Abstract
Antidepressants, given systemically, are widely used for the treatment of various chronic and neuropathic pain conditions in humans. In animal studies, antidepressants exhibit analgesic properties in nociceptive, inflammatory and neuropathic test systems, with outcomes depending on the specific agent, the particular test, the route of administration and the treatment method used. Although early studies focused on central (i.e., supraspinal, spinal) actions, more recent studies have demonstrated a local peripheral analgesic effect of antidepressants. These peripheral actions raise the possibility that topical formulations of antidepressants may be a useful alternative drug delivery system for analgesia. Antidepressants exhibit a number of pharmacological actions: they block reuptake of noradrenaline and 5-hydroxytryptamine, have direct and indirect actions on opioid receptors, inhibit histamine, cholinergic, 5-hydroxytryptamine and N-methyl-D-aspartate receptors, inhibit ion channel activity, and block adenosine uptake. The involvement of these mechanisms in both central and peripheral analgesia produced by antidepressants is considered. Data illustrating the preclinical peripheral analgesic actions of antidepressants are presented, as are some aspects of the mechanisms by which these actions occur.
Full text
PDF

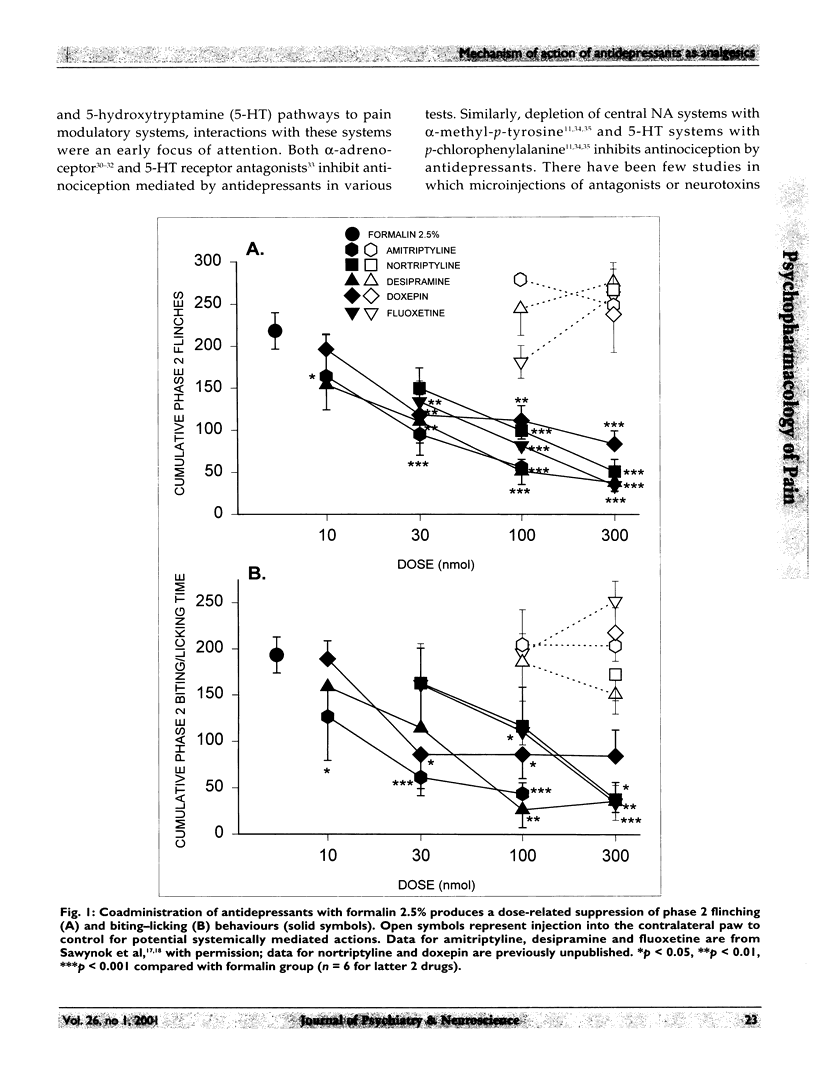
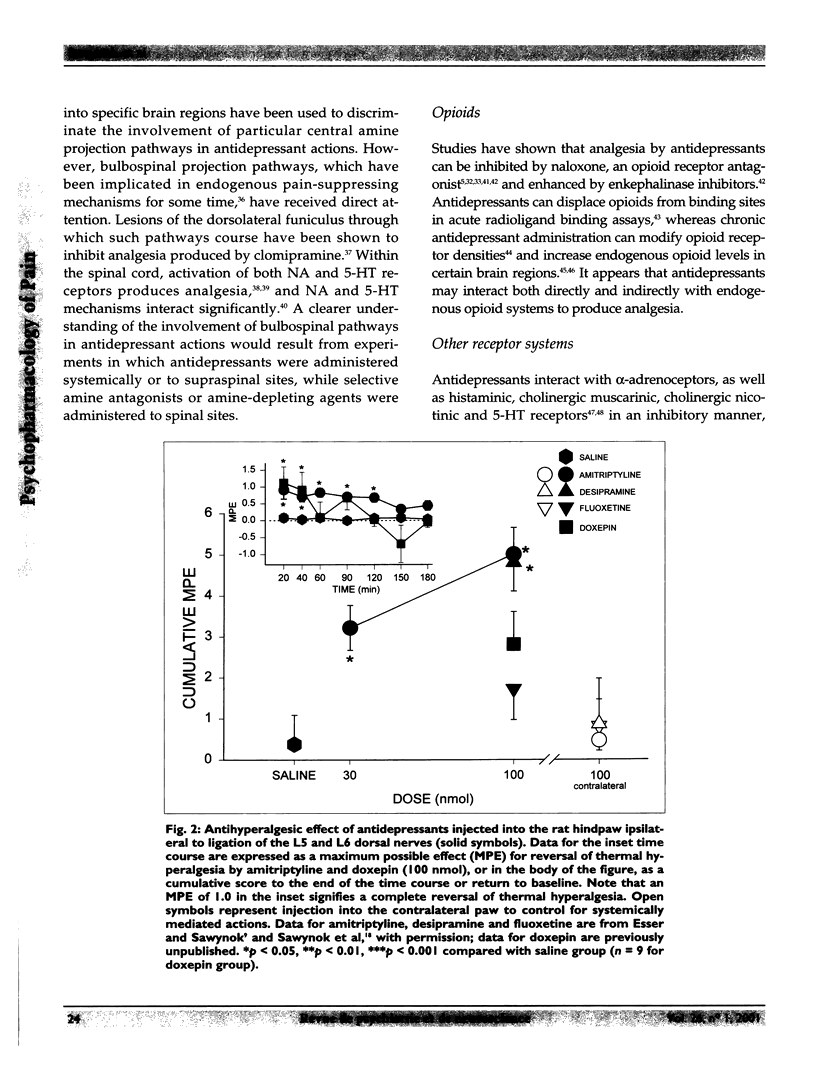

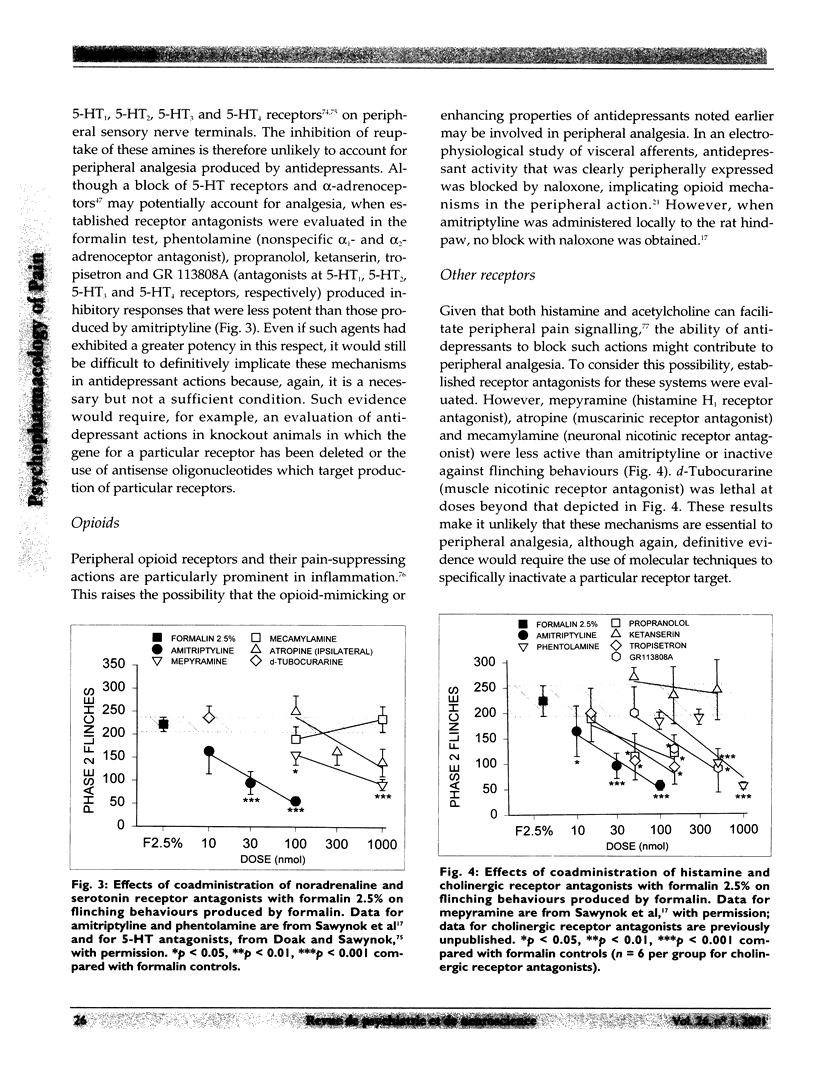



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad F., Feria M., Boada J. Chronic amitriptyline decreases autotomy following dorsal rhizotomy in rats. Neurosci Lett. 1989 Apr 24;99(1-2):187–190. doi: 10.1016/0304-3940(89)90287-5. [DOI] [PubMed] [Google Scholar]
- Abbott F. V., Hong Y., Blier P. Activation of 5-HT2A receptors potentiates pain produced by inflammatory mediators. Neuropharmacology. 1996 Jan;35(1):99–110. doi: 10.1016/0028-3908(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Abdi S., Lee D. H., Chung J. M. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg. 1998 Dec;87(6):1360–1366. [PubMed] [Google Scholar]
- Ardid D., Eschalier A., Lavarenne J. Evidence for a central but not a peripheral analgesic effect of clomipramine in rats. Pain. 1991 Apr;45(1):95–100. doi: 10.1016/0304-3959(91)90169-X. [DOI] [PubMed] [Google Scholar]
- Ardid D., Guilbaud G. Antinociceptive effects of acute and 'chronic' injections of tricyclic antidepressant drugs in a new model of mononeuropathy in rats. Pain. 1992 May;49(2):279–287. doi: 10.1016/0304-3959(92)90152-2. [DOI] [PubMed] [Google Scholar]
- Ardid D., Jourdan D., Mestre C., Villanueva L., Le Bars D., Eschalier A. Involvement of bulbospinal pathways in the antinociceptive effect of clomipramine in the rat. Brain Res. 1995 Oct 16;695(2):253–256. doi: 10.1016/0006-8993(95)00826-c. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978 Nov;4(5):451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Biegon A., Samuel D. Interaction of tricyclic antidepressants with opiate receptors. Biochem Pharmacol. 1980 Feb;29(3):460–462. doi: 10.1016/0006-2952(80)90531-6. [DOI] [PubMed] [Google Scholar]
- Butler S. H., Weil-Fugazza J., Godefroy F., Besson J. M. Reduction of arthritis and pain behaviour following chronic administration of amitriptyline or imipramine in rats with adjuvant-induced arthritis. Pain. 1985 Oct;23(2):159–175. doi: 10.1016/0304-3959(85)90057-0. [DOI] [PubMed] [Google Scholar]
- Cai Z., McCaslin P. P. Amitriptyline, desipramine, cyproheptadine and carbamazepine, in concentrations used therapeutically, reduce kainate- and N-methyl-D-aspartate-induced intracellular Ca2+ levels in neuronal culture. Eur J Pharmacol. 1992 Aug 14;219(1):53–57. doi: 10.1016/0014-2999(92)90579-s. [DOI] [PubMed] [Google Scholar]
- Carlton S. M., Zhou S. Attenuation of formalin-induced nociceptive behaviors following local peripheral injection of gabapentin. Pain. 1998 May;76(1-2):201–207. doi: 10.1016/s0304-3959(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Chaplan S. R., Malmberg A. B., Yaksh T. L. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997 Feb;280(2):829–838. [PubMed] [Google Scholar]
- Chaplan S. R., Pogrel J. W., Yaksh T. L. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994 Jun;269(3):1117–1123. [PubMed] [Google Scholar]
- Choi J. J., Huang G. J., Shafik E., Wu W. H., McArdle J. J. Imipramine's selective suppression of an L-type calcium channel in neurons of murine dorsal root ganglia involves G proteins. J Pharmacol Exp Ther. 1992 Oct;263(1):49–53. [PubMed] [Google Scholar]
- Coderre T. J., Katz J., Vaccarino A. L., Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993 Mar;52(3):259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Van Empel I. The utility of excitatory amino acid (EAA) antagonists as analgesic agents. I. Comparison of the antinociceptive activity of various classes of EAA antagonists in mechanical, thermal and chemical nociceptive tests. Pain. 1994 Dec;59(3):345–352. doi: 10.1016/0304-3959(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Courteix C., Bardin M., Chantelauze C., Lavarenne J., Eschalier A. Study of the sensitivity of the diabetes-induced pain model in rats to a range of analgesics. Pain. 1994 May;57(2):153–160. doi: 10.1016/0304-3959(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Davidson E. M., Carlton S. M. Intraplantar injection of dextrorphan, ketamine or memantine attenuates formalin-induced behaviors. Brain Res. 1998 Feb 23;785(1):136–142. doi: 10.1016/s0006-8993(97)01396-6. [DOI] [PubMed] [Google Scholar]
- Davidson E. M., Coggeshall R. E., Carlton S. M. Peripheral NMDA and non-NMDA glutamate receptors contribute to nociceptive behaviors in the rat formalin test. Neuroreport. 1997 Mar 3;8(4):941–946. doi: 10.1097/00001756-199703030-00025. [DOI] [PubMed] [Google Scholar]
- De Felipe M. C., De Ceballos M. L., Gil C., Fuentes J. A. Chronic antidepressant treatment increases enkephalin levels in n. accumbens and striatum of the rat. Eur J Pharmacol. 1985 May 28;112(1):119–122. doi: 10.1016/0014-2999(85)90247-x. [DOI] [PubMed] [Google Scholar]
- Doak G. J., Sawynok J. Formalin-induced nociceptive behavior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience. 1997 Oct;80(3):939–949. doi: 10.1016/s0306-4522(97)00066-3. [DOI] [PubMed] [Google Scholar]
- Eisenach J. C., Gebhart G. F. Intrathecal amitriptyline acts as an N-methyl-D-aspartate receptor antagonist in the presence of inflammatory hyperalgesia in rats. Anesthesiology. 1995 Nov;83(5):1046–1054. doi: 10.1097/00000542-199511000-00018. [DOI] [PubMed] [Google Scholar]
- Eschalier A., Montastruc J. L., Devoize J. L., Rigal F., Gaillard-Plaza G., Pechadre J. C. Influence of naloxone and methysergide on the analgesic effect of clomipramine in rats. Eur J Pharmacol. 1981 Aug 27;74(1):1–7. doi: 10.1016/0014-2999(81)90316-2. [DOI] [PubMed] [Google Scholar]
- Esser M. J., Sawynok J. Acute amitriptyline in a rat model of neuropathic pain: differential symptom and route effects. Pain. 1999 Apr;80(3):643–653. doi: 10.1016/S0304-3959(98)00261-9. [DOI] [PubMed] [Google Scholar]
- Esser M. J., Sawynok J. Caffeine blockade of the thermal antihyperalgesic effect of acute amitriptyline in a rat model of neuropathic pain. Eur J Pharmacol. 2000 Jul 7;399(2-3):131–139. doi: 10.1016/s0014-2999(00)00336-8. [DOI] [PubMed] [Google Scholar]
- Fryer J. D., Lukas R. J. Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J Neurochem. 1999 Mar;72(3):1117–1124. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- Galeotti N., Ghelardini C., Capaccioli S., Quattrone A., Nicolin A., Bartolini A. Blockade of clomipramine and amitriptyline analgesia by an antisense oligonucleotide to mKv1.1, a mouse Shaker-like K+ channel. Eur J Pharmacol. 1997 Jul 2;330(1):15–25. doi: 10.1016/s0014-2999(97)10134-0. [DOI] [PubMed] [Google Scholar]
- Gray A. M., Pache D. M., Sewell R. D. Do alpha2-adrenoceptors play an integral role in the antinociceptive mechanism of action of antidepressant compounds? Eur J Pharmacol. 1999 Aug 6;378(2):161–168. doi: 10.1016/s0014-2999(99)00464-1. [DOI] [PubMed] [Google Scholar]
- Gray A. M., Spencer P. S., Sewell R. D. The involvement of the opioidergic system in the antinociceptive mechanism of action of antidepressant compounds. Br J Pharmacol. 1998 Jun;124(4):669–674. doi: 10.1038/sj.bjp.0701882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H., Ogren S. O. Effects of antidepressant drugs on different receptors in the brain. Eur J Pharmacol. 1981 Mar 26;70(3):393–407. doi: 10.1016/0014-2999(81)90172-2. [DOI] [PubMed] [Google Scholar]
- Hamon M., Gozlan H., Bourgoin S., Benoliel J. J., Mauborgne A., Taquet H., Cesselin F., Mico J. A. Opioid receptors and neuropeptides in the CNS in rats treated chronically with amoxapine or amitriptyline. Neuropharmacology. 1987 Jun;26(6):531–539. doi: 10.1016/0028-3908(87)90144-4. [DOI] [PubMed] [Google Scholar]
- Hong Y., Abbott F. V. Contribution of peripheral alpha 1A-adrenoceptors to pain induced by formalin or by alpha-methyl-5-hydroxytryptamine plus noradrenaline. Eur J Pharmacol. 1996 Apr 22;301(1-3):41–48. doi: 10.1016/0014-2999(96)00009-x. [DOI] [PubMed] [Google Scholar]
- Hunter J. C., Gogas K. R., Hedley L. R., Jacobson L. O., Kassotakis L., Thompson J., Fontana D. J. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol. 1997 Apr 18;324(2-3):153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- Hwang A. S., Wilcox G. L. Analgesic properties of intrathecally administered heterocyclic antidepressants. Pain. 1987 Mar;28(3):343–355. doi: 10.1016/0304-3959(87)90068-6. [DOI] [PubMed] [Google Scholar]
- Isenberg K. E., Cicero T. J. Possible involvement of opiate receptors in the pharmacological profiles of antidepressant compounds. Eur J Pharmacol. 1984 Aug 3;103(1-2):57–63. doi: 10.1016/0014-2999(84)90189-4. [DOI] [PubMed] [Google Scholar]
- Iwashita T., Shimizu T. Imipramine inhibits intrathecal substance P-induced behavior and blocks spinal cord substance P receptors in mice. Brain Res. 1992 May 22;581(1):59–66. doi: 10.1016/0006-8993(92)90344-9. [DOI] [PubMed] [Google Scholar]
- Khasar S. G., Green P. G., Chou B., Levine J. D. Peripheral nociceptive effects of alpha 2-adrenergic receptor agonists in the rat. Neuroscience. 1995 May;66(2):427–432. doi: 10.1016/0306-4522(94)00562-j. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Chung J. M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992 Sep;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kral M. G., Xiong Z., Study R. E. Alteration of Na+ currents in dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1999 May;81(1-2):15–24. doi: 10.1016/s0304-3959(98)00264-4. [DOI] [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci. 1994 Aug;14(8):4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleane G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: a randomized, double-blind, placebo-controlled study. Br J Clin Pharmacol. 2000 Jun;49(6):574–579. doi: 10.1046/j.1365-2125.2000.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micó J. A., Gibert-Rahola J., Casas J., Rojas O., Serrano M. I., Serrano J. S. Implication of beta 1- and beta 2-adrenergic receptors in the antinociceptive effect of tricyclic antidepressants. Eur Neuropsychopharmacol. 1997 May;7(2):139–145. doi: 10.1016/s0924-977x(97)00411-2. [DOI] [PubMed] [Google Scholar]
- Mjellem N., Lund A., Hole K. Reduction of NMDA-induced behaviour after acute and chronic administration of desipramine in mice. Neuropharmacology. 1993 Jun;32(6):591–595. doi: 10.1016/0028-3908(93)90055-8. [DOI] [PubMed] [Google Scholar]
- Ogata N., Yoshii M., Narahashi T. Psychotropic drugs block voltage-gated ion channels in neuroblastoma cells. Brain Res. 1989 Jan 2;476(1):140–144. doi: 10.1016/0006-8993(89)91546-1. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The effect of various centrally active drugs on adenosine uptake by the central nervous system. Comp Biochem Physiol C. 1982;72(2):179–187. doi: 10.1016/0306-4492(82)90082-x. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Miller R. J. Tricyclic antidepressants block N-methyl-D-aspartate receptors: similarities to the action of zinc. Br J Pharmacol. 1988 Sep;95(1):95–102. doi: 10.1111/j.1476-5381.1988.tb16552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham M. C., Davies P. S., Fields H. L. Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol. 1995 Feb;37(2):246–253. doi: 10.1002/ana.410370216. [DOI] [PubMed] [Google Scholar]
- Sacerdote P., Brini A., Mantegazza P., Panerai A. E. A role for serotonin and beta-endorphin in the analgesia induced by some tricyclic antidepressant drugs. Pharmacol Biochem Behav. 1987 Jan;26(1):153–158. doi: 10.1016/0091-3057(87)90548-x. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998 Apr 17;347(1):1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Esser M. J., Reid A. R. Peripheral antinociceptive actions of desipramine and fluoxetine in an inflammatory and neuropathic pain test in the rat. Pain. 1999 Aug;82(2):149–158. doi: 10.1016/S0304-3959(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Reid A. R., Esser M. J. Peripheral antinociceptive action of amitriptyline in the rat formalin test: involvement of adenosine. Pain. 1999 Mar;80(1-2):45–55. doi: 10.1016/s0304-3959(98)00195-x. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Reid A. Interactions of descending serotonergic systems with other neurotransmitters in the modulation of nociception. Behav Brain Res. 1996;73(1-2):63–68. doi: 10.1016/0166-4328(96)00072-1. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Backer M. M., Pick C. G. The antinociceptive effect of venlafaxine in mice is mediated through opioid and adrenergic mechanisms. Neurosci Lett. 1999 Oct 1;273(2):85–88. doi: 10.1016/s0304-3940(99)00627-8. [DOI] [PubMed] [Google Scholar]
- Seltzer Z., Tal M., Sharav Y. Autotomy behavior in rats following peripheral deafferentation is suppressed by daily injections of amitriptyline, diazepam and saline. Pain. 1989 May;37(2):245–250. doi: 10.1016/0304-3959(89)90136-X. [DOI] [PubMed] [Google Scholar]
- Sierralta F., Pinardi G., Mendez M., Miranda H. F. Interaction of opioids with antidepressant-induced antinociception. Psychopharmacology (Berl) 1995 Dec;122(4):374–378. doi: 10.1007/BF02246269. [DOI] [PubMed] [Google Scholar]
- Sierralta F., Pinardi G., Miranda H. F. Effect of p-chlorophenylalanine and alpha-methyltyrosine on the antinociceptive effect of antidepressant drugs. Pharmacol Toxicol. 1995 Oct;77(4):276–280. doi: 10.1111/j.1600-0773.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Skolnick P., Layer R. T., Popik P., Nowak G., Paul I. A., Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996 Jan;29(1):23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- Spiegel K., Kalb R., Pasternak G. W. Analgesic activity of tricyclic antidepressants. Ann Neurol. 1983 Apr;13(4):462–465. doi: 10.1002/ana.410130418. [DOI] [PubMed] [Google Scholar]
- Stahl S. M. Basic psychopharmacology of antidepressants, part 1: Antidepressants have seven distinct mechanisms of action. J Clin Psychiatry. 1998;59 (Suppl 4):5–14. [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995 Jun 22;332(25):1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Su X., Gebhart G. F. Effects of tricyclic antidepressants on mechanosensitive pelvic nerve afferent fibers innervating the rat colon. Pain. 1998 May;76(1-2):105–114. doi: 10.1016/s0304-3959(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Taylor B. K., Peterson M. A., Basbaum A. I. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci. 1995 Nov;15(11):7575–7584. doi: 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tura B., Tura S. M. The analgesic effect of tricyclic antidepressants. Brain Res. 1990 Jun 4;518(1-2):19–22. doi: 10.1016/0006-8993(90)90948-b. [DOI] [PubMed] [Google Scholar]
- Valverde O., Micó J. A., Maldonado R., Mellado M., Gibert-Rahola J. Participation of opioid and monoaminergic mechanisms on the antinociceptive effect induced by tricyclic antidepressants in two behavioural pain tests in mice. Prog Neuropsychopharmacol Biol Psychiatry. 1994 Oct;18(6):1073–1092. doi: 10.1016/0278-5846(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Bowersox S. S., Pettus M., Gao D. Antinociceptive properties of fenfluramine, a serotonin reuptake inhibitor, in a rat model of neuropathy. J Pharmacol Exp Ther. 1999 Dec;291(3):1008–1016. [PubMed] [Google Scholar]
- Woolf C. J., Doubell T. P. The pathophysiology of chronic pain--increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994 Aug;4(4):525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]


