Abstract
The ribosomal protein S6 kinase (S6K) belongs to the AGC family of Ser/Thr kinases and is known to be involved in the regulation of protein synthesis and the G1/S transition of the cell cycle. There are two forms of S6K, termed S6Kα and S6Kβ, which have cytoplasmic and nuclear splice variants. Nucleocytoplasmic shuttling has been recently proposed for S6Kα, based on the use of the nuclear export inhibitor, leptomycin B. However, the molecular mechanisms regulating subcellular localization of S6Ks in response to mitogenic stimuli remain to be elucidated. Here we present data on the in vitro and in vivo phosphorylation of S6Kβ, but not S6Kα, by protein kinase C (PKC). The site of phosphorylation was identified as S486, which is located within the C-terminal nuclear localization signal. Mutational analysis and the use of phosphospecific antibodies provided evidence that PKC-mediated phosphorylation at S486 does not affect S6K activity but eliminates the function of its nuclear localization signal and causes retention of an activated form of the kinase in the cytoplasm. Taken together, this study uncovers a novel mechanism for the regulation of nucleocytoplasmic shuttling of S6KβII by PKC-mediated phosphorylation.
The ribosomal protein S6 (rpS6) kinase (S6K) belongs to the AGC family of Ser/Thr protein kinases which includes the protein kinase C's (PKCs), protein kinase B's, SGKs, and 90-kDa ribosomal S6 kinases (p90 RSKs). There are two forms of S6K, S6Kα and S6Kβ, which have cytoplasmic (S6KαII and S6KβII) and nuclear (S6KαI and S6KβI) variants derived from alternative splicings at the N terminus (2, 15). S6Kα and S6Kβ have a very high level of overall sequence similarity, with the greatest homology in the kinase and kinase extension domains. However, both kinases differ significantly at their N- and C-terminal regulatory regions, sharing only 28 and 25% homology, respectively. The C terminus of S6Kβ contains a specific proline-rich region, which is absent in S6Kα and might be involved in mediating protein-protein interactions with SH3 and WW domain-containing molecules. The presence of a PDZ domain-binding motif at the C terminus of S6Kα may direct the kinase into distinct signaling complexes (8).
The activity of S6K is regulated by phosphorylation and dephosphorylation events in cellular responses to various extracellular stimuli. The treatment of cells with growth factors, cytokines, and hormones leads to a rapid activation of S6K (10), while growth inhibitory agents, such as steroids and transforming growth factor β, suppress kinase activity (45, 52). The mechanism of activation of S6Kα has been studied in detail by various laboratories and was shown to be a multistep phosphorylation process involving several Ser/Thr kinases (14, 64).
No direct, highly specific S6K inhibitor has yet been identified. Under these circumstances, the use of two indirect inhibitors, namely wortmannin (a phosphatidylinositol 3-kinase [PI3-K] inhibitor) and rapamycin (an mTOR inhibitor), has been instrumental in dissecting signaling events involved in the regulation of both forms of S6K. Studies from numerous laboratories demonstrated that signals from the PI3-K and mTOR pathways are crucial for full activation of S6Kα (4, 7, 11, 20, 21).
rpS6 is the most widely studied physiological substrate of S6K. The phosphorylation of S6 protein was shown to closely correlate with the initiation of protein synthesis induced by various extracellular stimuli (16, 55). The transcriptional activator CREM, elongation factor 2 kinase, and the regulator of apoptosis, Bad 1, have also been shown to be phosphorylated by S6Kα in vitro and in vivo (13, 22, 63). However, the physiological relevance of these phosphorylations requires further investigation, since other protein kinases can phosphorylate these molecules at identical sites.
S6Kα was identified more than a decade ago, and S6Kβ was identified only recently, hence most functional studies have involved the p70/p85 isoforms of S6Kα. S6Kα was proposed to be involved in translational up-regulation of a subset of mRNAs that are characterized by the presence of an oligopyrimidine tract at their 5′ termini and generally encode ribosomal proteins and elongation factors (25). This view has been recently challenged by studies which show S6K-independent translation of mRNAs with oligopyrimidine tracts at their 5′ termini (58). Microinjection studies with neutralizing antibodies against S6Kα demonstrated its importance in mediating the G1/S transition of the cell cycle (29). Knockout studies of the S6Kα gene in mice and Drosophila melanogaster indicate that the kinase is a key player in the regulation of cell size, growth, and glucose homeostasis (36, 44, 53).
Accumulating evidence from several laboratories demonstrates that S6Kβ is activated similarly to S6Kα when cells are treated with fetal calf serum (FCS), insulin, or phorbol 12-myristate 13-acetate (PMA) (19, 28, 30, 32). Both kinases receive an input signal from common effectors of the PI3-K pathway, including PDK1, protein kinase B, PKCζ, Rac, and CDC42 (1, 6, 9, 32, 47, 50). However, some differences in the regulation of S6Kα and S6Kβ have started to emerge. Comparative analysis of both forms of S6K indicated that the basal activity of S6Kβ is more sensitive to activation by myristoylated PKCζ than is that of S6Kα (32). The same group has also reported that the C-terminal region of S6Kβ exerts a stronger inhibitory effect on the kinase than does the S6Kα C terminus. Moreover, a novel regulatory connection between the MEK/extracellular signal-regulated kinase and S6Kβ signaling pathways has recently been demonstrated (33, 42, 61).
As mentioned above, S6Kα and S6Kβ are each represented by two splice variants with distinct subcellular distributions. The 23- and 13-amino-acid extensions at the N-termini of S6KαI and S6KβI contain nuclear localization signals (NLSs) that target these isoforms constitutively to the nucleus (35, 49). The cytoplasmic form of S6Kα (S6KαII) or p70 S6K is predominantly cytosolic, but it can also accumulate in the nucleus when cells are treated with leptomycin B (LMB) (27). The presence of a functional NLS at the C terminus of S6Kβ, which is found in both splice variants, has recently been reported (28). The nuclear functions of S6Ks are not known.
The PKC family of lipid-dependent serine/threonine kinases has been implicated in a multitude of physiological processes, including protein synthesis, mitogenesis, cell survival, and transcriptional activation (34). Based on sequence homology, domain organization, and mode of activation, PKCs can be subdivided into 3 classes: classical PKCs (α, β, and γ) are activated by diacylglycerol (DAG) and calcium, novel PKCs (δ, ɛ, η, and θ) require DAG but not calcium, and atypical PKCs (ζ and ι/λ) require neither DAG nor calcium for their activation (38).
As mentioned above, both S6Kα and S6Kβ are rapidly activated when cells are treated with PMA, an activator of classical and novel PKCs. Furthermore, prolonged treatment of cells with PMA, which leads to down-regulation of DAG-activated PKCs, eliminates mitogenic activation of S6Kα (56, 57). Recently, the PI3-K- and PDK1-activated atypical PKC isoforms ζ and λ have been implicated in the regulation of S6Kα (1, 31, 50). Atypical PKCs have been found in complexes with S6Kα, but it is not yet known whether they can directly phosphorylate and modulate the function of S6Ks. Taken together, these data indicate that PKCs transduce signal(s) in activated cells to both forms of S6K, but the regulatory mechanisms and functional importance remain unclear.
We report here that S6KβII, but not S6KαII, is specifically phosphorylated by PKC at a site located in the middle of its C-terminal NLS. Using phosphospecific antibodies, we found that phosphorylation of S6KβII at S486 is strongly induced by PMA and to a lesser extent by epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), insulin, platelet-derived growth factor (PDGF), and FCS. Furthermore, we found that S486 phosphorylation does not effect S6K activity, but it eliminates the function of the C-terminal NLS. Mutational analysis of S6KβII provided evidence that S486 phosphorylation results in retention of an activated form of the kinase in the cytoplasm, possibly by blocking its nuclear import.
MATERIALS AND METHODS
Materials.
Restriction enzymes and DNA modifying enzymes were obtained from standard commercial sources and used according to the manufacturers' recommendations. Oligonucleotides were synthesized by Genosys, and phosphopeptides were synthesized by Eurogentec. Recombinant PKCs were purchased from Calbiochem. The anti-Myc 9E10 monoclonal antibody was from Santa Cruz Biotechnology. Monoclonal antibody to the EE tag was a gift from Julian Downward.
Construction of expression vectors.
The full-length coding sequences corresponding to both splicing forms of S6Kα and S6Kβ were amplified by PCR with rat S6Kα and human S6Kβ cDNAs as templates, respectively. The products of PCR amplification were then cloned into the BamHI/EcoRI sites of the pcDNA3.1 expression vector (Invitrogen) in frame with the amino-terminal EE tag epitope (MEFMPME). The C-terminal regions of S6Kα (amino acids 453 to 525) and S6Kβ (amino acids 442 to 495) were PCR amplified and cloned into the pET23d vector (Novagen) in frame with six-His tag sequences by using NcoI/EcoRI restriction sites. Mutated forms of S6Kα and S6Kβ were generated by using the QuikChange site-directed mutagenesis kit (Stratagene) as recommended by the manufacturer. All constructs were verified by restriction enzyme digestion and DNA sequencing. The generation of mammalian expression constructs for PKCs used in this study was previously described (31).
Cell culture and transient transfection.
Human embryonic kidney HEK 293 cells and human breast cancer MCF7 cells were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Inc.), 2 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. NIH 3T3 cells were grown in DMEM supplemented with 10% donor calf serum (Life Technologies, Inc.), 2 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. Transient transfections of HEK 293 cells were performed by using Lipofectamine according to the manufacturer's recommendations (Life Technologies, Inc.). NIH 3T3 cells were transfected by using the PolyFect reagent (Qiagen) according to the manufacturer's recommendations. At 24 h posttransfection, cells were starved in serum-free DMEM for 24 h and then stimulated with either 10% FBS, 1 μM PMA, 50 ng of EGF/ml, 50 ng of IGF-1/ml, 100 nM insulin, or 50 ng of PDGF/ml for the indicated time.
Adult rat ventricular cardiomyocytes (ARVC) were isolated from the hearts of adult rats as described previously (62). Isolated cardiomyocytes were seeded onto laminin-coated dishes, cultured overnight in medium 199 containing 1 g of glucose/liter, 0.68 mmol of glutamine/liter, 5 mmol of creatine/liter, 2 mmol of carnitine/liter, and 5 mmol of taurine/liter. The next day, the cells were treated with phenylephrine (10 μM), insulin (20 nM), or vehicle only for 30 min.
Immunoprecipitation and S6K assay.
HEK 293 cells were washed with ice-cold phosphate-buffered saline (PBS) and extracted with lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1% (vol/vol) Nonidet P-40, 2 mM EDTA, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 50 μg of leupeptin (Boehringer Mannheim)/ml, 0.5% aprotinin (Sigma), 1 mM phenylmethylsulfonyl fluoride (Sigma), and 3 mM benzamidine (Sigma). Whole-cell extracts were centrifuged at 10,000 × g for 15 min at 4°C, and recombinant EE-S6Ks were immunoprecipitated with the anti-EE monoclonal antibody immobilized on protein G-Sepharose beads (Amersham Pharmacia Biotech). Immune complexes were washed three times with lysis buffer followed by a single wash with kinase assay buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 10 mM β-glycerophosphate). The kinase reaction was initiated by resuspending the beads in 25 μl of kinase assay buffer supplemented with 1 μM protein kinase A inhibitor (Calbiochem), 50 μM ATP, 5 μCi of [γ-32P]ATP (Amersham Pharmacia Biotech), and 20 μg of 40S ribosomes isolated from rat liver. The reaction was carried out at 30°C for 10 min and terminated by the addition of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer and boiling the mixture for 5 min. Samples were subjected to SDS-10% PAGE, and the amount of 32P incorporated into the S6 protein was assessed by autoradiography and quantitated by phosphorimaging (Bio-Rad).
Expression of recombinant proteins in bacteria.
Recombinant His-tagged C-terminal regions of S6Kα and S6Kβ (His-S6KαC and His-S6KβC) were expressed in BLR21 DE3 cells. Expression was carried out at 22°C for 4 h in the presence of 1 mM isopropyl-β-d-galactosidase. Recombinant His-S6KαC and His-S6KβC were affinity purified by using Talon beads according to the manufacturer's recommendations. Purified proteins were dialyzed overnight at 4°C against 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 1 mM dithiothreitol in 50% glycerol and stored at −20°C.
In vitro phosphorylation of S6K by PKCs.
Recombinant EE-tagged S6KαII and S6KβII were immunoprecipitated from serum-starved HEK 293 cells with anti-EE monoclonal antibody immobilized on protein G-Sepharose. Beads were washed twice with lysis buffer containing 0.5 M NaCl and three times in PKC kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 100 μM CaCl2). Immune complexes and 1 μg of recombinant His-S6KαC, His-S6KβC, histone H1, or ɛ-peptide were incubated with 0.5 U of different PKC isoforms (Calbiochem)/ml at 30°C in buffer containing 100 μM ATP, 5 μCi of [γ-32P]ATP, 0.03% Triton X-100, 100 μg of phosphatidylserine/ml, and 20 μg of DAG/ml. After incubation for 10 min, reactions were terminated by the addition of SDS-PAGE sample buffer and boiling the mixtures for 5 min. The incorporation of 32P into recombinant EE-S6KαII, EE-S6KβII, His-S6KαC, His-S6KβC, histone H1, and ɛ-peptide was determined by phosphorimager analysis following SDS-5 to 17.5% PAGE.
Sample preparation and MS.
Recombinant EE-S6KβII and His-S6KβC were phosphorylated with PKCβΙΙ as described above but without [γ-32P]ATP. The products of the reaction were either directly analyzed by infrared and UV matrix-assisted laser desorption ionization (mass spectrometry) [MALDI (MS)] or first digested with modified trypsin (Promega, Southampton, United Kingdom) or endoproteinase Lys-C (Roche, Lewes, East Sussex, United Kingdom) in 25 mM ammonium bicarbonate buffer (pH 8) at 37°C and then analyzed. Prior to proteolysis some samples were separated by one-dimensional SDS-PAGE, and an in-gel digest (51) was performed on excised bands of interest.
MALDI samples were prepared by using the dried droplet method, which involves mixing 0.5 μl of the analyte solution with 0.5 to 1 μl of the matrix solution on the target and drying them by means of a warm stream of air. For all measurements, external calibration was performed by using calibration mixture 2 of the Sequazyme peptide mass standards kit (5 peptides/protein in the 1- to 6-kDa mass range) from Applied Biosystems, Warrington, Cheshire, United Kingdom.
All measurements were conducted on a Voyager Elite XL (Applied Biosystems, Framingham, Mass.) MALDI-time of flight mass spectrometer equipped with delayed extraction and a reflector analyzer for improved mass resolution and accuracy. The instrument has been modified to enable infrared MALDI measurements at 2.94 μm with a Q-switched Speser 15Q (Spektrum GmbH, Berlin, Germany) Er:YAG laser as well as UV MALDI measurements at 337 nm utilizing a VSL-337ND nitrogen laser (Laser Science, Inc., Franklin, Mass.) as supplied by the manufacturer of the mass spectrometer. The technical details regarding the experimental setup have been reported elsewhere (12).
Production of a phosphoserine-specific S6Kβ antibody.
Polyclonal antiserum that recognizes a specific phosphorylation site was raised against phosphopeptide corresponding to the C-terminal 11 amino acids (residues 481 to 491) of S6Kβ (SGTKKS486KRGRG) with serine 486 as a phosphorylated residue. The peptide was coupled to keyhole limpet hemocyanin and injected into rabbits by using standard immunization techniques. Rabbit antibody specific for pS486-S6Kβ was affinity purified by using antigenic peptide coupled to Actigel (Sterogene) and screened for antigen reactivity by immunoblot analysis.
Immunoblot analysis.
Protein samples were subjected to SDS-PAGE and transferred onto 0.45-μm-pore-size nitrocellulose or Immobilon-P membranes. After blocking with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween 20, the membranes were probed overnight at 4°C with anti-EE (1:1,500), anti-Myc, anti-pS486-S6Kβ (1:1,000), or anti-phospho rpS6 (Ser235) (Upstate Biotechnology) antibody. The immunoblots were washed four times for 15 min with Tris-buffered saline containing 0.1% Tween 20 and incubated with peroxidase-conjugated secondary antibodies for 40 min at room temperature. The antigen-antibody complexes were detected with the enhanced chemiluminescence system (Amersham Pharmacia Biotech).
Immunofluorescent staining and microscopy.
HEK 293 cells were plated onto poly-l-lysine-coated coverslips in 24-well dishes at a density of 2.5 × 104 cells per well and cultured overnight. The cells were then transfected with 0.5 μg of expression vectors containing various S6Kα and S6Kβ constructs. At 24 h posttransfection, the cells were starved in serum-free DMEM for 24 h and then stimulated with 1 μM PMA for 30 min. LMB-treated cells were cultured in the presence of 10 ng of LMB/ml for 16 h before stimulation. After a brief wash at room temperature with PBS, cells were fixed with 4% formaldehyde for 20 min and permeabilized with 0.2% Triton X-100 in PBS for 5 min. Nonspecific binding was blocked by incubation with 0.5% bovine serum albumin in PBS for 30 min. The cells were then incubated with anti-EE (1:1,500) (mouse) or rabbit anti-pS486 (1:800) antibodies for 2 h at room temperature. After extensive washing with PBS, the samples were incubated for 45 min with goat fluorescein isothiocyanate-conjugated anti-mouse or anti-rabbit antibodies (1:200), respectively. Finally, the coverslips were extensively rinsed with PBS, air dried, and mounted onto microscope slides. Immunofluorescent staining was analyzed with a laser scanning microscope (LSM51D; Zeiss, Oberkochen, Germany).
RESULTS
S6Kβ but not S6Kα is phosphorylated in vitro at the C terminus by different isoforms of PKC.
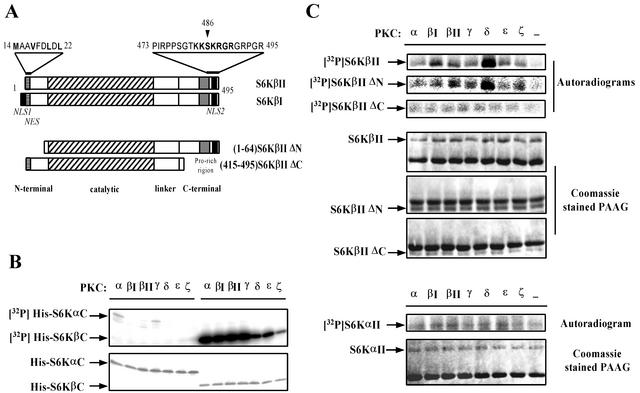
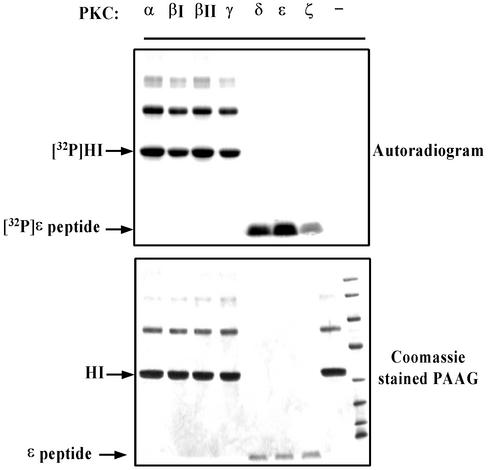
Inspection of the amino acid sequence of S6Kβ revealed a potential PKC phosphorylation site located within the C-terminal regulatory region (Fig. 1A). S6Kα displays a low level of identity with S6Kβ at the C terminus and does not contain consensus sequences for phosphorylation by PKC. To test whether PKC phosphorylates S6Kβ, we initially employed an in vitro kinase assay. The C-terminal regions of S6Kα and S6Kβ (His-S6KαC and His-S6KβC), expressed in bacteria as His-tag fusion proteins, were used as substrates in a PKC phosphorylation assay. As shown in Fig. 1B, all PKC isoforms tested efficiently phosphorylated His-S6KβC, whereas no significant phosphorylation of His-S6KαC was observed under similar conditions. The activities of the PKC isoforms were analyzed with histone H1 or ɛ-peptide as substrates (Fig. 2). It should be noted that the efficiency of His-S6KβC phosphorylation by PKCs correlated with their specific activities.
FIG. 1.
S6KβII, but not S6KαII, is phosphorylated at the C terminus by different PKC isoforms in vitro. (A) Schematic representation of S6KβI and S6KβII and their deletion mutants, which lack amino- and carboxyl-terminal sequences. Major domain boundaries are indicated. Structural features are indicated as follows: grey boxes indicate unique proline-rich sequences of S6Kβ; solid black boxes indicate NLSs (NLS1 and NLS2); striped boxes correspond to potential NESs. The N- and C-terminal amino acid sequences, containing NES and NLS, are shown above the diagrams. All recombinant constructs carry an N-terminal EE-tag sequence, and deleted amino acids are indicated. (B) In vitro phosphorylation of bacterially expressed His-S6KαC and His-S6KβC by various PKCs. Affinity-purified His-tagged S6Kα and S6Kβ C-terminal peptides were incubated in the presence of different recombinant PKC isoforms and [γ-32P]ATP. The reaction mixtures were separated by SDS-PAGE and stained with Coomassie. The dried gel was analyzed by autoradiography. (C) In vitro phosphorylation of recombinant full-length S6KαII, S6KβII, and deleted S6KβII mutants by PKCs. HEK 293 cells transiently transfected with wild-type EE-S6KαII, EE-S6KβII, EE-S6KβIIΔN, or EE-S6KβIIΔC were serum starved for 24 h, and recombinant proteins were immunoprecipitated with anti-EE-tag antibody. The immunoprecipitates were incubated with [γ-32P]ATP in the absence or presence of different recombinant PKC isoforms. The reaction mixtures were analyzed as described above.
FIG. 2.
Analysis of enzymatic activities of recombinant PKC isoforms. In vitro kinase assays were performed as described in Materials and Methods. HI, histone H1.
Next, we investigated whether full-length S6KαII and S6KβII could serve as substrates for PKCs in an in vitro kinase assay. In this experiment, transiently expressed EE-tagged forms of S6KαII and S6KβII were immunoprecipitated from serum-starved HEK 293 cells and subjected to in vitro phosphorylation by different isoforms of PKC. The results demonstrated that all isoforms of PKC readily phosphorylated full-length S6KβII but failed to use S6KαII as a substrate (Fig. 1C). We have also observed a higher efficiency of S6KβII phosphorylation by PKCβΙ, PKCβΙΙ, and PKCδ (2-, 1.5-, and 4-fold increases, respectively) when compared with other isoforms.
To confirm that the PKC phosphorylation site is located within the C terminus of S6Kβ, we created N- and C-terminal deletion mutants and tested whether they were phosphorylated by PKCs under the conditions described for the full-length kinases. As shown in Fig. 1C, deletion of the N-terminal region of S6Kβ did not affect the efficiency and the pattern of phosphorylation by PKC isoforms. However, the removal of the C terminus completely abolished PKC-mediated phosphorylation of S6KβII. The data presented above clearly indicate that S6Kβ can be phosphorylated by PKC in vitro and that the site(s) of phosphorylation is located at the C terminus.
Identification of PKC phosphorylation site(s) and characterization of phosphospecific antibodies.
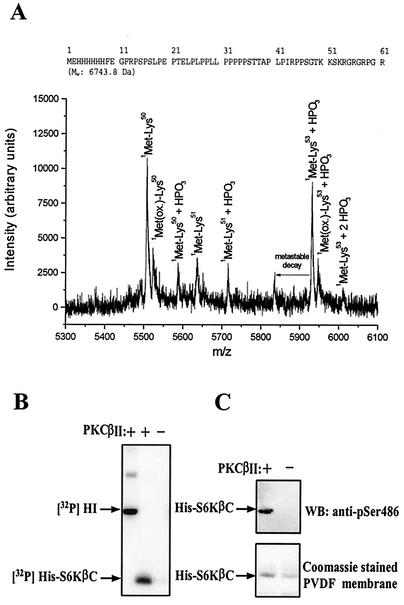
The precise identification of the PKC phosphorylation site(s) in S6KβII was carried out by MS. Affinity-purified His-S6KβC was used as a substrate for PKCβΙΙ in the presence of cold ATP. The products of the reaction were digested by trypsin or endoproteinase Lys-C, and the resulting peptides were analyzed by MS. Initial MALDI (MS) analysis of the intact or trypsin in-gel-digested His-S6KβC was inconclusive with regard to PKC phosphorylation. However, proteolysis with the endoproteinase Lys-C produced phosphorylation-indicative peptides (Fig. 3A). The recorded peptide ions from the MALDI (MS) analysis show that the main phosphorylation is located in the KS486K sequence stretch, suggesting serine as the phosphorylation site. The stoichiometry of S6KβII phosphorylation by PKCβΙΙ was found to be approximately 1 mol of phosphate per mol of S6KβII.
FIG. 3.
Identification of PKC phosphorylation site and characterization of phosphospecific S6Kβ antibody. (A) Mass spectroscopy analysis of PKC phosphorylation site in S6KβII. The amino acid sequence of His-S6KβC is shown on top. (B and C) Analysis of specificity of anti-pS486 antibody. Bacterially expressed His-S6KβC was incubated with [γ-32P]ATP in the presence (+) or absence −) of recombinant PKCβII. Samples were resolved by SDS-PAGE, transferred onto nitrocellulose membranes, and analyzed by autoradiography (B) or immunoblotting with anti-pS486 antibody (C). HI, histone H1; PVDF, polyvinylidene difluoride; WB, Western blot.
Phosphospecific antibodies are a powerful tool for investigating the physiological importance of protein phosphorylations. We therefore generated an antibody that specifically recognizes S6Kβ phosphorylated at S486. The antibodies were raised in rabbits and affinity purified on Actigel beads coupled with antigenic peptide. Recombinant His-S6KβC prephosphorylated with PKCβΙΙ was used to test the specificity of the antibodies generated. As shown in Fig. 3B and C, affinity-purified anti-pS486 antibody specifically recognized His-S6KβC only when it was prephosphorylated by PKCβΙΙ. Furthermore, the recognition of phosphorylated His-S6KβC by anti-pS486 antibody was abolished by preincubation with the phosphorylated form, but not with the nonphosphorylated form, of the antigenic peptide (data not shown).
Phosphorylation of S6KβII at S486 in cellular responses to mitogenic stimuli.
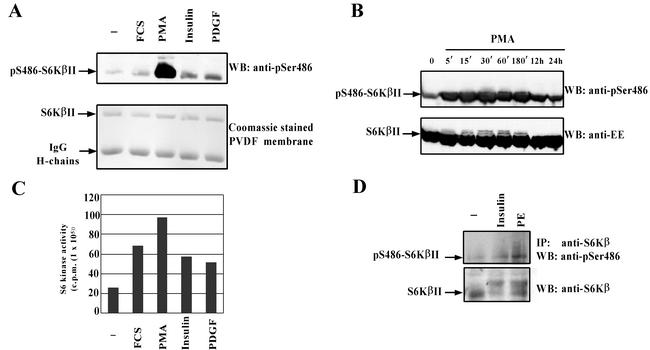
The availability of a phosphospecific antibody has allowed us to study the phosphorylation status of S6KβII at S486 in response to various extracellular stimuli. We found that treatment of HEK 293 cells transiently overexpressing EE-S6KβII with PMA induced a significant (up to 15-fold) increase in S486 phosphorylation (Fig. 4A). A time course stimulation of cells with PMA demonstrates that phosphorylation of S486 is very rapid and reaches a peak at 30 min but is still detectable even 24 h after induction (Fig. 4B). Noticeably, phosphorylation at S486 parallels the activation profile of S6KβII, as seen from the mobility shift of activated forms of the kinase (Fig. 4B).
FIG. 4.
S6KβII is phosphorylated at Ser486 in response to different mitogenic stimuli. HEK 293 cells were transiently transfected with wild-type EE-S6KβII, serum starved, and stimulated with 10% FCS, 1 μM PMA, 100 nM insulin, 50 ng of PDGF/ml, or vehicle alone (−). Recombinant S6KβII was immunoprecipitated with anti-EE antibody and used for the in vitro S6K assay (B) or analyzed by Western blotting (WB) with anti-pS486 antibody (A). (C) Time course phosphorylation of S6KβII at Ser486 in PMA-treated HEK 293 cells. HEK 293 cells were transiently transfected with wild-type EE-S6KβII, serum starved for 24 h, and stimulated with 1 μM PMA for the indicated period of time. Cell lysates were analyzed by Western blotting with anti-pS486 or anti-EE antibodies. (D) Phosphorylation of endogenous S6Kβ at Ser486 in phenylephrine (PE)-stimulated cardiomyocytes. Isolated cardiomyocytes were treated with 10 μM phenylephrine, 10 nM insulin, or vehicle alone for 30 min. Native S6Kβ was immunoprecipitated (IP) from lysed cells with anti-C-terminal antibodies. Immune complexes were separated by SDS-PAGE and immunoblotted with anti-pS486 antibody. The results presented have been reproduced in three independent experiments. IgG, immunoglobulin G.
We consistently observed an increase (1.5- to 3-fold) in S486 phosphorylation when starved HEK 293 cells were treated with FCS, insulin, or PDGF (Fig. 4A). In the case of FCS stimulation, the changes in S486 phosphorylation followed a time course similar to that seen for PMA (http://www.ludwig.ucl.ac.uk/cellreg-html/research.htm). When we compared the increase in the S6K activity of exogenously expressed S6KβII with the extent of S486 phosphorylation in response to PMA, FCS, insulin, and PDGF, no obvious correlation was observed (Fig. 4A and C). These results suggested that phosphorylation of S6Kβ at S486 might not affect its kinase activity or its activation by other kinases.
We have recently demonstrated that S6KβII is expressed at high levels in cardiomyocytes (ARVC) and is activated by treatment with insulin or phenylephrine (61). In contrast to S6Kα, which is known to be activated in cardiomyocytes via the PI3-K and mTOR signaling pathways, the activity of S6Kβ can also be regulated in a MEK-dependent manner. Moreover, studies from other laboratories show that treatment of cardiomyocytes with insulin and phenylephrine induces rapid activation of PKC (43, 46).
Therefore, this cellular model was used to investigate whether endogenous S6Kβ is phosphorylated at S486 in response to insulin and phenylephrine. We treated ARVC with 20 nM insulin or 10 μM phenylephrine for 30 min, and the endogenous S6Kβ was immunoprecipitated with the C-terminal polyclonal antibodies. Western blot analysis of immune complexes, resolved by SDS-PAGE, with anti-pS486 antibodies indicated that S6Kβ is specifically phosphorylated at S486 in cardiomyocytes treated with insulin and phenylephrine (Fig. 4D). Thus, endogenous S6Kβ in primary cells undergoes phosphorylation at S486 in response to a physiological agonist that activates PKC.
PKC mediates phosphorylation of S6KβII at S486 and rpS6 in vivo.
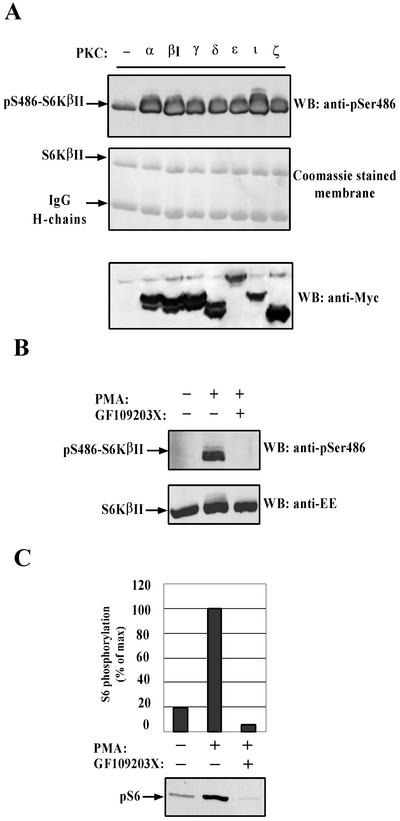
The in vitro phosphorylation studies and the ability of PMA to induce S6KβII phosphorylation at S486 strongly suggested the involvement of PKC. In order to examine whether PKC could mediate phosphorylation of S6KβII at S486 in vivo, EE-S6KβII was transiently coexpressed with various Myc-tagged PKCs in HEK 293 cells. Two days after transfection, S6KβII was immunoprecipitated with anti-EE antibodies, resolved by SDS-PAGE, and immunoblotted with anti-pS486 antibodies. The results indicated that coexpression of any PKC isoform with S6KβII induces strong phosphorylation of S486 (Fig. 5A). Coomassie staining of the polyvinylidene difluoride membrane showed that an equal amount of EE-S6KβII was immunoprecipitated from all transfected cells. Western blotting of total cell lysates with anti-Myc antibodies confirmed that all PKC isoforms were expressed at approximately equal levels (Fig. 5A, bottom section).
FIG. 5.
In vivo phosphorylation of S6KβII at Ser486 and rpS6 phosphorylation are mediated by PKC. (A) Coexpression of various PKCs with S6KβII induces phosphorylation at Ser486 in HEK 293 cells. HEK 293 cells were cotransfected with EE-S6KβII and various Myc-PKCs. Recombinant S6Kβ was immunoprecipitated with anti-EE-tag antibody and analyzed by Western blotting (WB) with anti-pS486 antibody. Expression levels of transiently expressed PKCs were analyzed in whole-cell extracts with anti-Myc antibody. (B) Effect of PKC inhibitor GF109203X on Ser486 phosphorylation. HEK 293 cells were transiently transfected with wild-type EE-S6KβII, serum starved, and stimulated with 1 μM PMA. A 1 μM concentration of GF109203X was added for 30 min prior to stimulation. (C) Effect of GF109203X on PMA-stimulated phosphorylation of rpS6. MCF7 cells were serum starved for 24 h and then treated with 1 μM PMA or vehicle alone for 30 min. A 1 μM concentration of GF109203X was added for 30 min prior to stimulation. Phosphorylation of S6 protein was analyzed in whole-cell extracts with anti-phospho-rpS6 (Ser235) antibody. IgG, immunoglobulin G; +, present; −, absent.
To further establish that S6KβII is a target for PKC-mediated phosphorylation in vivo, we tested the effect of a PKC inhibitor, GF109203X, on S486 phosphorylation in response to PMA. As shown in Fig. 5B, treatment of HEK 293 cells expressing EE-S6KβII with a 1 μM concentration of GF109203X completely eliminated PMA-induced phosphorylation at S486. Collectively, the results presented above strongly suggest that PKCs mediate the in vivo phosphorylation of S6KβII at S486.
rpS6 is known to be a physiological substrate for both S6Kα and S6Kβ (53). Phosphorylation of rpS6 is one of the earliest events detected following mitogenic stimulation, and it correlates with polysome formation and the initiation of protein synthesis (55). Multiple studies have shown that different mitogenic stimuli employ distinct signaling pathways to mediate rpS6 phosphorylation and the initiation of protein synthesis. Taking this into account, it was interesting to examine the contribution of PKC signaling to in vivo phosphorylation of rpS6. MCF7 cells were chosen for this study since they express large quantities of both S6KαII and S6KβII, as determined by immunoblot and Northern blot analysis (data not shown). The treatment of serum-starved MCF7 cells with PMA induces a fivefold increase in the level of rpS6 phosphorylation at S235 (Fig. 5C). This increase was completely inhibited by 1 μM GF109203X, strongly indicating that signaling via PKC is important for rpS6 phosphorylation in response to PMA.
PKC-mediated phosphorylation of S6KβII at Ser486 does not affect S6K activity.
Since S6K is activated by multiple Ser/Thr phosphorylations, it was important to investigate the effect of S486 phosphorylation on S6KβII activity. In order to explore the upstream regulation of S486 phosphorylation, we used two indirect inhibitors of S6K, rapamycin (mTOR pathway) and wortmannin (PI3-K pathway).
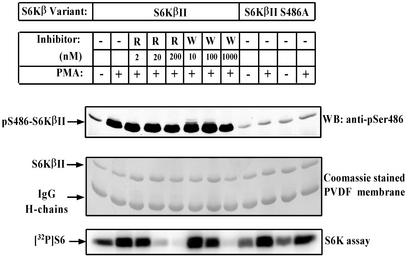
The treatment of serum-starved HEK 293 cells with PMA induced a fourfold increase in the activity of recombinant S6KβII towards rpS6 (Fig. 6). As expected, pretreatment of cells with rapamycin or wortmannin blocked PMA-induced activation of S6KβII. Noticeably, rapamycin did not exert any obvious effect on PMA-induced phosphorylation of S486 while wortmannin showed a slight inhibition at very high concentrations (Fig. 6).
FIG. 6.
PKC-mediated phosphorylation of S6KβII at Ser486 is insensitive to specific TOR/FRAP and PI3-K inhibitors and does not effect S6K activity. HEK 293 cells were transiently transfected with wild-type EE-S6KβII or EE-S6KβII S486A and incubated in the presence (+) or absence (−) of 1 μM PMA for 30 min after 24 h of starvation. Rapamycin (R) or wortmannin (W) was added for 30 min before cell stimulation. Recombinant S6KβII was immunoprecipitated with anti-EE-tag antibody and used for the in vitro S6K assay or analyzed by immunoblotting with anti-pS486 antibody. IgG, immunoglobulin G; PVDF, polyvinylidene difluoride; WB, Western blot.
These results have also been confirmed by in vitro studies. In these experiments, EE-S6KβII was immunoprecipitated from serum-starved HEK 293 cells and phosphorylated with different PKC isoforms in the presence of cold ATP. After washing, S6K activity towards rpS6 was measured. These experiments revealed that prephosphorylation of S6KβII by PKCs does not affect its S6K activity (http://www.ludwig.ucl.ac.uk/cellreg-html/research.htm).
To gain further insight into the importance of PKC-mediated phosphorylation of S6KβII, we mutated serine 486 to alanine. It is important to note that anti-pS486 antibodies did not recognize the mutated form of S6KβII overexpressed in HEK 293 cells, confirming their specificity (http://www.ludwig.ucl.ac.uk/cellreg-html/research.htm). Moreover, the activity of the S486A mutant was found to be similar to that of thewild-type kinase in HEK 293 cells treated or not treated with PMA (Fig. 6). Taken together, the results demonstrate that PKC-mediated phosphorylation of S6KβII at S486 does not effect the activity of the kinase in response to mitogenic stimuli.
Effect of PMA and LMB on subcellular localization of S6Kβ.
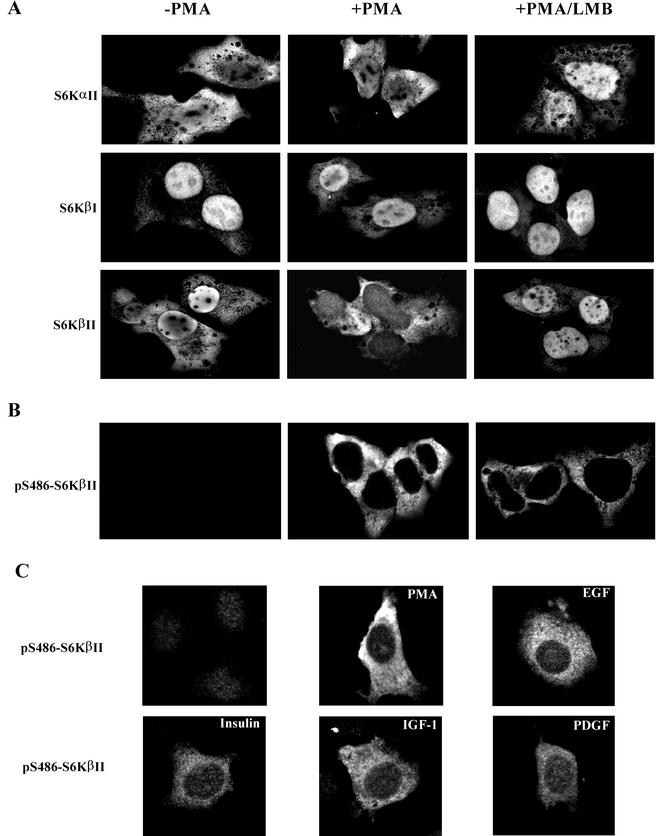
Since S486 is located within the C-terminal nuclear localization sequence, it was reasoned that PKC-mediated phosphorylation of this site might modulate the subcellular localization of S6Kβ. To test this possibility, we initially examined the subcellular localization of EE-S6Ks in transiently transfected HEK 293 cells stimulated with PMA. As shown in Fig. 7A, EE-S6KαII was mainly localized in the cytoplasm of serum-starved cells and PMA stimulation did not change its pattern of distribution. However, pretreatment of cells with LMB leads to accumulation of EE-S6KαII in the nucleus, suggesting dynamic nucleocytoplasmic shuttling. These data are in agreement with studies carried out by other groups (27, 28).
FIG.7.
Analysis of subcellular localization of S6Kα and S6Kβ by confocal microscopy. (A) HEK 293 cells were transiently transfected with wild-type EE-S6KαII, EE-S6KβI, or EE-S6KβII, serum starved for 24 h, and stimulated with 1 μM PMA (+PMA) for 30 min or vehicle alone (−PMA). Treatment of cells with LMB (10 ng/ml) was carried out for 16 h before the stimulation with PMA. Cells were fixed, probed with anti-EE antibody and fluorescein isothiocyanate-labeled anti-mouse immunoglobulin G, and analyzed by confocal microscopy. (B) Subcellular localization of pSer486-S6KβII in HEK 293 cells treated with PMA and LMB. HEK 293 cells were transfected with EE-S6KβII and treated in the same way as described above. After fixation and probing with anti-pS486 antibody, confocal microscopy analysis was carried out. (C) Subcellular localization of pSer486-S6KβII in NIH 3T3 cells treated with PMA, EGF, IGF-1, insulin, or PDGF. Transient transfection of NIH 3T3 cells and confocal microscopy were performed as described in Materials and Methods.
By contrast, EE-S6KβII is found predominantly in the nucleus of serum-starved cells and shifts to the cytoplasm after PMA treatment (Fig. 7A). Moreover, LMB prevents PMA-stimulated accumulation of EE-S6KβII in the cytoplasm as seen by the retention of the kinase in the nucleus. This finding indicates that S6KβII may shuttle between the nucleus and the cytosol during the course of activation. In contrast, the nuclear localization of EE-S6KβI, which contains two NLS sequences, one at the N terminus and another at the C terminus, is not affected by treatment with PMA or LMB (Fig. 7A).
Taken together, these results suggest the existence of nucleocytoplasmic shuttling for S6KβII, which is LMB sensitive and could be regulated by the PKC signaling pathway.
Phosphorylation at S486 eliminates the function of the NLS in S6KβII.
The data presented above prompted us to study the subcellular distribution of pS486-S6KβII in PMA-stimulated cells with phosphospecific antibodies. Confocal immunofluorescence microscopy clearly indicated that pS486-S6KβII is localized exclusively in the cytoplasm of PMA-treated cells (Fig. 7B). No signal was detected in serum-starved cells, confirming once again the specificity of the phosphospecific antibodies. It was interesting to study whether blocking nuclear export with LMB affected the subcellular localization of pS486-S6KβII. As shown in Fig. 7B, the pattern of pS486-S6KβII distribution did not change when cells were treated with both PMA and LMB. No changes in the subcellular localization of pS486-S6KβII were observed when cells were treated with PMA in the presence of rapamycin (data not shown). Altogether, these results strongly suggest that PMA-induced phosphorylation of S6KβII at S486 takes place in the cytoplasm and prevents translocation of the kinase to the nucleus. Moreover, we have analyzed the phosphorylation and subcellular localization of pS486-S6KβII in NIH 3T3 cells stimulated with other mitogenic stimuli. Figure 7C shows that S6KβII is phosphorylated at S486 in response to EGF, IGF-1, insulin, or PDGF, and the phosphorylated protein is localized in the cytoplasm. However, the immunofluorescent signal is significantly weaker when compared to that of PMA stimulation. These data are in agreement with anti-pS486-S6Kβ immunoblot analysis presented in Fig. 4A.
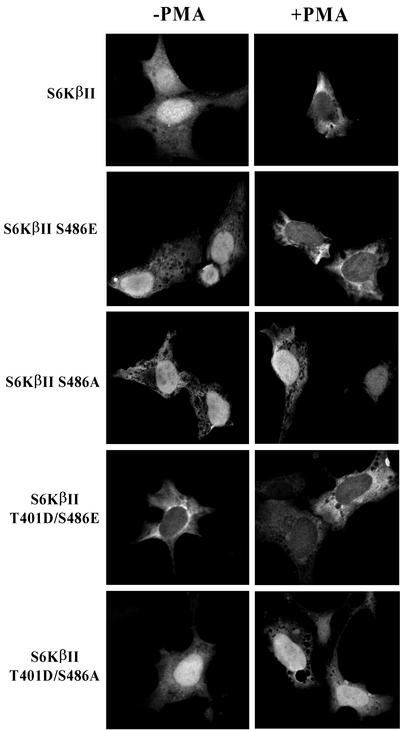
Substitution of the phosphorylation site with acidic amino acids mimics, in many cases, the phosphorylation of that site in the protein of interest and therefore provides an excellent model for functional studies. To this end we have generated an S486E mutant of S6KβII and analyzed its subcellular localization with the expectation that it would be present only in the cytoplasm of transfected cells. Unexpectedly, the S486E mutant behaved similarly to the wild-type protein in serum-starved and PMA-stimulated cells (Fig. 8). However, we found that the S486A mutant is predominantly localized in the nucleus of serum-starved cells and does not accumulate in the cytoplasm in response to PMA. A likely explanation of the observed differences in the subcellular localization of S486E and S486A mutants is that regulation of nucleocytoplasmic shuttling by phosphorylation of S6KβII at this site requires the kinase to be in an activated state. To test this hypothesis, we created a double mutant of S6KβII bearing T401D and S486E substitutions. It has previously been demonstrated that a T401D (equivalent to T412 in S6Kα) mutant is constitutively active and possesses fourfold-higher S6K activity than the wild-type S6KβII (60). The results of immunofluorescence analysis unambiguously demonstrate that the T401D/S486E mutant is retained in the cytoplasm of serum-starved and PMA-treated cells (Fig. 8). The importance of S486 phosphorylation in controlling nuclear shuttling of the activated form of S6KβII was further confirmed with the use of a T401D/S486A mutant. This mutant was found to be localized predominantly in the nucleus of serum-starved cells and did not accumulate in the cytoplasm in response to PMA.
FIG. 8.
Subcellular localization of S6KβII mutants in HEK 293 cells. Plasmids carrying EE-S6KβII, EE-S6KβII S486E, EE-S6KβII S486A, EE-S6KβII T401D/S486E, or EE-S6KβII T401D/S486A were transfected into HEK 293 cells. After 24 h, cells were serum starved and stimulated for 30 min with 1 μM PMA (+PMA) or vehicle alone (−PMA). Fixed cells were incubated with anti-EE antibody and analyzed by immunofluorescence.
Taken together, the results of immunofluorescence microscopy clearly demonstrate that PMA-mediated phosphorylation of S6KβII at S486 regulates nucleocytoplasmic shuttling of an activated form of the kinase. Since S486 is located in the middle of the C-terminal NLS, we propose that phosphorylation of this residue eliminates its function.
DISCUSSION
In this study we have addressed the role of PKC signaling in the regulation of nucleocytoplasmic shuttling of S6KβII. We found that S6Kβ, but not S6Kα, is phosphorylated by PKCs in vitro and in vivo. The site of phosphorylation was identified by MS as S486, which is located in the C-terminal regulatory domain. Furthermore, the use of phosphospecific antibodies indicated that S486 phosphorylation is induced by various mitogenic stimuli, including PMA, FCS, EGF, IGF-1, insulin, and PDGF.
Studies from different laboratories demonstrated that activation of S6Kα is a multistep phosphorylation process, involving at least nine sites and various S/T kinases (14, 64). Most of these sites are conserved in S6Kβ, with the exception of one (equivalent to T444 in S6Kα), indicating a very similar mode of activation. In contrast, this study clearly demonstrates that PKC-mediated phosphorylation of S6KβII at S486 is not involved in the regulation of its kinase activity.
What is the importance of S486 phosphorylation for the cellular functions of S6KβII? In agreement with the results of previous studies, we detected S6KαII mainly in the cytoplasm, whereas S6KβII was predominately nuclear (27, 28). The presence of a functional NLS at the C terminus of S6KβII has been recently reported by Koh et al. The authors also found that mutation of Lys487 to Met in the KKSK487RGR sequence of S6KβII relocates the kinase from the nucleus to the cytoplasm. Since S486 is located in the middle of the C-terminal NLS, we focused our efforts on elucidating the effect of PKC-mediated phosphorylation of this site on the subcellular localization of S6KβII. Following this assumption, we found that treatment of cells with PMA induced rapid translocation of S6KβII from the nucleus to the cytoplasm, whereas no changes in the subcellular localization of S6KαII were observed. Furthermore, this translocation was blocked completely by LMB, a specific inhibitor of CRM1-mediated nuclear export, indicating the existence of nucleocytoplasmic shuttling for S6KβII. Interestingly, PMA and LMB do not affect the subcellular localization of S6KβI, whose exclusive nuclear distribution is determined by the presence of two NLSs.
A continuous shuttling of S6KβII between the nucleus and the cytoplasm may require the presence of both the NLS and nuclear export signal (NES) sequences in S6KβII. During the last few years, a short leucine-rich consensus sequence was identified in a variety of signaling molecules and shown to possess nuclear export properties (18, 39). Inspection of the amino acid sequence allowed us to identify a potential NES located at the N terminus of S6KβII (Fig. 1A). This sequence resembles the Crm1 consensus sequence, which is known to be LMB sensitive. The nuclear export receptor for S6KβII remains to be identified. We are currently investigating the function of this potential NES by mutational analysis and confocal microscopy. Preliminary data indicate that the N-terminal region of S6KβII possesses a functional NES which is LMB sensitive (T. Valovka, unpublished data).
Many proteins are transported constitutively into and out of the nucleus by members of the β-importin family of nuclear transport receptors (17). In contrast to constitutive transport, regulated transport occurs only in response to specific cellular signals and involves a specific NLS receptor, usually α-importin (26). The docking of proteins that contain classical or bipartite types of NLS to the cytoplasmic side of the nuclear pore is mediated by an α-importin/β-importin heterodimer and Ran GTPase. The formation of this multiprotein complex can be influenced directly by posttranslational modifications, such as phosphorylation, acetylation, and methylation (24). Is nuclear transport of S6KβII driven by this mode of regulation?
Mutational analysis of the S486 site allowed us to gain insight into the regulation of S6KβII nucleocytoplasmic shuttling by PKCs. We observed that an S486A mutant of S6KβII does not accumulate in the cytoplasm in response to PMA, indicating that phosphorylation of S486 might be necessary for this event to occur. However, when we tested the subcellular localization of the S486E mutant, we found unexpectedly that it behaves similarly to the wild-type S6KβII. Therefore, phosphorylation of S6KβII at S486 is not sufficient on its own to confer cytoplasmic localization of the kinase. Further mutational studies of S486 and T401 (equivalent to T412 in S6KαII) uncovered the dependence of nucleocytoplasmic shuttling of S6KβII on the activated state of the kinase.
A possible explanation of these findings is that S6KβII has to be in an activated state, in which the structure unfolds, making both NES and NLS operational. The structure of S6K has not been solved, and in the absence of crystallographic data, the primary structure of S6K has been functionally dissected into four domains. Based on these studies, a model for S6K activation has been proposed which implies that active conformation of the kinase is achieved by coordinated phosphorylations at three regions: the C-terminal autoinhibitory domain, by Ser-Pro-directed kinases; the activation loop in the kinase domain, by PDK1; and the conserved hydrophobic site in the kinase-extension domain (48). It is believed that in unstimulated cells, the interaction between the N- and C-terminal regulatory domains keeps the kinase domain in a locked conformation. Following mitogen stimulation, multiple phosphorylations open the structure by initially unlocking the N-terminal domain and subsequently releasing the C-terminal autoinhibitory domain. In agreement with this model, PMA-induced activation of S6KβII may release the N-terminal domain, making the NES operational. This may shift the steady-state constants for nuclear export and import, establishing an altered equilibrium in the nucleocytoplasmic shuttling of S6KβII.
Using pS486 phosphospecific antibodies, we discovered that pS486-S6KβII is exclusively localized in the cytoplasm of PMA-treated cells and that LMB does not alter its localization. These data strongly suggest that phosphorylation of S6KβII at S486 occurs in the cytoplasm of PMA-stimulated cells. Moreover, phosphorylation of S6KβII at S486 coincides with the depletion of the kinase from the nucleus and subsequent accumulation in the cytoplasm (Valovka, unpublished). We propose that phosphorylation of S6KβII at S486 eliminates the function of its sole NLS, and as a result, the kinase is confined to the cytoplasm. This mode of regulation (NLS masking) is common among signaling molecules and has been reported for DAG kinase ζ, Ca2+/calmodulin-dependent protein kinase II, and the forkhead transcription factor AFX (5, 23, 59).
What is the physiological relevance of S6KβII translocation from the nucleus to the cytoplasm in response to mitogenic stimuli? One possible explanation is that it brings the kinase in close proximity to its substrate(s), such as rpS6. Knockout studies of the S6Kα gene in mice showed that the S6 protein is a physiological substrate for S6Kβ (53). Mitogen-induced phosphorylation of rpS6 is associated with the initiation of protein synthesis of a specific pool of mRNA whose gene products are involved in ribosomal biogenesis (25).
Based on the data presented here and current knowledge of signaling via S6Ks, we propose a model to explain nucleocytoplasmic shuttling of S6KβII in response to mitogenic stimuli, such as PMA (Fig. 9). In unstimulated cells, S6KβII adopts an inactive conformation and is mainly localized in the nucleus. In this state, S6KβII import must be faster than export or the kinase may be in complex with an anchoring protein in the nucleus. Treatment of cells with PMA or other mitogenic stimuli triggers the activation of classical and novel PKCs and downstream signaling molecules, including S6KβII. The fact that exclusive nuclear forms of S6K, S6KαI and S6KβI, are activated in response to mitogenic stimuli suggests that all components required for multistep phosphorylation and/or activation of S6KβII are present in the nucleus (35, 49). Activation of S6KβII may unfold the kinase, releasing the N-terminal NES from its intramolecular interactions. In this state, the kinase may be transported to the cytoplasm by Crm1-facilitated nuclear export. Phosphorylation of S6KβII by activated forms of PKC may be essential for inactivating the function of its C-terminal NLS. The addition of negative charges within the NLS or flanking regions may eliminate the interaction with the NLS receptor. Given that negatively charged sequences of the NLS receptor are thought to bind to the positively charged NLS of nuclear-targeted proteins for nuclear import to occur (54), it is not surprising that the presence of a negative charge within the NLS may inhibit this interaction. Retention of the activated form of S6KβII in the cytoplasm could be required for phosphorylation of rpS6 and initiation of protein synthesis. It is well documented that PMA-activated protein synthesis is a key event for the induction of cell growth and proliferation (3, 37, 40, 41). Dephosphorylation of S486 in response to environmental changes can unmask the C-terminal NLS, making it available for importin-dependent nuclear import.
FIG. 9.
Subcellular localization of S6KβII is regulated by PKC. See the text for details.
In conclusion, this report describes for the first time mitogen-regulated nucleocytoplasmic shuttling of S6KβII and deciphers a critical role of PKC signaling in this process.
Acknowledgments
We thank M. Griffin for excellent technical assistance.
This work was supported in part by grants from the Wellcome Trust (055427/Z/98), the British Heart Foundation (PE99/004), and The Royal Society (FSU/CEE/JP). T.V. was supported by the Overseas Research Students Awards Scheme (ORS/2000061024).
REFERENCES
- 1.Akimoto, K., M. Nakaya, T. Yamanaka, J. Tanaka, S. Matsuda, Q. P. Weng, J. Avruch, and S. Ohno. 1998. Atypical protein kinase C lambda binds and regulates p70 S6 kinase. Biochem. J. 335:417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avruch, J., C. Belham, Q. Weng, K. Hara, and K. Yonezawa. 2001. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog. Mol. Subcell. Biol. 26:115-154. [DOI] [PubMed] [Google Scholar]
- 3.Brostrom, M. A., K. V. Chin, C. Cade, D. Gmitter, and C. O. Brostrom. 1987. Stimulation of protein synthesis in pituitary cells by phorbol esters and cyclic AMP. Evidence for rapid induction of a component of translational initiation. J. Biol. Chem. 262:16515-16523. [PubMed] [Google Scholar]
- 4.Brown, E. J., P. A. Beal, C. T. Keith, J. Chen, T. B. Shin, and S. L. Schreiber. 1995. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377:441-446. [DOI] [PubMed] [Google Scholar]
- 5.Brownawell, A. M., G. J. Kops, I. G. Macara, and B. M. Burgering. 2001. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell. Biol. 21:3534-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 7.Burnett, P. E., R. K. Barrow, N. A. Cohen, S. H. Snyder, and D. M. Sabatini. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA 95:1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett, P. E., S. Blackshaw, M. M. Lai, I. A. Qureshi, A. F. Burnett, D. M. Sabatini, and S. H. Snyder. 1998. Neurabin is a synaptic protein linking p70 S6 kinase and the neuronal cytoskeleton. Proc. Natl. Acad. Sci. USA 95:8351-8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, M. M., and J. Blenis. 1996. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 85:573-583. [DOI] [PubMed] [Google Scholar]
- 10.Chou, M. M., and J. Blenis. 1995. The 70 kDa S6 kinase: regulation of a kinase with multiple roles in mitogenic signalling. Curr. Opin. Cell Biol. 7:806-814. [DOI] [PubMed] [Google Scholar]
- 11.Chung, J., T. C. Grammer, K. P. Lemon, A. Kazlauskas, and J. Blenis. 1994. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature 370:71-75. [DOI] [PubMed] [Google Scholar]
- 12.Cramer, R., W. J. Richter, E. Stimson, and A. L. Burlingame. 1998. Analysis of phospho- and glycopolypeptides with infrared matrix-assisted laser desorption and ionization. Anal. Chem. 70:4939-4944. [DOI] [PubMed] [Google Scholar]
- 13.de Groot, R. P., L. M. Ballou, and P. Sassone-Corsi. 1994. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell 79:81-91. [DOI] [PubMed] [Google Scholar]
- 14.Dennis, P. B., N. Pullen, R. B. Pearson, S. C. Kozma, and G. Thomas. 1998. Phosphorylation sites in the autoinhibitory domain participate in p70(s6k) activation loop phosphorylation. J. Biol. Chem. 273:14845-14852. [DOI] [PubMed] [Google Scholar]
- 15.Dufner, A., and G. Thomas. 1999. Ribosomal S6 kinase signaling and the control of translation. Exp. Cell Res. 253:100-109. [DOI] [PubMed] [Google Scholar]
- 16.Duncan, R., and E. H. McConkey. 1982. Preferential utilization of phosphorylated 40-S ribosomal subunits during initiation complex formation. Eur. J. Biochem. 123:535-538. [DOI] [PubMed] [Google Scholar]
- 17.Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 18.Gorlich, D., and I. W. Mattaj. 1996. Nucleocytoplasmic transport. Science 271:1513-1518. [DOI] [PubMed] [Google Scholar]
- 19.Gout, I., T. Minami, K. Hara, Y. Tsujishita, V. Filonenko, M. D. Waterfield, and K. Yonezawa. 1998. Molecular cloning and characterization of a novel p70 S6 kinase, p70 S6 kinase beta containing a proline-rich region. J. Biol. Chem. 273:30061-30064. [DOI] [PubMed] [Google Scholar]
- 20.Han, J. W., R. B. Pearson, P. B. Dennis, and G. Thomas. 1995. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J. Biol. Chem. 270:21396-21403. [DOI] [PubMed] [Google Scholar]
- 21.Hara, K., K. Yonezawa, Q. P. Weng, M. T. Kozlowski, C. Belham, and J. Avruch. 1998. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 273:14484-14494. [DOI] [PubMed] [Google Scholar]
- 22.Harada, H., J. S. Andersen, M. Mann, N. Terada, and S. J. Korsmeyer. 2001. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA 98:9666-9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heist, E. K., M. Srinivasan, and H. Schulman. 1998. Phosphorylation at the nuclear localization signal of Ca2+/calmodulin-dependent protein kinase II blocks its nuclear targeting. J. Biol. Chem. 273:19763-19771. [DOI] [PubMed] [Google Scholar]
- 24.Jans, D. A., and S. Hubner. 1996. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol. Rev. 76:651-685. [DOI] [PubMed] [Google Scholar]
- 25.Jefferies, H. B., S. Fumagalli, P. B. Dennis, C. Reinhard, R. B. Pearson, and G. Thomas. 1997. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 16:3693-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaffman, A., and E. K. O'Shea. 1999. Regulation of nuclear localization: a key to a door. Annu. Rev. Cell Dev. Biol. 15:291-339. [DOI] [PubMed] [Google Scholar]
- 27.Kim, J. E., and J. Chen. 2000. Cytoplasmic-nuclear shuttling of FKBP12-rapamycin-associated protein is involved in rapamycin-sensitive signaling and translation initiation. Proc. Natl. Acad. Sci. USA 97:14340-14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh, H., K. Jee, B. Lee, J. Kim, D. Kim, Y. H. Yun, J. W. Kim, H. S. Choi, and J. Chung. 1999. Cloning and characterization of a nuclear S6 kinase, S6 kinase-related kinase (SRK); a novel nuclear target of Akt. Oncogene 18:5115-5119. [DOI] [PubMed] [Google Scholar]
- 29.Lane, H. A., A. Fernandez, N. J. Lamb, and G. Thomas. 1993. p70s6k function is essential for G1 progression. Nature 363:170-172. [DOI] [PubMed] [Google Scholar]
- 30.Lee-Fruman, K. K., C. J. Kuo, J. Lippincott, N. Terada, and J. Blenis. 1999. Characterization of S6K2, a novel kinase homologous to S6K1. Oncogene 18:5108-5114. [DOI] [PubMed] [Google Scholar]
- 31.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 32.Martin, K. A., S. S. Schalm, C. Richardson, A. Romanelli, K. L. Keon, and J. Blenis. 2001. Regulation of ribosomal S6 kinase 2 by effectors of the phosphoinositide 3-kinase pathway. J. Biol. Chem. 276:7884-7891. [DOI] [PubMed] [Google Scholar]
- 33.Martin, K. A., S. S. Schalm, A. Romanelli, K. L. Keon, and J. Blenis. 2001. Ribosomal S6 kinase 2 inhibition by a potent C-terminal repressor domain is relieved by mitogen-activated protein-extracellular signal-regulated kinase kinase-regulated phosphorylation. J. Biol. Chem. 276:7892-7898. [DOI] [PubMed] [Google Scholar]
- 34.Mellor, H., and P. J. Parker. 1998. The extended protein kinase C superfamily. Biochem. J. 332:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minami, T., K. Hara, N. Oshiro, S. Ueoku, K. Yoshino, C. Tokunaga, Y. Shirai, N. Saito, I. Gout, and K. Yonezawa. 2001. Distinct regulatory mechanism for p70 S6 kinase beta from that for p70 S6 kinase alpha. Genes Cells 6:1003-1015. [DOI] [PubMed] [Google Scholar]
- 36.Montagne, J., M. J. Stewart, H. Stocker, E. Hafen, S. C. Kozma, and G. Thomas. 1999. Drosophila S6 kinase: a regulator of cell size. Science 285:2126-2129. [DOI] [PubMed] [Google Scholar]
- 37.Morley, S. J., and J. A. Traugh. 1990. Differential stimulation of phosphorylation of initiation factors eIF-4F, eIF-4B, eIF-3, and ribosomal protein S6 by insulin and phorbol esters. J. Biol. Chem. 265:10611-10616. [PubMed] [Google Scholar]
- 38.Newton, A. C. 1995. Protein kinase C: structure, function, and regulation. J. Biol. Chem. 270:28495-28498. [DOI] [PubMed] [Google Scholar]
- 39.Nigg, E. A. 1997. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature 386:779-787. [DOI] [PubMed] [Google Scholar]
- 40.Nishizuka, Y. 1992. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258:607-614. [DOI] [PubMed] [Google Scholar]
- 41.Nishizuka, Y. 1984. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693-698. [DOI] [PubMed] [Google Scholar]
- 42.Pardo, O. E., A. Arcaro, G. Salerno, T. D. Tetley, T. Valovka, I. Gout, and M. J. Seckl. 2001. Novel cross talk between MEK and S6K2 in FGF-2 induced proliferation of SCLC cells. Oncogene 20:7658-7667. [DOI] [PubMed] [Google Scholar]
- 43.Pellieux, C., T. Sauthier, A. Domenighetti, D. J. Marsh, R. D. Palmiter, H. R. Brunner, and T. Pedrazzini. 2000. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proc. Natl. Acad. Sci. USA 97:1595-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pende, M., S. C. Kozma, M. Jaquet, V. Oorschot, R. Burcelin, Y. Le Marchand-Brustel, J. Klumperman, B. Thorens, and G. Thomas. 2000. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408:994-997. [DOI] [PubMed] [Google Scholar]
- 45.Petritsch, C., H. Beug, A. Balmain, and M. Oft. 2000. TGF-beta inhibits p70 S6 kinase via protein phosphatase 2A to induce G(1) arrest. Genes Dev. 14:3093-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puceat, M., R. Hilal-Dandan, B. Strulovici, L. L. Brunton, and J. H. Brown. 1994. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J. Biol. Chem. 269:16938-16944. [PubMed] [Google Scholar]
- 47.Pullen, N., P. B. Dennis, M. Andjelkovic, A. Dufner, S. C. Kozma, B. A. Hemmings, and G. Thomas. 1998. Phosphorylation and activation of p70s6k by PDK1. Science 279:707-710. [DOI] [PubMed] [Google Scholar]
- 48.Pullen, N., and G. Thomas. 1997. The modular phosphorylation and activation of p70s6k. FEBS Lett. 410:78-82. [DOI] [PubMed] [Google Scholar]
- 49.Reinhard, C., A. Fernandez, N. J. Lamb, and G. Thomas. 1994. Nuclear localization of p85s6k: functional requirement for entry into S phase. EMBO J. 13:1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romanelli, A., K. A. Martin, A. Toker, and J. Blenis. 1999. p70 S6 kinase is regulated by protein kinase Cζ and participates in a phosphoinositide 3-kinase-regulated signalling complex. Mol. Cell Biol. 19:2921-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeld, J., J. Capdevielle, J. C. Guillemot, and P. Ferrara. 1992. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 203:173-179. [DOI] [PubMed] [Google Scholar]
- 52.Shah, O. J., S. R. Kimball, and L. S. Jefferson. 2000. Among translational effectors, p70S6k is uniquely sensitive to inhibition by glucocorticoids. Biochem. J. 347:389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shima, H., M. Pende, Y. Chen, S. Fumagalli, G. Thomas, and S. C. Kozma. 1998. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 17:6649-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silver, P. A. 1991. How proteins enter the nucleus. Cell 64:489-497. [DOI] [PubMed] [Google Scholar]
- 55.Stewart, M. J., and G. Thomas. 1994. Mitogenesis and protein synthesis: a role for ribosomal protein S6 phosphorylation? Bioessays 16:809-815. [DOI] [PubMed] [Google Scholar]
- 56.Susa, M., A. R. Olivier, D. Fabbro, and G. Thomas. 1989. EGF induces biphasic S6 kinase activation: late phase is protein kinase C-dependent and contributes to mitogenicity. Cell 57:817-824. [DOI] [PubMed] [Google Scholar]
- 57.Susa, M., D. Vulevic, H. A. Lane, and G. Thomas. 1992. Inhibition or down-regulation of protein kinase C attenuates late phase p70s6k activation induced by epidermal growth factor but not by platelet-derived growth factor or insulin. J. Biol. Chem. 267:6905-6909. [PubMed] [Google Scholar]
- 58.Tang, H., E. Hornstein, M. Stolovich, G. Levy, M. Livingstone, D. Templeton, J. Avruch, and O. Meyuhas. 2001. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol. Cell. Biol. 21:8671-8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Topham, M. K., M. Bunting, G. A. Zimmerman, T. M. McIntyre, P. J. Blackshear, and S. M. Prescott. 1998. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature 394:697-700. [DOI] [PubMed] [Google Scholar]
- 60.Valovka, T., V. Filonenko, S. Palchevsky, M. Velikiy, L. Drobot, M. D. Waterfield, G. Matsuka, and I. Gout. 1999. Functional and regulatory properties of p70S6 kinase β. Biopolym. Cell 15:1-7. [Google Scholar]
- 61.Wang, L., I. Gout, and C. G. Proud. 2001. Cross-talk between the ERK and p70 S6 kinase (S6K) signaling pathways. MEK-dependent activation of S6K2 in cardiomyocytes. J. Biol. Chem. 276:32670-32677. [DOI] [PubMed] [Google Scholar]
- 62.Wang, L., X. Wang, and C. G. Proud. 2000. Activation of mRNA translation in rat cardiac myocytes by insulin involves multiple rapamycin-sensitive steps. Am. J. Physiol. Heart Circ. Physiol. 278:H1056-H1068. [DOI] [PubMed] [Google Scholar]
- 63.Wang, X., W. Li, M. Williams, N. Terada, D. R. Alessi, and C. G. Proud. 2001. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 20:4370-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weng, Q. P., M. Kozlowski, C. Belham, A. Zhang, M. J. Comb, and J. Avruch. 1998. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J. Biol. Chem. 273:16621-16629. [DOI] [PubMed] [Google Scholar]