CCR5+/+-derived CD4+ T cells, RAG1−/− recipients of CCR5−/−-derived CD4+ T cells were able to enter the CNS. This accumulation suggested that chemokine receptors other than CCR5 are allowing these cells to gain access into the CNS. Therefore, chemokine receptor gene expressions by M133-147-expanded CD4+ T cells from CCR5+/+ and CCR5−/− mice were compared. As shown in Fig. 2, there are similar gene expression profiles for chemokine receptors by antigen-expanded CD4+ T cells from either CCR5+/+ or CCR5−/− mice. Transcripts for CCR1, CXCR2, CXCR4, CCR2, and CXCR3 are expressed by both populations of cells. In addition, CCR5 transcripts are prominently expressed in CD4+ T cells from wild-type mice but are not present in CD4+ T cells from CCR5−/− mice. Quantitative analysis of signal intensity revealed a greater-than-twofold reduction in transcript levels for chemokine receptors CCR1, CCR2, and CXCR3 present in CD4+ T cells from CCR5−/− mice compared to CCR5+/+ mice (Table 2). Previous studies have demonstrated that these chemokine receptors help T cells gain access to the CNS during periods of inflammation (5, 25, 27, 42). Therefore, these data indicate that antigen-specific CCR5−/−-derived CD4+ T cells are able to migrate into the CNS of MHV-infected mice. However, their ability to do so is compromised, in part, by the lack of CCR5 as well as the muted expression of CCR1, CCR2, and CXCR3.
TABLE 1.
CCR5 enhances CD4+-T-cell accumulation within the CNS
| Experimental condition | Day Posttransfer | No. of M133-147-specific cells transferred | Titer (mean ± SEM)a | CD4 (mean ± SEM)b |
|---|---|---|---|---|
| RAG1−/− | NAc | NA | 5.4 ± 0.1 (7) | 0 (3) |
| CCR5+/+ (CD4)→RAG1−/− | 9 | 2.0 × 106 | <2.0 (6) | 2.2 × 105 ± 1.4 × 104 (3) |
| CCR5−/− (CD4)→RAG1−/− | 9 | 2.0 × 106 | 4.3 ± 0.1 (7)d,e | 5.4 × 104 ± 3.3 × 103 (3)d |
Titer data is presented as PFU per gram of brain tissue (L/og10). Titers were determined at day 12 p.i. The number of mice used is shown in parentheses.
Flow data are presented as total number of cells within the gated population. The number of mice used is shown in parentheses.
NA, not applicable.
P < 0.003 compared to CCR5+/+ (CD4)→RAG1−/−.
P < 0.001 compared to RAG1−/−.
FIG. 2.
Chemokine receptor gene expression from M133-147-expanded CD4+ T cells. Total RNA from M133-147-expanded CD4+ T cells from either CCR5+/+ or CCR5−/− mice was subjected to RNase protection assay analysis with a multitemplate chemokine receptor probe set. Each lane represents pooled RNA from CD4+ T cells isolated from six individual mice. Increased receptor expression is indicated on the right.
TABLE 2.
Chemokine receptor mRNA expression in M133-147-expanded CD4+ T cells
| Experimental condition | Expressiona
|
|||||
|---|---|---|---|---|---|---|
| CCR1 | CXCR2 | CXCR4 | CCR5 | CCR2 | CXCR3 | |
| Sham infection | 0 | 0 | 0 | 0 | 0 | 0 |
| CCR5+/+ (CD4) | 54.6 | 22.0 | 43.8 | 76.5 | 78.7 | 35.2 |
| CCR5−/− (CD4) | 22.5 | 18.8 | 30.2 | 0 | 33.5 | 15.6 |
All data are presented as normalized units representing the ratio of band intensity to that of the GAPDH control.
CCR5+/+ CD4+ T cells amplify demyelination.
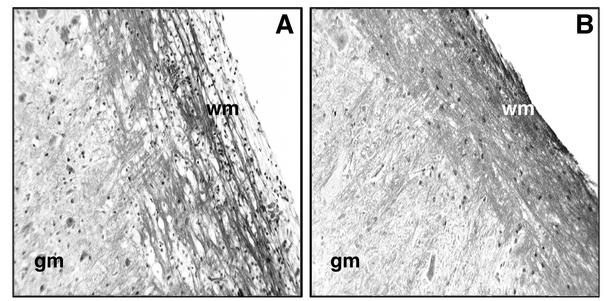
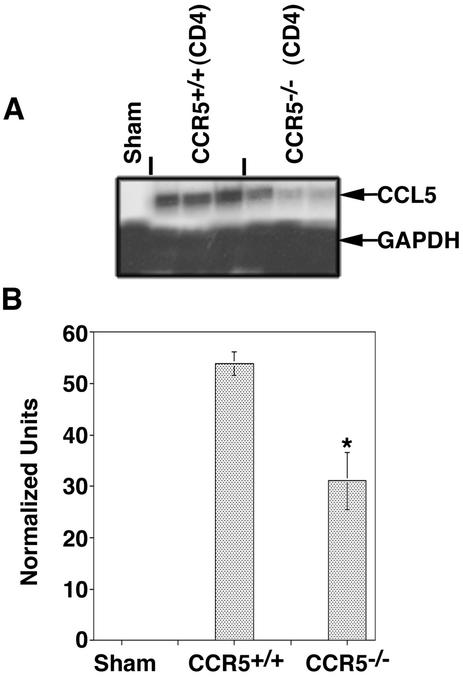
Adoptive transfer of M133-147-expanded CD4+ T cells from CCR5+/+ mice into MHV-infected RAG1−/− mice resulted in pronounced clinical disease at day 12 p.i. that was significantly (P < 0.003) more severe (average score, 2.5 ± 0.1; n = 6) than that in RAG1−/− recipients of CD4+ T cells from CCR5−/− mice (0.8± 0.1; n = 7). The increase in clinical disease severity in CCR5+/+ recipient mice correlated with increased numbers of CD4+ T cells present within the CNS, suggesting that demyelination may be more severe in this population of mice (21). Histologic analysis of spinal cords revealed a significant increase (P < 0.05) in the severity of demyelination in RAG1−/− mice that received CCR5+/+-derived CD4+ T cells (average score, 2.4 ± 0.1; n = 4) compared to CD4+ T cells obtained from CCR5−/− mice (average score, 0.7 ± 0.1; n = 5) (Fig. 3; Table 3). A previous study from our laboratory demonstrated that CD4+ T cells amplify demyelination in MHV-infected mice by attracting macrophages into the CNS through the release of chemokines, including CCL5 (21). Consistent with these observations is the demonstration of an approximate 60% reduction (P < 0.003) in macrophage accumulation (determined by measuring CD45high/F480+ cells) within the CNS of RAG1−/− mice that received CD4+ T cells from CCR5−/− mice compared to RAG1−/− recipients of CCR5+/+-derived CD4+ T cells (Table 3). Correlating with the diminished CD4+-T-cell and macrophage accumulation within the CNS of RAG1−/− mice receiving CCR5−/−-derived CD4+ T cells were decreased levels (approximately twofold) of CCL5 transcript expression within the CNS compared to those in RAG1−/− mice that received CD4+ T cells from CCR5+/+ mice (Fig. 4).
FIG. 3.
Representative LFB staining of spinal cords from RAG1−/− mice following adoptive transfer of M133-147-expanded CD4+ T cells. (A) MHV-infected RAG1−/− recipients of CD4+ T cells from CCR5+/+ mice show demyelination and cellular infiltration within white matter (wm). gm, grey matter. (B) Spinal cords from RAG1−/− recipients of CD4+ T cells obtained from CCR5−/− mice display limited demyelination. Magnification, ×200. Spinal cords were analyzed at day 12 p.i. and day 9 posttransfer.
TABLE 3.
M133-147-expanded CD4+ T cells amplify demyelination
| Experimental condition | CD45high/F480 (mean ± SEM)a | Demyelination (mean ± SEM)b |
|---|---|---|
| RAG1−/− | 2.3 × 105 ± 2.7 × 104 (6) | 0 (5) |
| CCR5+/+ (CD4)→RAG1−/− | 8.6 × 105 ± 5.8 × 104 (3) | 2.4 ± 0.1 (4) |
| CCR5−/− (CD4)→RAG1−/− | 3.7 × 105 ± 2.9 × 104 (3)c | 0.7 ± 0.1 (5)c |
Flow data are presented as total number of cells within the gated population. The number in parentheses indicates the number of mice used.
Demyelination is scored as described in Materials and Methods. The number in parentheses indicates the number of mice used.
P < 0.003 compared to CCR5+/+ (CD4)→RAG1−/−.
FIG. 4.
CCL5 mRNA expression. (A) Total RNA was isolated from the brains of RAG1−/− recipients of M133-147-expanded CCR5+/+ and CCR5−/−-derived CD4+ T cells and subjected to RNase protection assay analysis to assess CCL5 gene expression. Each lane represents an individual mouse. (B) Quantitative analysis of CCL5 transcript expressed. Densitometric analysis of each lane representing a brain sample from an individual mouse was preformed on the scanned autoradiograph in panel A. *, P < 0.05 compared to RAG1−/− recipient of CCR5+/+-derived CD4+ T cells. Data are means ± SEMs.
DISCUSSION
Animal models of demyelination including experimental autoimmune encephalomyelitis, Theiler's murine encephalomyelitis virus, and MHV have provided valuable insights into how T cells may contribute to the immunopathology of human demyelinating diseases such as MS (9, 26, 31, 33). Recent studies indicate that both CD4+ and CD8+ T cells enhance demyelination in MHV-infected mice, although the mechanisms by which these cells contribute to disease may differ (21, 26, 38, 39, 53). Pewe and Perlman (39) have recently reported an IFN-γ-dependent mechanism by which CD8+ T cells enhance demyelination in MHV-infected mice by attracting macrophages into the CNS. In contrast, the inability of CD4+ T cells to produce IFN-γ correlated with increased demyelination in MHV-infected mice (38). Other studies have indicated that CD4+ T cells, rather than CD8+ T cells, amplify demyelination by attracting macrophages into the CNS, which then participate in white matter destruction. In support of this are studies demonstrating that demyelination is significantly reduced in MHV-infected CD4−/− mice compared to infected CD8−/− mice (21). MHV-infected CD4−/− mice exhibited a marked decrease in the numbers of macrophages present within the CNS compared to CD8−/− mice. In addition, antibody targeting of the T-cell chemoattractant CXCL10 in MHV-infected mice selectively reduced CD4+-T-cell accumulation within the CNS, but not that of CD8+ T cells, and this resulted in improved neurologic disease, reduced macrophage infiltration and demyelination, and a marked increase in the number of remyelinated axons (26). Accumulating evidence indicates that one mechanism by which CD4+ T cells contribute to demyelinating disease in MHV-infected mice is through the release of the macrophage chemoattractant CCL5 (21). In support of this is the recent demonstration that treatment of MHV-infected mice with neutralizing antisera specific for CCL5 resulted in diminished macrophage accumulation within the CNS and that this correlated with reduced demyelination (21). The data presented in the present study support and extend these previous observations. CCR5+/+ CD4+ T cells specific for the M133-147 epitope are able to traffic into the CNS of MHV-infected mice and amplify demyelination by increasing CCL5 expression, which serves to attract macrophages into the CNS. In contrast, CCR5−/− CD4+ T cells exhibited a limited ability to gain access into the CNS of infected mice, and this correlates with reduced CCL5 expression, diminished macrophage accumulation, and a reduction in the severity of demyelination.
CCR5 is a member of the CC family of chemokine receptors that is expressed on a variety of leukocytes, including T cells and macrophages. Expression of CCR5 has been shown to be important in the host defense following viral infection. Infection of CCR5−/− mice with influenza A virus results in increased mortality accompanied by enhanced macrophage accumulation within the lungs of infected mice, which correlated with increased chemokine expression within the lungs of infected mice (6). In addition, CCR5 is important in the host defense following infection with Cryptococcus neoformans and Listeria monocytogenes. In both models, a lack of CCR5 resulted in delayed clearance of the microbial pathogen from infected tissues as well as altered immune responses and leukocyte trafficking, indicating that CCR5 signaling was important in regulating these processes (14, 15, 56). The data presented clearly indicate that CCR5 signaling does not regulate either CD4+-T-cell expansion or IFN-γ production following MHV infection (Fig. 1A; see Results). This is consistent with a report by Sato et al., (43), who demonstrated no deficiencies in IFN-γ production by activated CD4+ T cells from CCR5−/− mice compared to CCR5+/+ mice. Therefore, CCR5 signaling is not required for antigen-specific expansion or IFN-γ secretion by CD4+ T cells in the MHV model.
Expression of CCR5 has been shown to regulate leukocyte trafficking during periods of inflammation. Lichterfeld et al. (21a) have shown that CCR1 and CCR5 are down regulated on CD4+ T cells in patients with chronic hepatitis C virus infections. Reduced expression of these receptors was associated with reduced migration of CD4+ T cells in response to chemotactic signals and ultimately reduced trafficking of these cells to the liver, suggesting a functional role for these chemokine receptors in viral control. Huffnagle and colleagues (14) have shown that CCR5−/− mice exhibited significant defects in leukocyte migration into the brain following C. neoformans infection, which correlated with the presence of increased levels of cryptococcal antigen. In addition, studies with MS patients indicate that CCR5 is important in allowing inflammatory cells to traffic into the CNS. CCR5 is detected on T cells, monocytes, and microglia present within the CNS of individuals with MS, and CCR5 expression is associated with areas undergoing demyelination (4, 45, 48, 49). These data suggest that CCR5 enables leukocytes to migrate into the CNS of MS patients and contribute to disease pathogenesis. In support of this are genetic studies examining the effects of the homozygous delta32 CCR5 gene mutation on the development and severity of symptoms in MS patients. Although individuals with this deletion were not protected from MS, there was an associated lower risk of recurrent clinical disease activity (44). The results presented here support these earlier observations by demonstrating the relevance of CCR5 signaling in allowing virus-specific CD4+ T cells to traffic and accumulate within the CNS of MHV-infected mice (Table 1). Analysis of chemokine receptor mRNA transcripts present in this population of cells revealed increased expression of CCR1, CCR2, CCR5, and CXCR3 compared to antigen-specific CCR5−/− CD4+ T cells. These data indicate that CCR5 signaling enhances expression of these receptors, which confers the ability of these cells to enter the CNS (Fig. 2 and Table 2). The fact that a low percentage CCR5−/− CD4+ T cells were able to migrate into the CNS of MHV-infected mice reflects the absence of CCR5 as well as the reduced levels of CCR2 and CXCR3, which have been shown to contribute to T-cell migration to the CNS of MHV-infected mice (5, 24). In addition, although CXCR2 and CXCR4 were expressed equally on CD4+ T cells from either CCR5+/+ and CCR5−/− mice, these receptors are not normally associated with Th1 inflammatory diseases. The CCR5−/− CD4+ T cells that do enter the CNS are exerting a protective, albeit limited, effect, as evidenced by the reduced viral burden within the CNS of RAG1−/− recipients of CCR5−/−-derived CD4+ T cells compared to control RAG1−/− mice. This most likely reflects the fact that these cells are able to secrete IFN-γ following antigen stimulation (Fig. 1A; see Results).
In conclusion, these studies support and extend previous studies from our laboratory that indicate that chemokines and chemokine receptors are important in regulating T-cell and macrophage activation and trafficking into the CNS following MHV infection (5, 10, 21, 23, 26). Therefore, it is possible to put forth a model with regard to chemokine signaling as it relates to T-cell activation, host defense, and disease development within the CNS of MHV-infected mice. Based on the results presented, CCR5 signaling enhances expression of the chemokine receptors CCR1, CCR2, and CXCR3 on CD4+ T cells following exposure to the M133-147 epitope. Increased expression of these receptors enhances the ability of CD4+ T cells to respond to their chemokine ligands CCL2, CCL5, CCL9, and CXCL10 that are expressed within the CNS of MHV-infected mice during acute disease (5, 19, 21, 25, 26). Increased infiltration of virus-specific CD4+ T cells aids in the elimination of the bulk of virus and protects mice from death. However, virus persists within white matter tracts, and this results in chronic expression of CXCL10, which serves to attract activated CD4+ T cells into the CNS (26). Once present, these cells amplify demyelination by influencing expression of CCL5, which serves to attract activated macrophages into the CNS via signaling through CCR5 expressed on the surface of these cells (10, 21). The collective evidence highlights the diverse and nonredundant roles of chemokines and their receptors in participating in neuroinflammation and further supports targeting of these molecules for the treatment of neuroinflammatory diseases.
Acknowledgments
This work was supported by National Institutes of Health grants 37336 and 41249 to T.E.L. W.G.G. was supported by National Institutes of Health training grant A107319-12.
REFERENCES
- 1.Akaike, T., E. Weihe, M. Schaefer, Z. F. Fu, Y. M. Zheng, W. Vogel, H. Schmidt, H. Koprowski, and B. Dietzschold. 1995. Effect of neurotropic virus infection on neuronal and inducible nitric oxide synthase activity in rat brain. J. Neurovirol. 1:118-125. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, C., T. Jensen, A. Nansen, O. Marker, and A. R. Thomsen. 1999. CD4+ T cell-mediated protection against a lethal outcome of systemic infection with vesicular stomatitis virus requires CD40 ligand expression, but not IFN-gamma or IL-4. Int. Immunol. 11:2035-2042. [DOI] [PubMed] [Google Scholar]
- 3.Asensio, V. C., and I. L. Campbell. 1997. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J. Virol. 71:7832-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balashov, K. E., J. B. Rottman, H. L. Weiner, and W. W. Hancock. 1999. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1α and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 96:6873-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, B. P., W. A. Kuziel, and T. E. Lane. 2001. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J. Immunol. 167:4585-4592. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, T. C., M. A. Beck, W. A. Kuziel, F. Henderson, and N. Maeda. 2000. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 156:1951-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorries, R. 2001. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr. Top. Microbiol. Immunol. 253:219-245. [DOI] [PubMed] [Google Scholar]
- 8.Finke, D., U. G. Brinckmann, V. ter Meulen, and U. G. Liebert. 1995. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J. Virol. 69:5469-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass, W. G., B. P. Chen, M. T. Liu, and T. E. Lane. 2002. Mouse hepatitis virus infection of the central nervous system: chemokine-mediated regulation of host defense and disease. Viral Immunol. 15:261-272. [DOI] [PubMed] [Google Scholar]
- 10.Glass, W. G., M. T. Liu, W. A. Kuziel, and T. E. Lane. 2001. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology 288:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gogate, N., M. Bakhiet, K. Kristensson, E. Norrby, and T. Olsson. 1991. Gamma interferon expression and major histocompatibility complex induction during measles and vesicular stomatitis virus infections of the brain. J. Neuroimmunol. 31:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano, N., T. Murakami, K. Fujiwara, and M. Matsumoto. 1978. Utility of mouse cell line DBT for propagation and assay of mouse hepatitis virus. Jpn. J. Exp. Med. 48:71-75. [PubMed] [Google Scholar]
- 13.Houtman, J. J., and J. O. Fleming. 1996. Pathogenesis of mouse hepatitis virus-induced demyelination. J. Neurovirol. 2:361-376. [DOI] [PubMed] [Google Scholar]
- 14.Huffnagle, G. B., L. K. McNeil, R. A. McDonald, J. W. Murphy, G. B. Toews, N. Maeda, and W. A. Kuziel. 1999. Cutting edge: role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J. Immunol. 163:4642-4646. [PubMed] [Google Scholar]
- 15.Huffnagle, G. B., T. R. Traynor, R. A. McDonald, M. A. Olszewski, D. M. Lindell, A. C. Herring, and G. B. Toews. 2000. Leukocyte recruitment during pulmonary Cryptococcus neoformans infection. Immunopharmacology 48:231-236. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, A. J., J. Upshaw, K. D. Pavelko, M. Rodriguez, and L. R. Pease. 2001. Preservation of motor function by inhibition of CD8+ virus peptide-specific T cells in Theiler's virus infection. FASEB J. 15:2760-2762. [DOI] [PubMed] [Google Scholar]
- 17.Kang, B. S., M. A. Lyman, and B. S. Kim. 2002. The majority of infiltrating CD8+ T cells in the central nervous system of susceptible SJL/J mice infected with Theiler's virus are virus specific and fully functional. J. Virol. 76:6577-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundig, T. M., H. Hengartner, and R. M. Zinkernagel. 1993. T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J. Immunol. 150:2316-2321. [PubMed] [Google Scholar]
- 19.Lane, T. E., V. C. Asensio, N. Yu, A. D. Paoletti, I. L. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160:970-978. [PubMed] [Google Scholar]
- 20.Lane, T. E., and M. J. Buchmeier. 1997. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol 5:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane, T. E., M. T. Liu, B. P. Chen, V. C. Asensio, R. M. Samawi, A. D. Paoletti, I. L. Campbell, S. L. Kunkel, H. S. Fox, and M. J. Buchmeier. 2000. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 74:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Lichterfeld, M., L. Leifeld, H. D. Nischalke, J. K. Rockstroh, L. Hess, T. Sauerbruch, and U. Spengler. 2002. Reduced CC chemokine receptor (CCR) 1 and CCR5 surface expression on peripheral blood T lymphocytes from patients with chronic hepatitis C infection. J. Infect. Dis. 185:1803-1807. [DOI] [PubMed] [Google Scholar]
- 22.Lin, M. T., S. A. Stohlman, and D. R. Hinton. 1997. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 71:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, M. T., D. Armstrong, T. A. Hamilton, and T. E. Lane. 2001. Expression of Mig (monokine induced by interferon-gamma) is important in T lymphocyte recruitment and host defense following viral infection of the central nervous system. J. Immunol. 166:1790-1795. [DOI] [PubMed] [Google Scholar]
- 24.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 25.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, T. A. Hamilton, D. A. Armstrong, and T. E. Lane. 2001. The CXC chemokines IP-10 and Mig are essential in host defense following infection with a neurotropic coronavirus. Adv. Exp. Med. Biol. 494:323-327. [DOI] [PubMed] [Google Scholar]
- 26.Liu, M. T., H. S. Keirstead, and T. E. Lane. 2001. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J. Immunol. 167:4091-4097. [DOI] [PubMed] [Google Scholar]
- 27.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 28.Marten, N. W., S. A. Stohlman, R. D. Atkinson, D. R. Hinton, J. O. Fleming, and C. C. Bergmann. 2000. Contributions of CD8+ T cells and viral spread to demyelinating disease. J. Immunol. 164:4080-4088. [DOI] [PubMed] [Google Scholar]
- 29.Marten, N. W., S. A. Stohlman, and C. C. Bergmann. 2001. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 14:1-18. [DOI] [PubMed] [Google Scholar]
- 30.Medana, I. M., A. Gallimore, A. Oxenius, M. M. Martinic, H. Wekerle, and H. Neumann. 2000. MHC class I-restricted killing of neurons by virus-specific CD8+ T lymphocytes is effected through the Fas/FasL, but not the perforin pathway. Eur. J. Immunol. 30:3623-3633. [DOI] [PubMed] [Google Scholar]
- 31.Miller, D. J., J. J. Bright, S. Sriram, and M. Rodriguez. 1997. Successful treatment of established relapsing experimental autoimmune encephalomyelitis in mice with a monoclonal natural autoantibody. J. Neuroimmunol. 75:204-209. [DOI] [PubMed] [Google Scholar]
- 32.Murray, P. D., D. B. McGavern, L. R. Pease, and M. Rodriguez. 2002. Cellular sources and targets of IFN-gamma-mediated protection against viral demyelination and neurological deficits. Eur. J. Immunol. 32:606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neville, K. L., J. Padilla, and S. D. Miller. 2002. Myelin-specific tolerance attenuates the progression of a virus-induced demyelinating disease: implications for the treatment of MS. J. Neuroimmunol. 123:18-29. [DOI] [PubMed] [Google Scholar]
- 34.Palma, J. P., H. G. Lee, M. Mohindru, B. S. Kang, M. Dal Canto, S. D. Miller, and B. S. Kim. 2001. Enhanced susceptibility to Theiler's virus-induced demyelinating disease in perforin-deficient mice. J. Neuroimmunol. 116:125-135. [DOI] [PubMed] [Google Scholar]
- 35.Parra, B., C. C. Bergmann, D. R. Hinton, R. Atkinson, and S. A. Stohlman. 2001. IFN-gamma secreted by virus-specific CD8+ T cells contribute to CNS viral clearance. Adv. Exp. Med. Biol. 494:335-340. [DOI] [PubMed] [Google Scholar]
- 36.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 37.Pasquetto, V., S. F. Wieland, S. L. Uprichard, M. Tripodi, and F. V. Chisari. 2002. Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte cultures. J. Virol. 76:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pewe, L., J. Haring, and S. Perlman. 2002. CD4 T-cell-mediated demyelination is increased in the absence of gamma interferon in mice infected with mouse hepatitis virus. J. Virol. 76:7329-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pewe, L., and S. Perlman. 2002. Cutting edge: CD8 T cell-mediated demyelination is IFN-gamma dependent in mice infected with a neurotropic coronavirus. J. Immunol. 168:1547-1551. [DOI] [PubMed] [Google Scholar]
- 40.Ransohoff, R. M., T. Wei, K. D. Pavelko, J. C. Lee, P. D. Murray, and M. Rodriguez. 2002. Chemokine expression in the central nervous system of mice with a viral disease resembling multiple sclerosis: roles of CD4+ and CD8+ T cells and viral persistence. J. Virol. 76:2217-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson, J. H., and A. A. Delvig. 2002. Diversity in MHC class II antigen presentation. Immunology 105:252-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rottman, J. B., A. J. Slavin, R. Silva, H. L. Weiner, C. G. Gerard, and W. W. Hancock. 2000. Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur. J. Immunol. 30:2372-2377. [DOI] [PubMed] [Google Scholar]
- 43.Sato, N., W. A. Kuziel, P. C. Melby, R. L. Reddick, V. Kostecki, W. Zhao, N. Maeda, S. K. Ahuja, and S. S. Ahuja. 1999. Defects in the generation of IFN-gamma are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1 alpha-, or CCR2-deficient mice. J. Immunol. 163:5519-5525. [PubMed] [Google Scholar]
- 44.Sellebjerg, F., H. O. Madsen, C. V. Jensen, J. Jensen, and P. Garred. 2000. CCR5 δ32, matrix metalloproteinase-9 and disease activity in multiple sclerosis. J. Neuroimmunol. 102:98-106. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen, T. L., M. Tani, J. Jensen, V. Pierce, C. Lucchinetti, V. A. Folcik, S. Qin, J. Rottman, F. Sellebjerg, R. M. Strieter, J. L. Frederiksen, and R. M. Ransohoff. 1999. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 103:807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stohlman, S. A., C. C. Bergmann, D. J. Cua, M. T. Lin, S. Ho, W. Wei, and D. R. Hinton. 1998. Apoptosis of JHMV-specific CTL in the CNS in the absence of CD4+ T cells. Adv. Exp. Med. Biol. 440:425-430. [DOI] [PubMed] [Google Scholar]
- 47.Stohlman, S. A., S. Kyuwa, J. M. Polo, D. Brady, M. M. Lai, and C. C. Bergmann. 1993. Characterization of mouse hepatitis virus-specific cytotoxic T cells derived from the central nervous system of mice infected with the JHM strain. J. Virol. 67:7050-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strunk, T., S. Bubel, B. Mascher, P. Schlenke, H. Kirchner, and K. P. Wandinger. 2000. Increased numbers of CCR5+ interferon-gamma- and tumor necrosis factor-alpha-secreting T lymphocytes in multiple sclerosis patients. Ann. Neurol. 47:269-273. [PubMed] [Google Scholar]
- 49.Trebst, C., T. L. Sorensen, P. Kivisakk, M. K. Cathcart, J. Hesselgesser, R. Horuk, F. Sellebjerg, H. Lassmann, and R. M. Ransohoff. 2001. CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am. J. Pathol. 159:1701-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vollstedt, S., M. Franchini, G. Alber, M. Ackermann, and M. Suter. 2001. Interleukin-12- and gamma interferon-dependent innate immunity are essential and sufficient for long-term survival of passively immunized mice infected with herpes simplex virus type 1. J. Virol. 75:9596-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh, C. M., M. Matloubian, C. C. Liu, R. Ueda, C. G. Kurahara, J. L. Christensen, M. T. Huang, J. D. Young, R. Ahmed, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williamson, J. S., and S. A. Stohlman. 1990. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J. Virol. 64:4589-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, G. F., A. A. Dandekar, L. Pewe, and S. Perlman. 2000. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 165:2278-2286. [DOI] [PubMed] [Google Scholar]
- 54.Xue, S., A. Jaszewski, and S. Perlman. 1995. Identification of a CD4+ T cell epitope within the M protein of a neurotropic coronavirus. Virology 208:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi, K., N. Goto, S. Kyuwa, M. Hayami, and Y. Toyoda. 1991. Protection of mice from a lethal coronavirus infection in the central nervous system by adoptive transfer of virus-specific T cell clones. J. Neuroimmunol. 32:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, Y., T. Kurihara, R. P. Ryseck, Y. Yang, C. Ryan, J. Loy, G. Warr, and R. Bravo. 1998. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J. Immunol. 160:4018-4025. [PubMed] [Google Scholar]
- 57.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]