Abstract
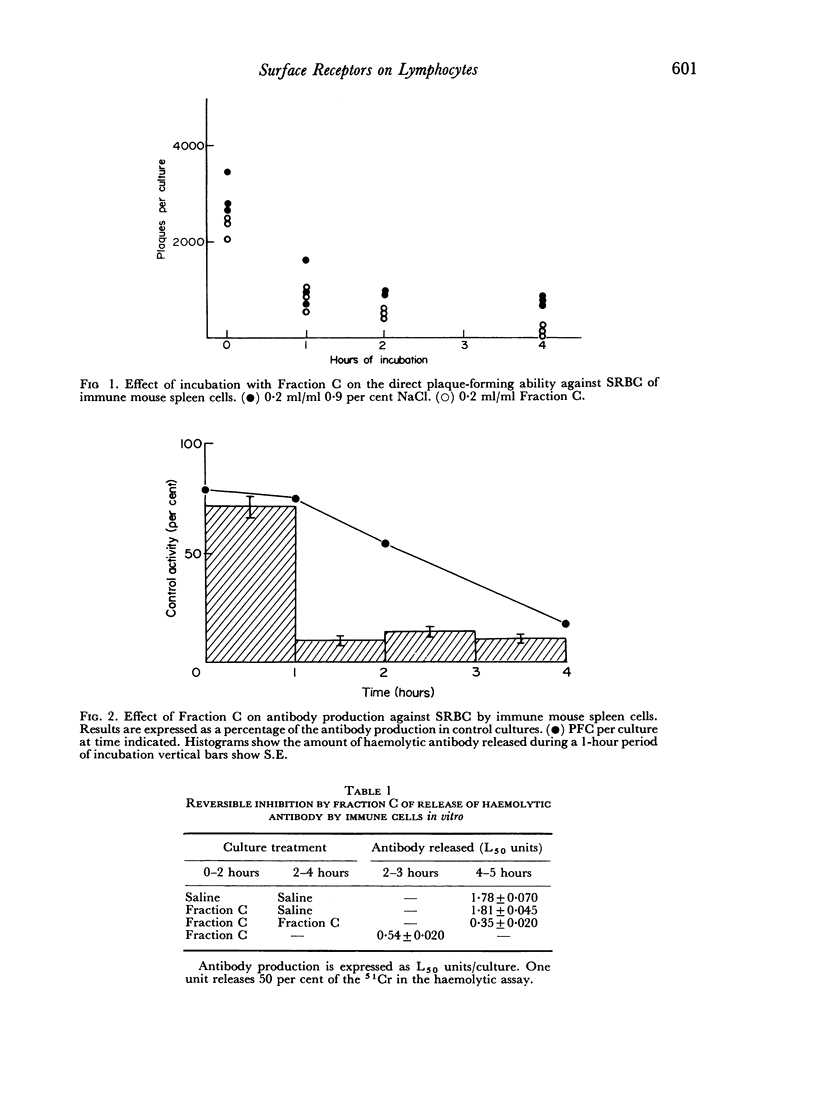
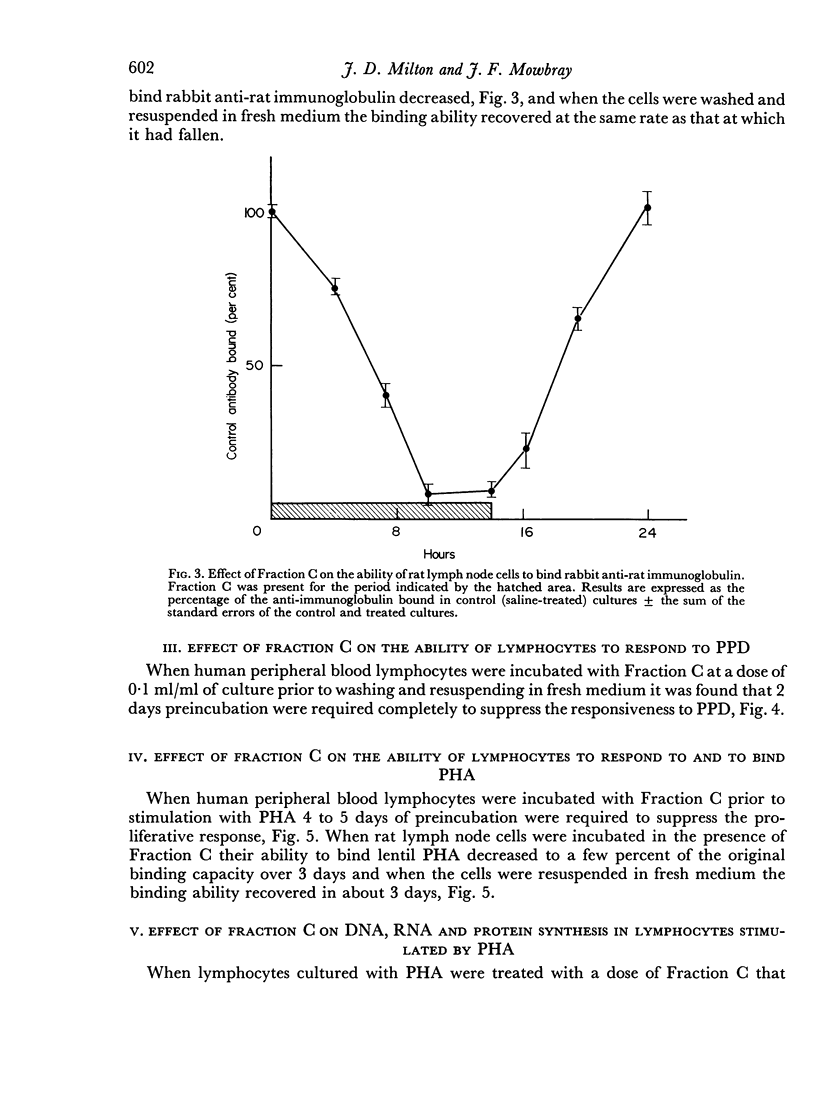
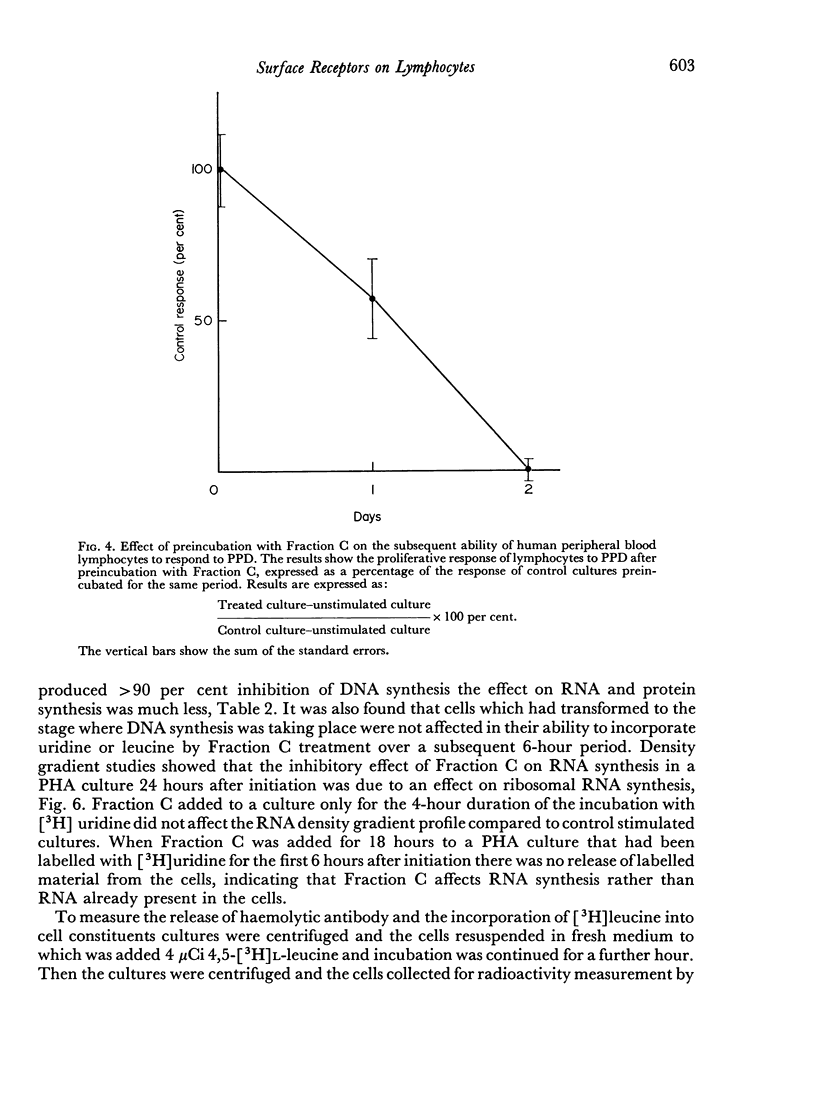
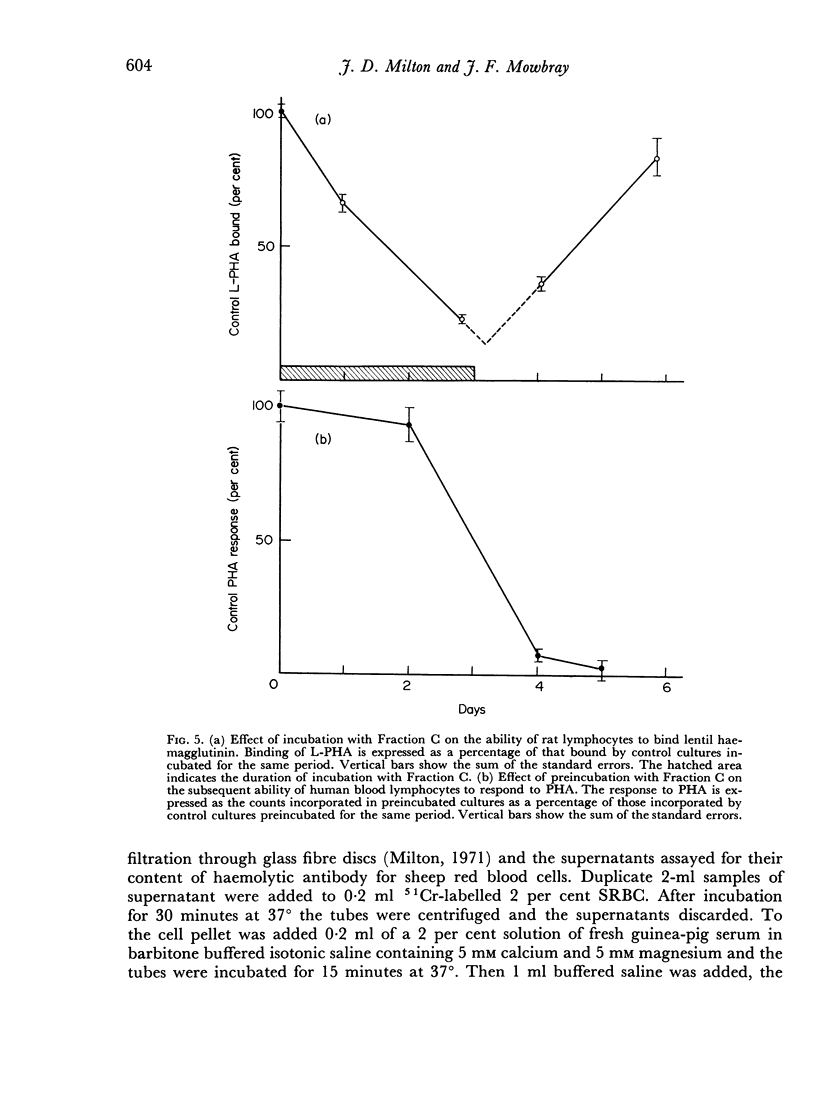
An α-2-glycoprotein from bovine serum (Fraction C) with immunosuppressive properties in vivo and anti-proliferative activity in vitro has been shown to reversibly inhibit the synthesis of cell surface receptors in vitro. The proliferative response of human lymphocytes in vitro to PHA and PPD can be inhibited by prolonged preincubation with Fraction C, which also inhibits the ability of lymphoid cells to bind PHA, 3–4 days for 90 per cent inhibition, and anti-immunoglobulin, 10 hours for 90 per cent inhibition. This inhibition is reversible and the binding ability recovers after removal of Fraction C at a similar rate to that at which it declined. Fraction C also rapidly and reversibly inhibits the secretion of antibody by immune spleen cells in vitro. The anti-proliferative effect of Fraction C is shown to involve an effect on ribosomal RNA synthesis and it is suggested that the mechanism of this action depends on the inhibition of protein synthesis, probably mediated by an effect on newly synthesized messenger RNA. The demonstration of different turnover rates of cell surface components on different types of cells suggests that it may be possible to use Fraction C to affect responsiveness of one type of lymphocyte while leaving another type unaffected.
Full text
PDF

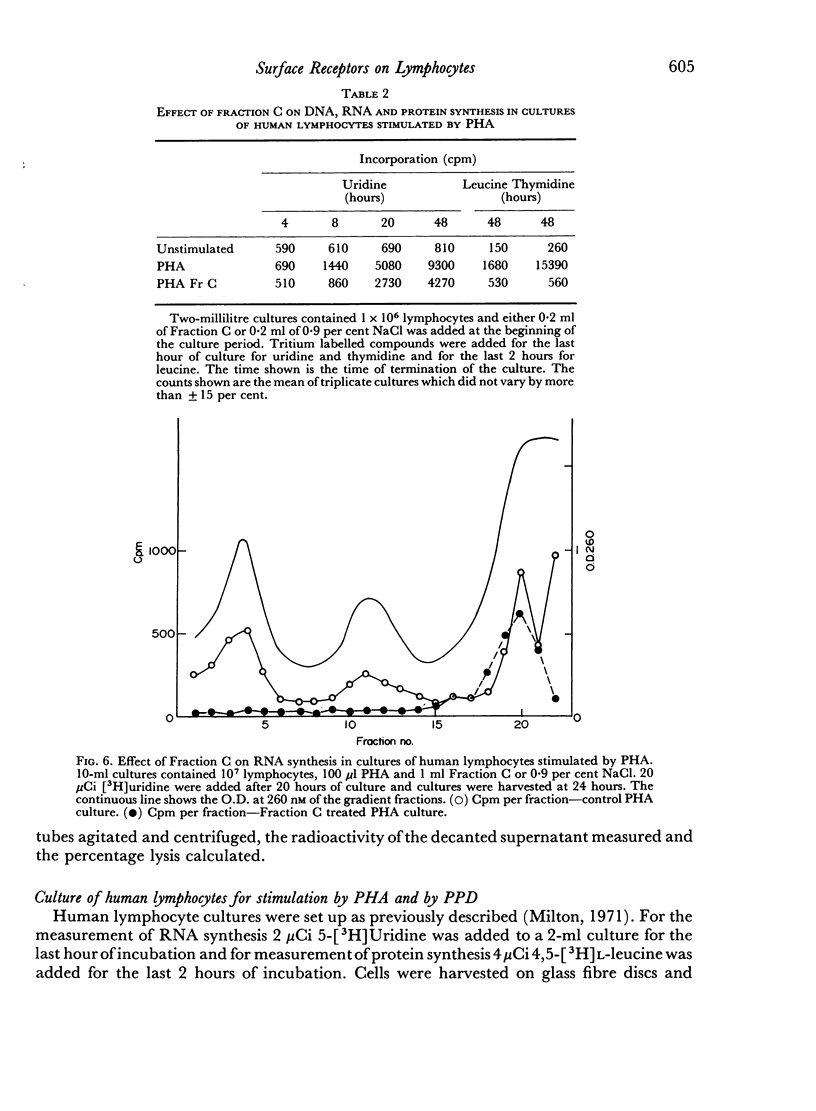

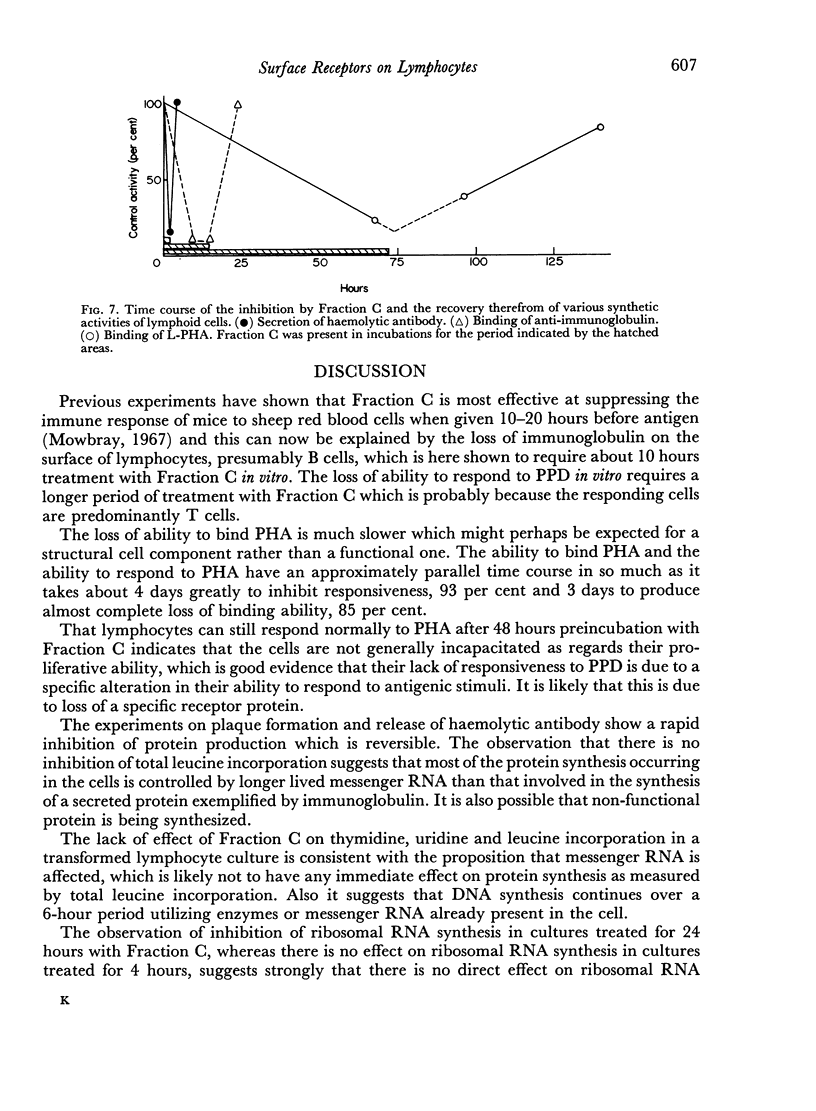

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter C. B., Milton J. D., Mowbray J. F., Butterworth A. E. Immunosuppressive properties of a ribonuclease-containing fraction from bacterial cultures. Immunology. 1972 Aug;23(2):171–182. [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., Gibson E. M. Control of synthesis and wastage of ribosomal ribonucleic acid in lymphocytes. II. The role of protein synthesis. J Biol Chem. 1971 Aug 25;246(16):5059–5066. [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Stein M. D., Young N. M., Leon M. A., Dyckes D. F. Studies on a phytohemagglutinin from the lentil. II. Multiple forms of Lens culinaris hemagglutinin. J Biol Chem. 1971 Mar 25;246(6):1590–1595. [PubMed] [Google Scholar]
- Kay J. E., Korner A. Effect of cycloheximide on protein and ribonucleic acid synthesis in cultured human lymphocytes. Biochem J. 1966 Sep;100(3):815–822. doi: 10.1042/bj1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STECK T. L., HOELZLWALLACH D. F. THE BINDING OF KIDNEY-BEAN PHYTOHEMAGGLUTININ BY EHRLICH ASCITES CARCINOMA. Biochim Biophys Acta. 1965 Mar 8;97:510–522. [PubMed] [Google Scholar]


