Abstract
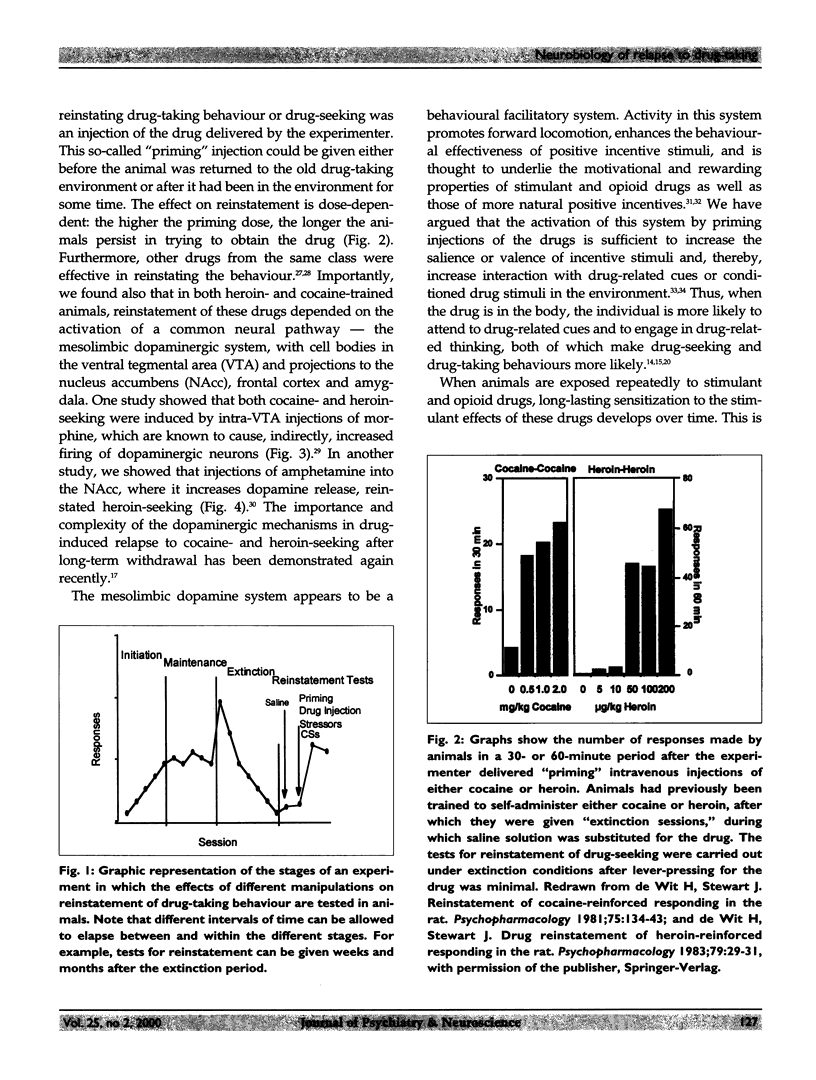
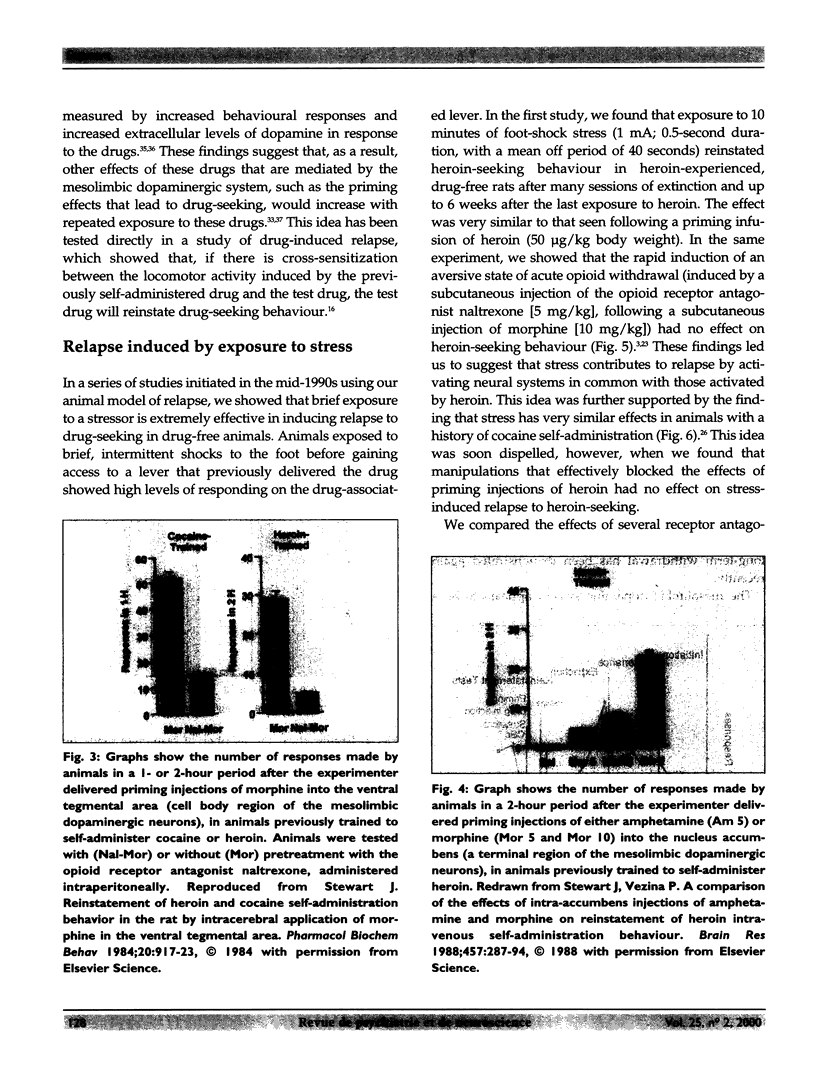
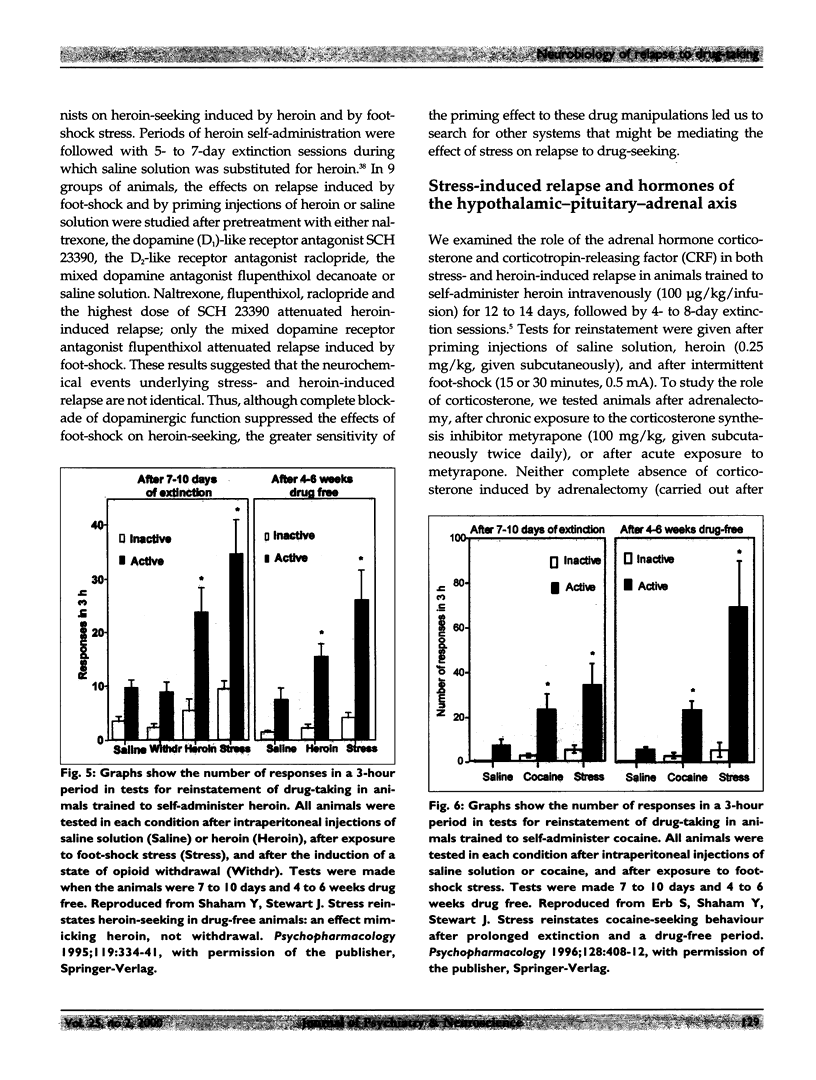
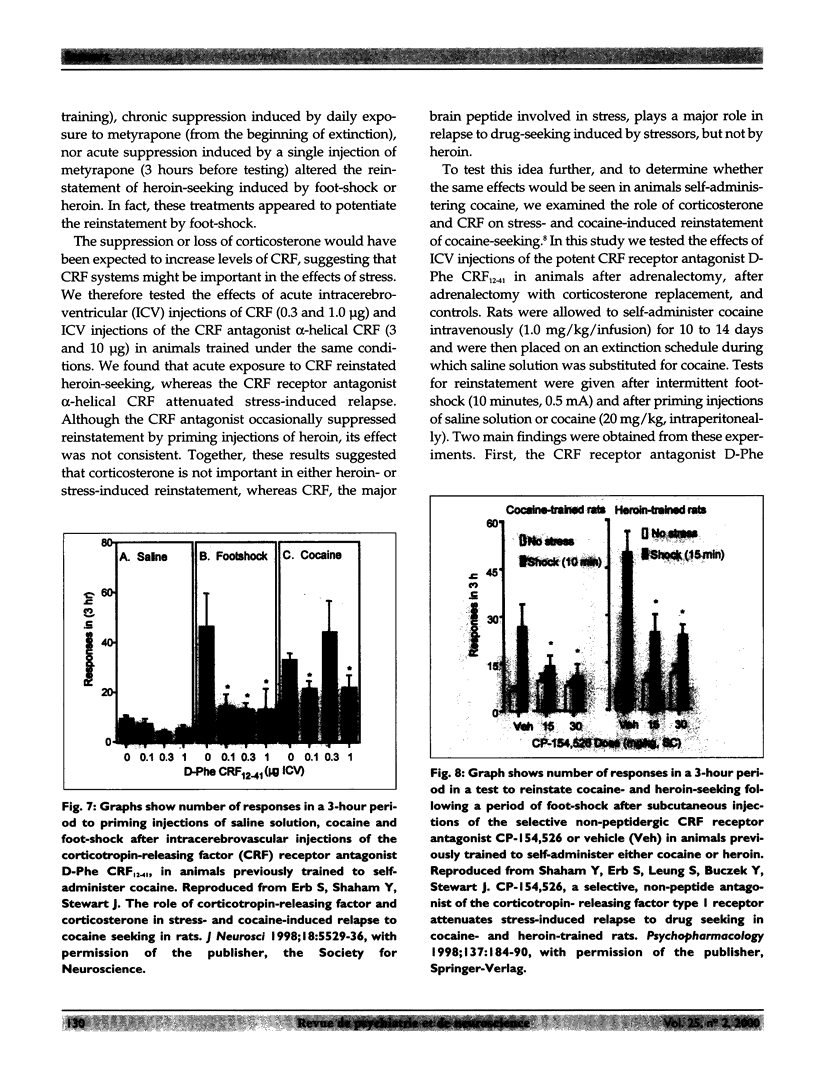
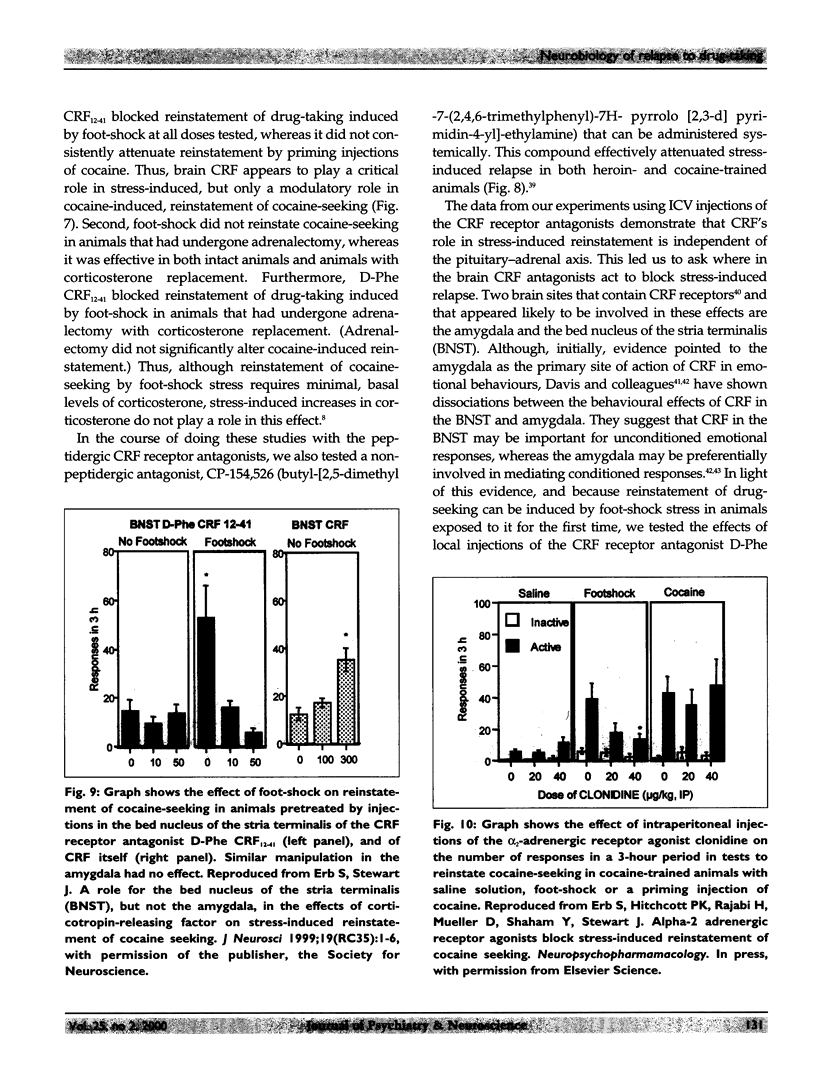
Relapse is a major characteristic of drug addiction, and remains the primary problem in treating drug abuse. Without an understanding of the factors that determine renewed drug-seeking, the urge to use drugs, and the persistent craving for them, it is unlikely that health care professionals can provide effective treatment. Using an animal model of relapse, the author and her team are studying factors that induce reinstatement of drug-taking behaviour after short and long periods of abstinence, and they are exploring the neurobiological basis of these effects. In their experiments, rats are trained to self-administer drugs intravenously by pressing 1 of 2 levers. During a subsequent period, the drug is no longer available, but the rats are free to try to obtain the drug (a period of "extinction training"). After extinction of responding, the investigators test for the ability of various events to reinitiate drug-seeking. On this background of renewed drug-seeking or relapse, the investigators search for pharmacological and neurochemical manipulations that might block or attenuate such behaviour. They have found that the 2 most effective events for reinstating responding after both short and long drug-free periods are re-exposure to the drug itself and exposure to a brief period of stress. The critical neurochemical pathways mediating drug-induced relapse are not identical to those mediating stress-induced relapse. Relapse induced by "priming" injections of heroin or cocaine involves activation of the mesolimbic dopaminergic pathways, whereas relapse induced by stress involves actions of corticotropin-releasing factor (CRF) in the brain, and of brain noradrenergic (NE) systems. In addition, evidence shows that CRF and NE may interact at the level of the bed nucleus of the stria terminalis in stress-induced relapse. By contrast, relapse induced by "priming" injections of drugs is relatively unaffected by manipulation of CRF and NE systems of the brain.
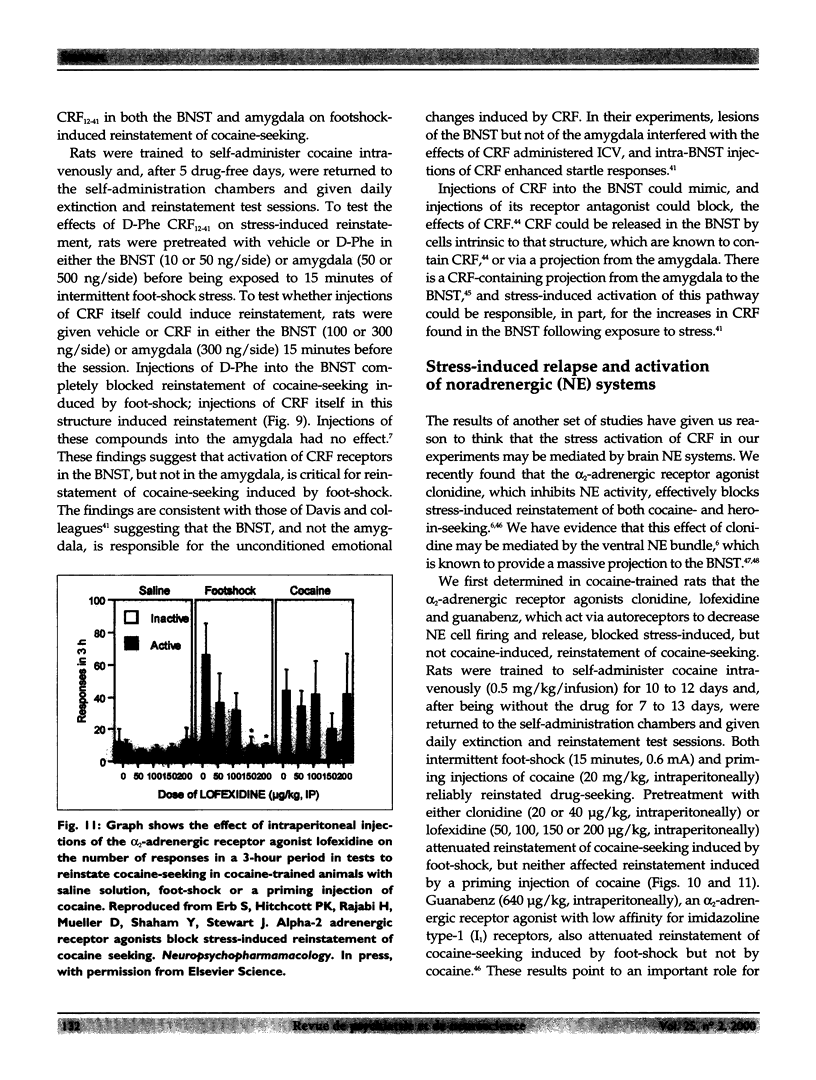
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. H., Koob G. F. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997 Aug;132(3):289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Albert D. J., Walsh M. L. The inhibitory modulation of agonistic behavior in the rat brain: a review. Neurosci Biobehav Rev. 1982 Summer;6(2):125–143. doi: 10.1016/0149-7634(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Breiter H. C., Gollub R. L., Weisskoff R. M., Kennedy D. N., Makris N., Berke J. D., Goodman J. M., Kantor H. L., Gastfriend D. R., Riorden J. P. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997 Sep;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Buczek Y., Lê A. D., Wang A., Stewart J., Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999 May;144(2):183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Carnwath T., Hardman J. Randomised double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998 May 1;50(3):251–254. doi: 10.1016/s0376-8716(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Chalmers D. T., Lovenberg T. W., De Souza E. B. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995 Oct;15(10):6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress A. R., Mozley P. D., McElgin W., Fitzgerald J., Reivich M., O'Brien C. P. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999 Jan;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. M., Smith S. G. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976 Oct-Dec;11(4):222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- De Vries T. J., Schoffelmeer A. N., Binnekade R., Vanderschuren L. J. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 1999 Apr;143(3):254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- Eng H., Lund K., Campenot R. B. Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J Neurosci. 1999 Jan 1;19(1):1–9. doi: 10.1523/JNEUROSCI.19-01-00001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S., Shaham Y., Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996 Dec;128(4):408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S., Shaham Y., Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998 Jul 15;18(14):5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B. J., Parkinson J. A., Olmstead M. C., Arroyo M., Robledo P., Robbins T. W. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999 Jun 29;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Gewirtz J. C., McNish K. A., Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry. 1998 May;22(4):625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Jaffe J. H., Cascella N. G., Kumor K. M., Sherer M. A. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97(1):59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Kahn A., Mumford J. P., Rogers G. A., Beckford H. Double-blind study of lofexidine and clonidine in the detoxification of opiate addicts in hospital. Drug Alcohol Depend. 1997 Jan 10;44(1):57–61. doi: 10.1016/s0376-8716(96)01316-6. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991 Sep-Dec;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kelley A. E. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999 Jun 29;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Lee Y., Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997 Aug 15;17(16):6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. K., Strang J., Su L. W., Tsai C. J., Hu W. H. Double-blind randomised controlled trial of lofexidine versus clonidine in the treatment of heroin withdrawal. Drug Alcohol Depend. 1997 Nov 25;48(2):127–133. doi: 10.1016/s0376-8716(97)00116-6. [DOI] [PubMed] [Google Scholar]
- Lê A. D., Quan B., Juzytch W., Fletcher P. J., Joharchi N., Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998 Jan;135(2):169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Madeja M. Do neurons have a reserve of sodium channels for the generation of action potentials? A study on acutely isolated CA1 neurons from the guinea-pig hippocampus. Eur J Neurosci. 2000 Jan;12(1):1–7. doi: 10.1046/j.1460-9568.2000.00871.x. [DOI] [PubMed] [Google Scholar]
- McDougle C. J., Black J. E., Malison R. T., Zimmermann R. C., Kosten T. R., Heninger G. R., Price L. H. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry. 1994 Sep;51(9):713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- Meil W. M., See R. E. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997 Sep;87(2):139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Numan M., Numan M. J. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997 May;9(5):369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Numan M., Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996 Jan;29(1):23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M., Sheehan T. P. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997 Jan 15;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Robbins S. J., Ehrman R. N., Childress A. R., O'Brien C. P. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999 Feb 1;53(3):223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Becker J. B. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986 Jun;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep-Dec;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sakanaka M., Shibasaki T., Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986 Sep 24;382(2):213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Self D. W., Barnhart W. J., Lehman D. A., Nestler E. J. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996 Mar 15;271(5255):1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Adamson L. K., Grocki S., Corrigall W. A. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 1997 Apr;130(4):396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Leung S., Buczek Y., Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998 May;137(2):184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Funk D., Erb S., Brown T. J., Walker C. D., Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997 Apr 1;17(7):2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Rajabi H., Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996 Mar 1;16(5):1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl) 1996 Jun;125(4):385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995 Jun;119(3):334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaikh M. B., Brutus M., Siegel H. E., Siegel A. Regulation of feline aggression by the bed nucleus of stria terminalis. Brain Res Bull. 1986 Feb;16(2):179–182. doi: 10.1016/0361-9230(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Shaikh M. B., Brutus M., Siegel H. E., Siegel A. Topographically organized midbrain modulation of predatory and defensive aggression in the cat. Brain Res. 1985 Jun 17;336(2):308–312. doi: 10.1016/0006-8993(85)90657-2. [DOI] [PubMed] [Google Scholar]
- Sinha R., Catapano D., O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999 Mar;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984 Jun;20(6):917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Stewart J., Vezina P. A comparison of the effects of intra-accumbens injections of amphetamine and morphine on reinstatement of heroin intravenous self-administration behavior. Brain Res. 1988 Aug 9;457(2):287–294. doi: 10.1016/0006-8993(88)90698-1. [DOI] [PubMed] [Google Scholar]
- Stewart J., de Wit H., Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984 Apr;91(2):251–268. [PubMed] [Google Scholar]
- Stretch R., Gerber G. J. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can J Psychol. 1973 Jun;27(2):168–177. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- Terenzi M. G., Ingram C. D. A combined immunocytochemical and retrograde tracing study of noradrenergic connections between the caudal medulla and bed nuclei of the stria terminalis. Brain Res. 1995 Feb 20;672(1-2):289–297. doi: 10.1016/0006-8993(94)01453-o. [DOI] [PubMed] [Google Scholar]
- Veinante P., Stoeckel M. E., Freund-Mercier M. J. GABA- and peptide-immunoreactivities co-localize in the rat central extended amygdala. Neuroreport. 1997 Sep 8;8(13):2985–2989. doi: 10.1097/00001756-199709080-00035. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997 Dec 1;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner E. A., Kosten T. R., O'Connor P. G. Pharmacotherapy for opioid and cocaine abuse. Med Clin North Am. 1997 Jul;81(4):909–925. doi: 10.1016/s0025-7125(05)70555-1. [DOI] [PubMed] [Google Scholar]
- Wise R. A. Neurobiology of addiction. Curr Opin Neurobiol. 1996 Apr;6(2):243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- de Wit H., Chutuape M.A. Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol. 1993 Feb;4(1):29–36. [PubMed] [Google Scholar]
- de Wit H., Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 1983;79(1):29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- de Wit H., Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]


