Abstract
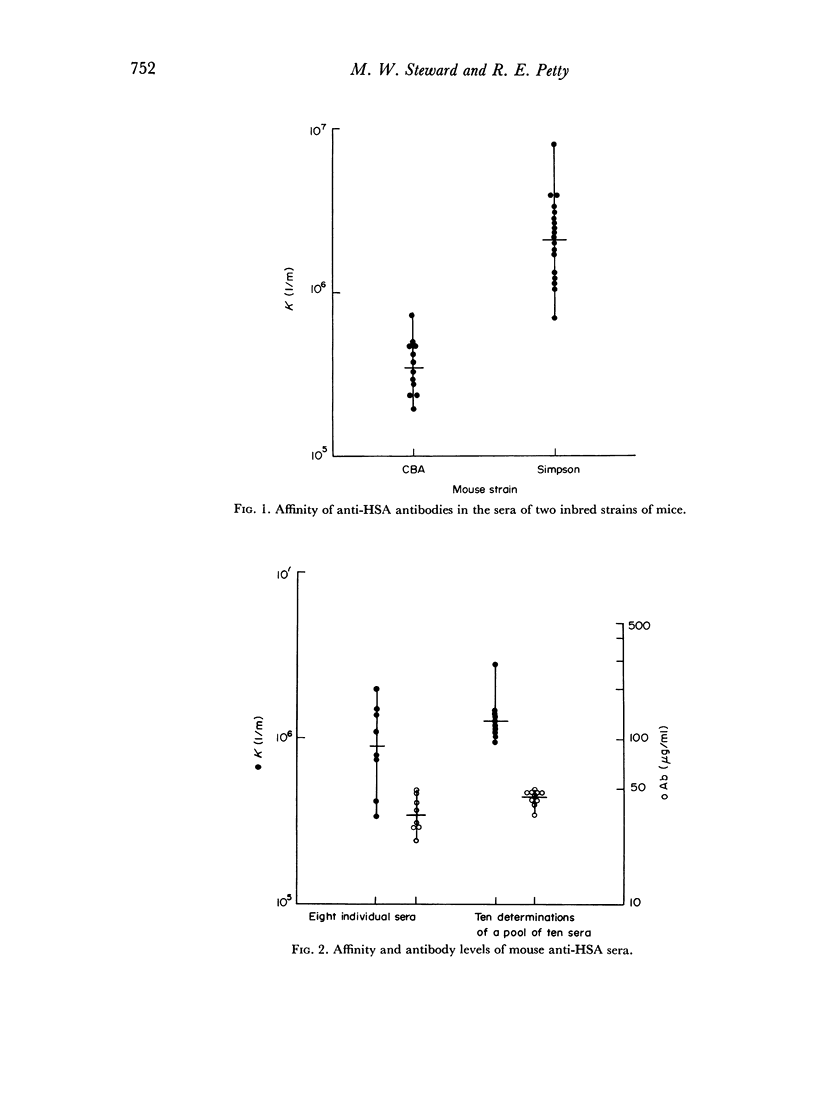
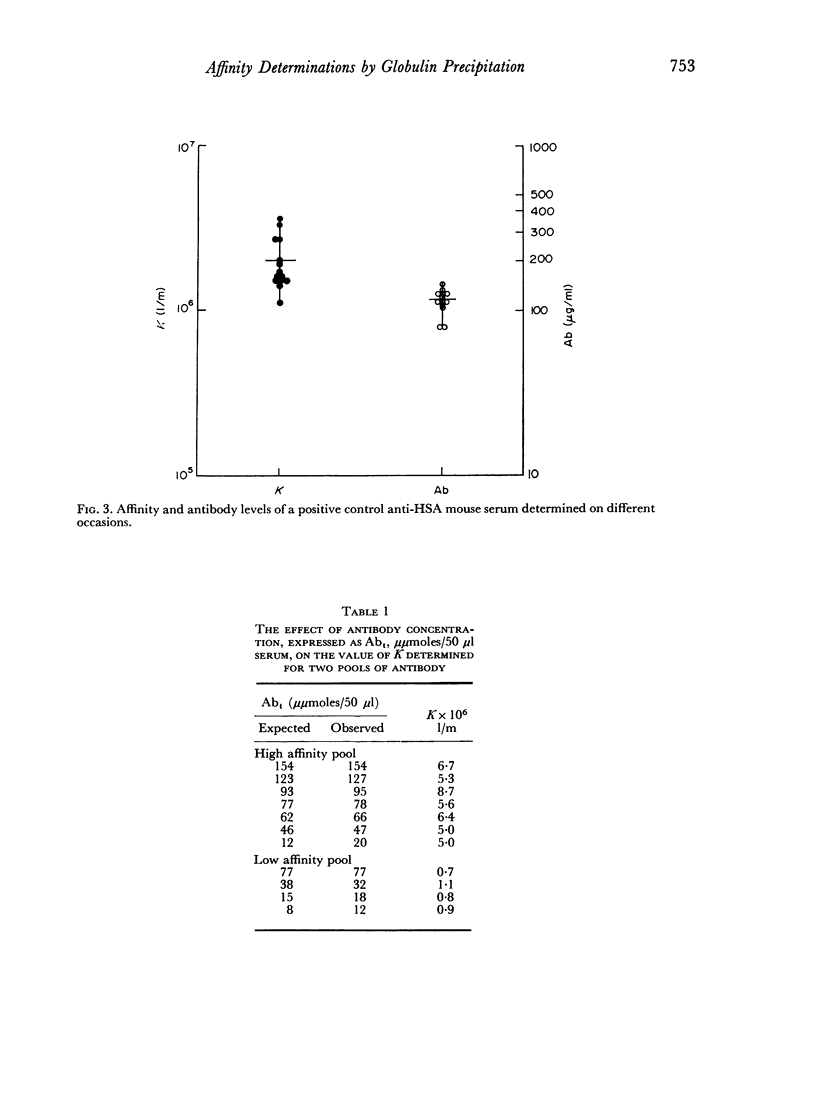
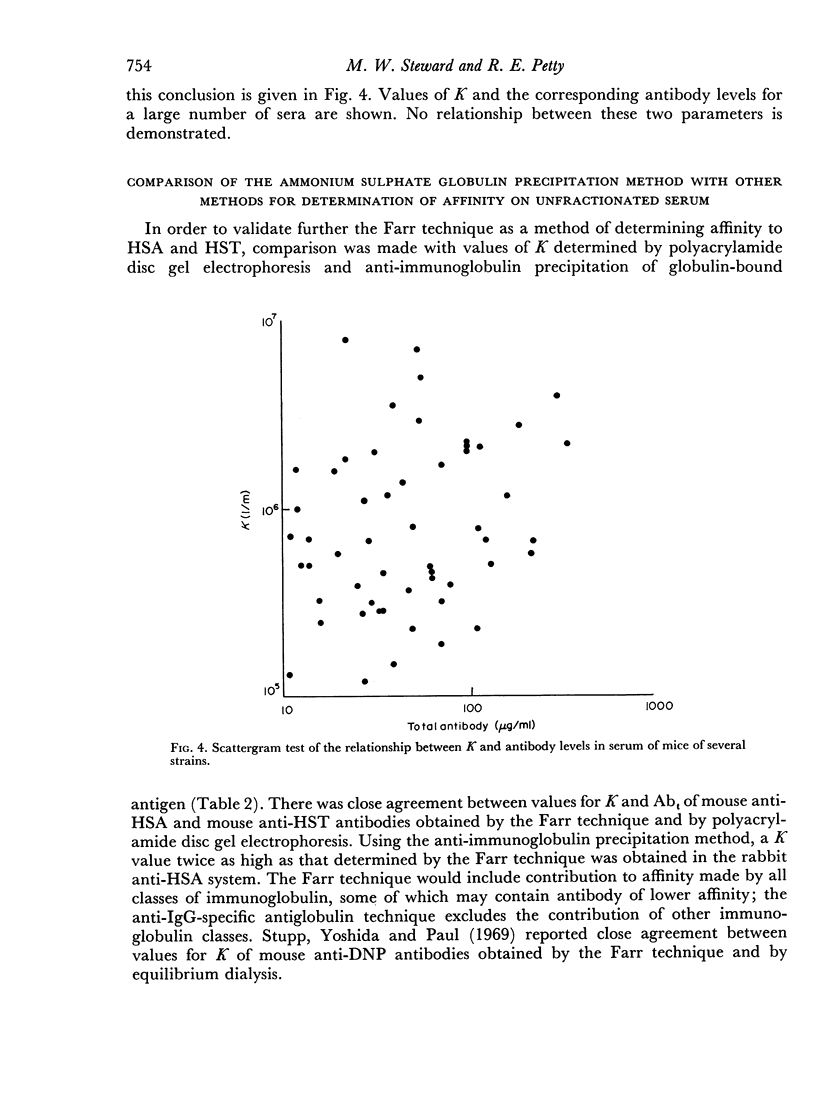
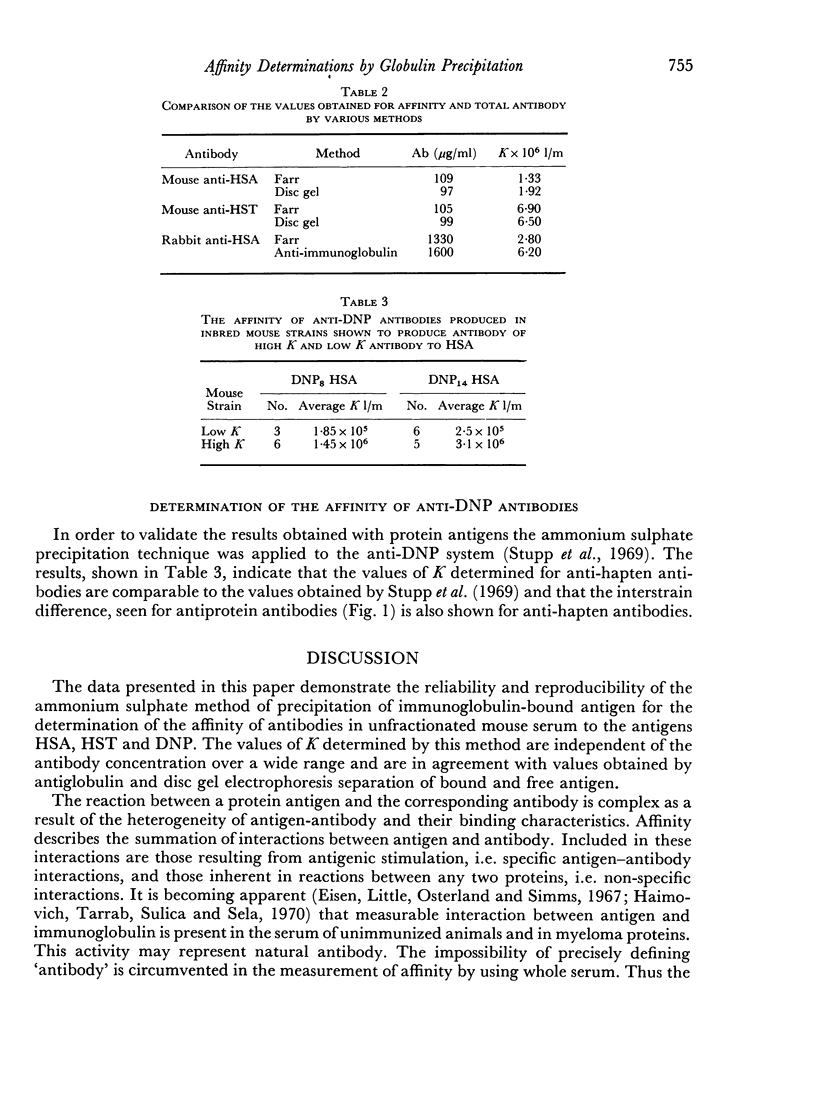
The use of a modification of the ammonium sulphate globulin precipitation technique for the determination of antibody affinity is reported. This method is shown to provide reproducible and valid measurements for the comparison of affinity of unselected and unpurified antibody to human serum albumin and transferrin in different mouse strains.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DANDLIKER W. B., SCHAPIRO H. C., MEDUSKI J. W., ALONSO R., FEIGEN G. A., HAMRICK J. R., Jr APPLICATION OF FLUORESCENCE POLARIZATION TO THE ANTIGEN-ANTIBODY REACTION. THEORY AND EXPERIMENTAL METHOD. Immunochemistry. 1964 Oct;1:165–191. doi: 10.1016/0019-2791(64)90041-2. [DOI] [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Haimovich J., Tarrab R., Sulica A., Sela M. Antibdies of different specificities in normal rabbit sera. J Immunol. 1970 Apr;104(4):1033–1034. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Minden P., Anthony B. F., Farr R. S. A comparison of seven procedures to detect the primary binding of antigen by antibody. J Immunol. 1969 Apr;102(4):832–841. [PubMed] [Google Scholar]
- NISONOFF A., PRESSMAN D. Heterogeneity and average combining constants of antibodies from individual rabbits. J Immunol. 1958 Jun;80(6):417–428. [PubMed] [Google Scholar]
- Parker C. W., Yoo T. J., Johnson M. C., Godt S. M. Fluorescent probes for the study of the antibody-hapten reaction. I. Binding of the 5-dimethylaminonaphthalene-1-sulfonamido group by homologous rabbit antibody. Biochemistry. 1967 Nov;6(11):3408–3416. doi: 10.1021/bi00863a011. [DOI] [PubMed] [Google Scholar]
- Soothill J. F., Steward M. W. The immunopathological significance of the heterogeneity of antibody affinity. Clin Exp Immunol. 1971 Aug;9(2):193–199. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Todd C. W., Kindt T. J., David G. S. Low molecular weight mercaptoethanol sensitive antibody in rabbits. Immunochemistry. 1969 Sep;6(5):649–658. doi: 10.1016/0019-2791(67)90130-9. [DOI] [PubMed] [Google Scholar]
- Stupp Y., Yoshida T., Paul W. E. Determination of antibody-hapten equilibrium constants by an ammonium sulfate precipitation technique. J Immunol. 1969 Sep;103(3):625–627. [PubMed] [Google Scholar]
- Velick S. F., Parker C. W., Eisen H. N. EXCITATION ENERGY TRANSFER AND THE QUANTITATIVE STUDY OF THE ANTIBODY HAPTEN REACTION. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1470–1482. doi: 10.1073/pnas.46.11.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]


