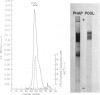
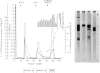
Abstract
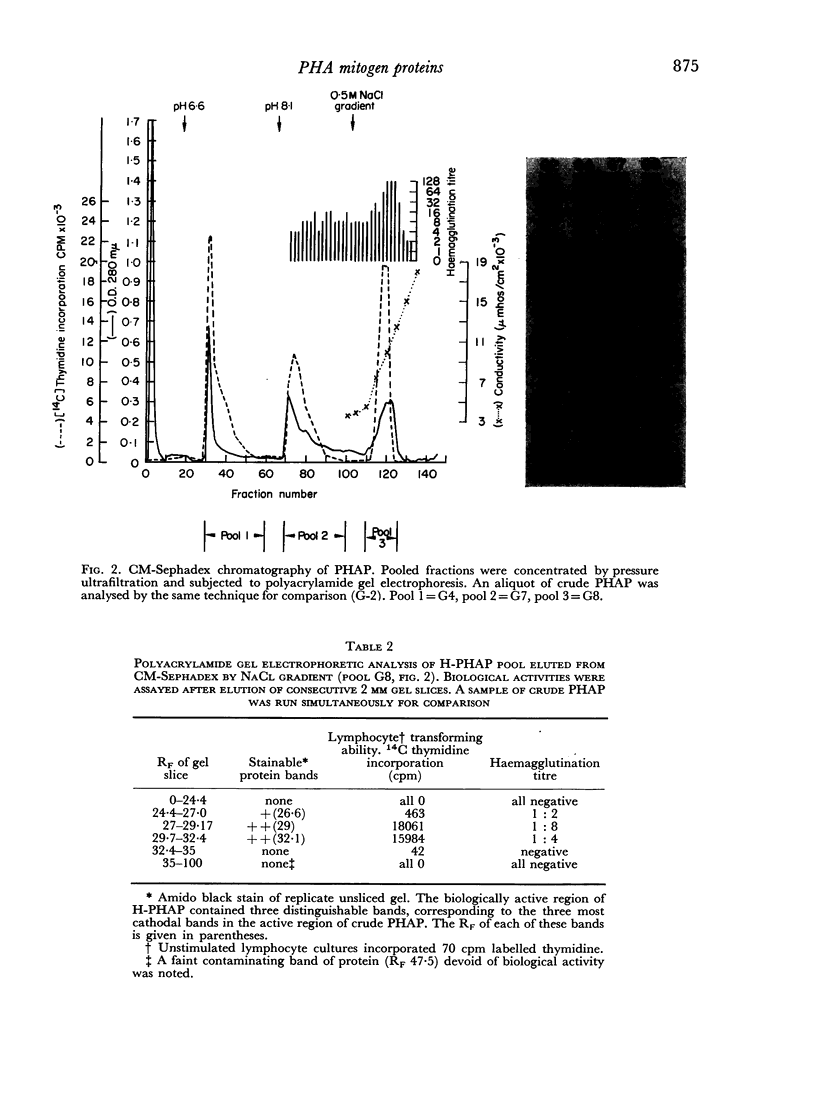
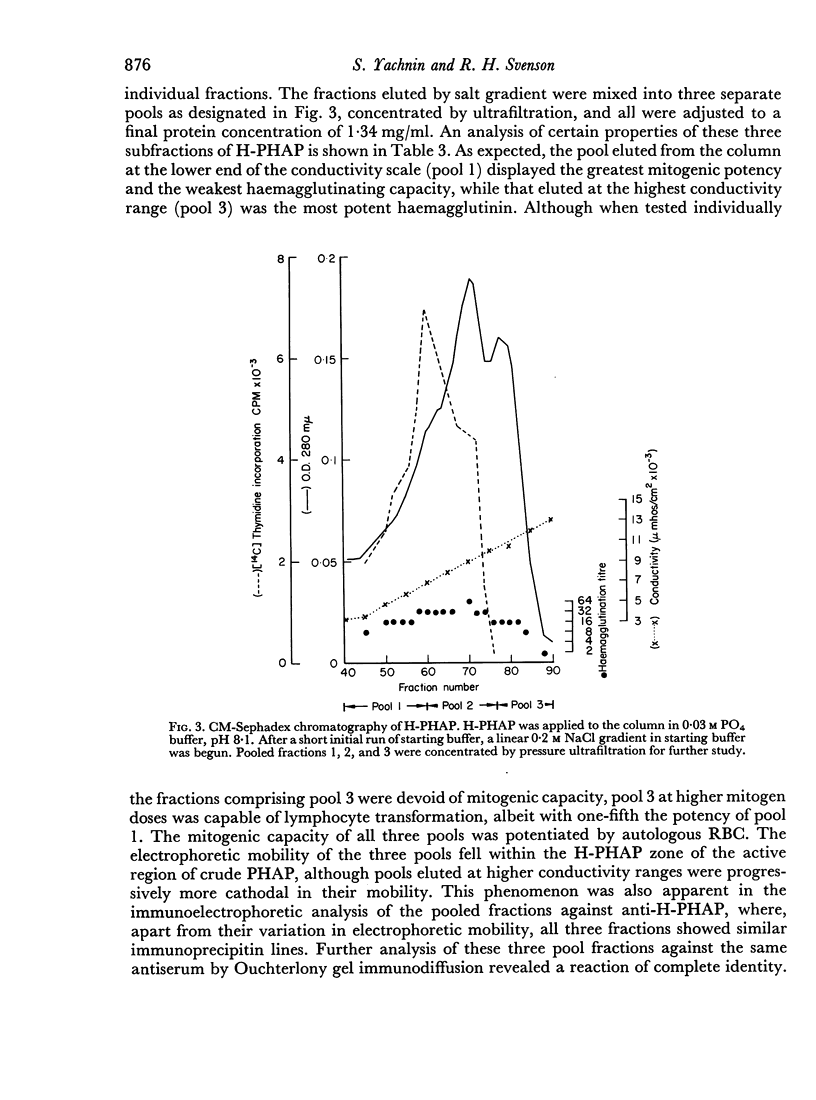
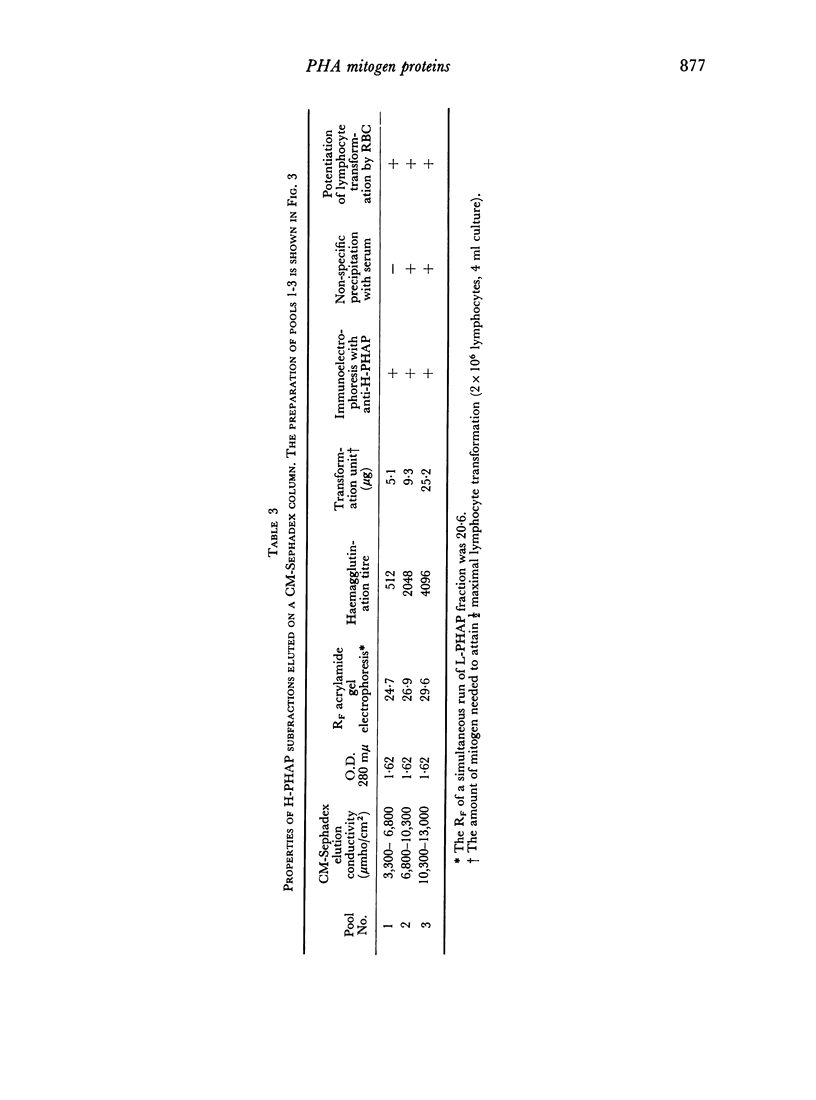
A commercially available phytohaemagglutinin derived from Phaseolus vulgaris (PHAP) was subjected to CM Sephadex and Sephadex G-150 chromatography. Two major mitogenic fractions were isolated; one (L-PHAP), appeared homogeneous by polyacrylamide gel electrophoresis, and was virtually lacking in haemagglutinating activity, while the other (H-PHAP) was a potent haemagglutinin. H-PHAP was a complex material as shown by its behaviour on CM Sephadex chromatography. Electrophoretic analysis revealed three distinct bands of protein, all migrating cathodally to L-PHAP. With increasing cathodal mobility H-PHAP subfractions showed diminishing mitogenicity, increasing haemagglutinating potency, and the appearance of the ability to precipitate serum proteins non-specifically. The latter property, present in crude PHAP, was not displayed by L-PHAP. The mitogenic activity of all H-PHAP subfractions was potentiated by the presence of autologous red blood cells.
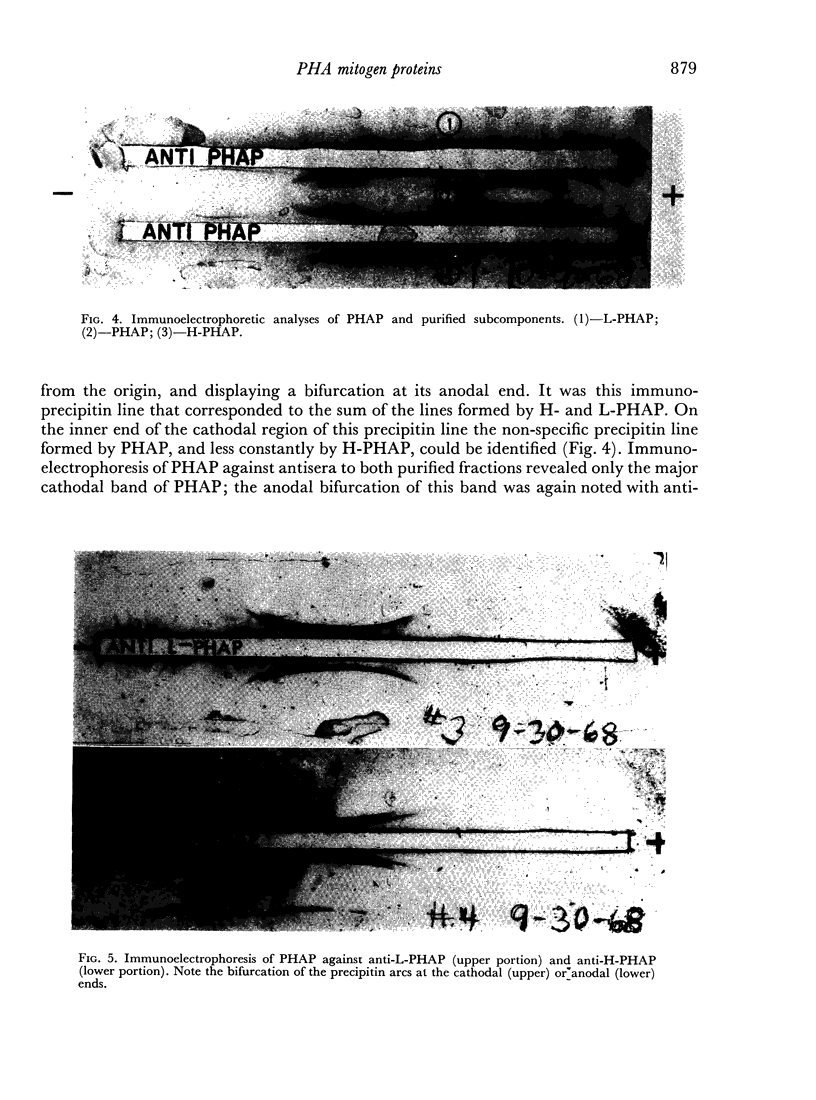
While L- and H-PHAP were closely related by immunological criteria, distinct differences were noted by three methods of analysis. The most cathodal of the H-PHAP subfractions was found to be immunodeficient by comparison with crude PHAP, L-PHAP, and the remaining H-PHAP subfractions. The implications of these findings with respect to the biological activity of PHAP and to its subunit structure are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. W., Svenson R. H., Yachnin S. Purification of mitogenic proteins derived from Phaseolus vulgaris: isolation of potent and weak phytohemagglutinins possessing mitogenic activity. Proc Natl Acad Sci U S A. 1969 Jun;63(2):334–341. doi: 10.1073/pnas.63.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie R. B., Horne C. H., Wilkinson P. C. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966 Dec 3;2(7475):1224–1226. doi: 10.1016/s0140-6736(66)92305-1. [DOI] [PubMed] [Google Scholar]
- Holland N. H., Holland P. Haemagglutinating, precipitating and lymphocyte-stimulating factors of phytohaemagglutinin. Nature. 1965 Sep 18;207(5003):1307–1308. doi: 10.1038/2071307a0. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O. LACTATE DEHYDROGENASE--STRUCTURE AND FUNCTION. Brookhaven Symp Biol. 1964 Dec;17:131–153. [PubMed] [Google Scholar]
- Kornfeld S., Kornfeld R. Solubilization and partial characterization of a phytohemagglutinin receptor site from human erythrocytes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1439–1446. doi: 10.1073/pnas.63.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. H. Immunological studies of phytohaemagglutinin. I. Reaction between phytohaemagglutinin and normal sera. Immunology. 1968 May;14(5):713–724. [PMC free article] [PubMed] [Google Scholar]
- Osunkoya B. O., Williams A. I. Precipitation of serum proteins by phytohaemagglutinin. Clin Exp Immunol. 1971 Feb;8(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- Rigas D. A., Head C. The dissociation of phytohemagglutinin of Phaseolus vulgaris by 8.0 M urea and the separation of the mitogenic from the erythroagglutinating activity. Biochem Biophys Res Commun. 1969 Mar 10;34(5):633–639. doi: 10.1016/0006-291x(69)90785-2. [DOI] [PubMed] [Google Scholar]