Abstract
The transmission of antibodies across the gut of suckling pouch-young was investigated in three species of marsupials (Setonix brachyurus, Macropus eugenii and Trichosurus vulpecula) from Australia.
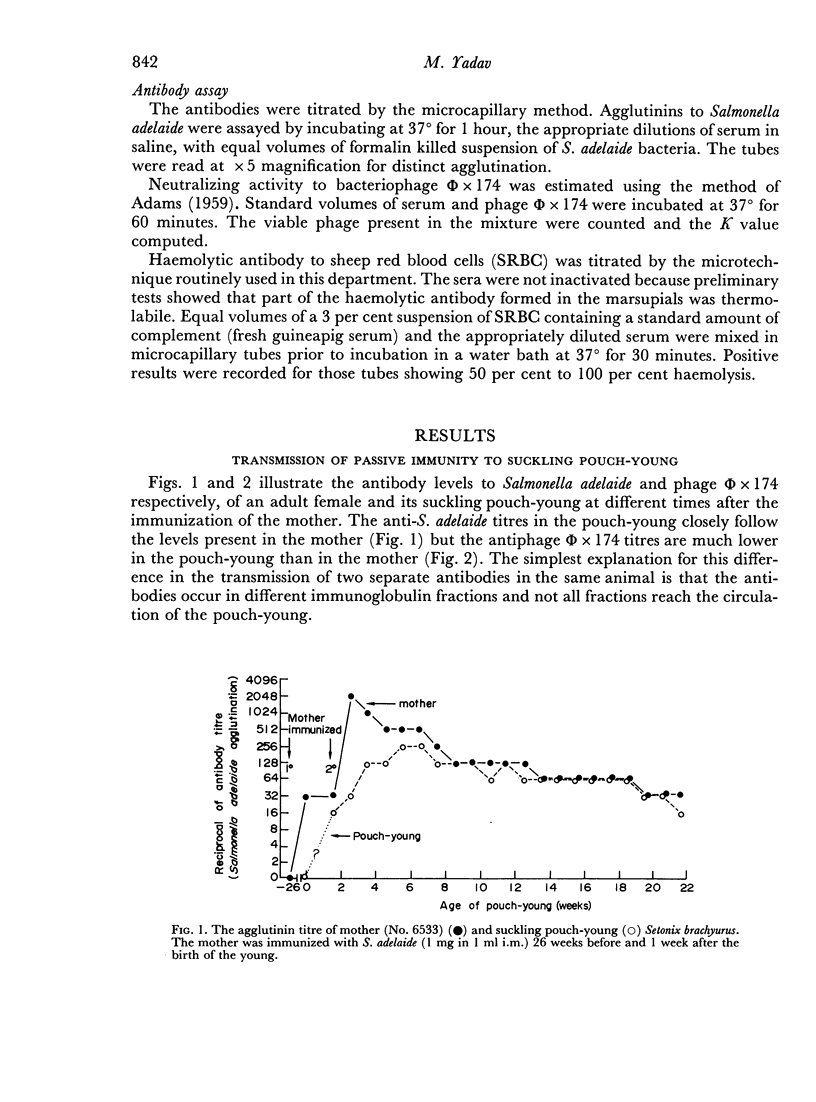
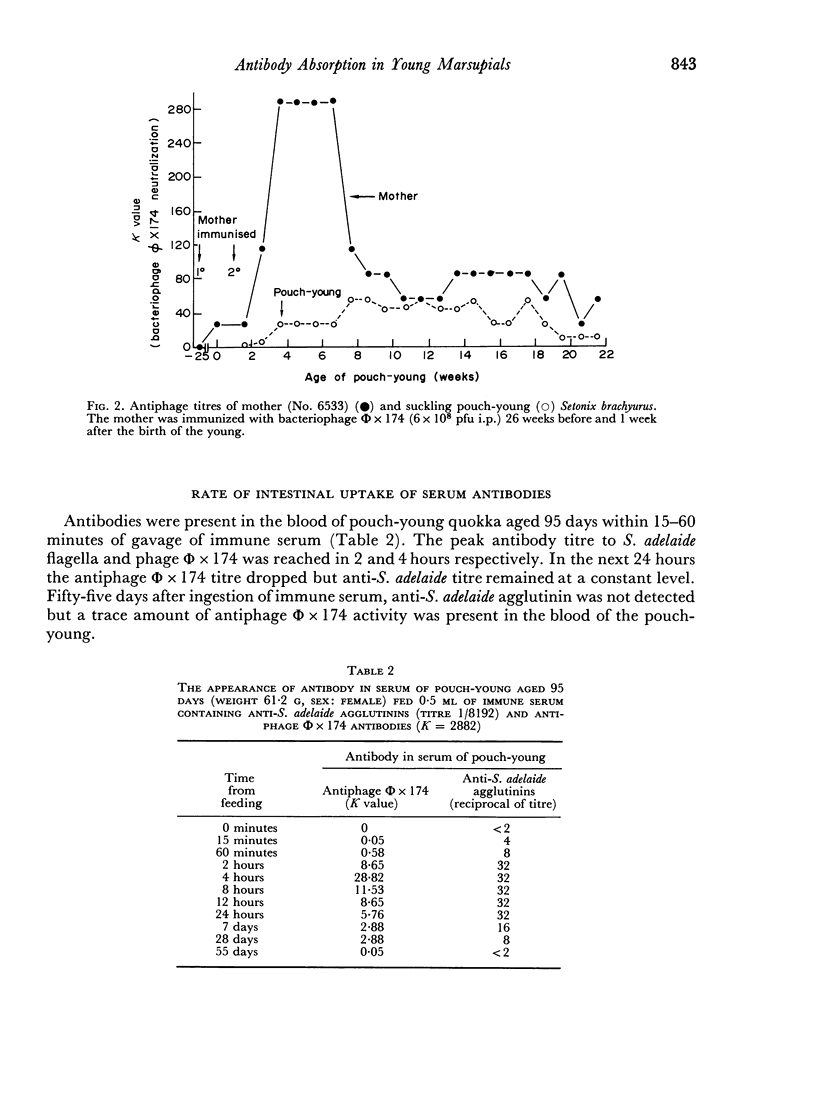
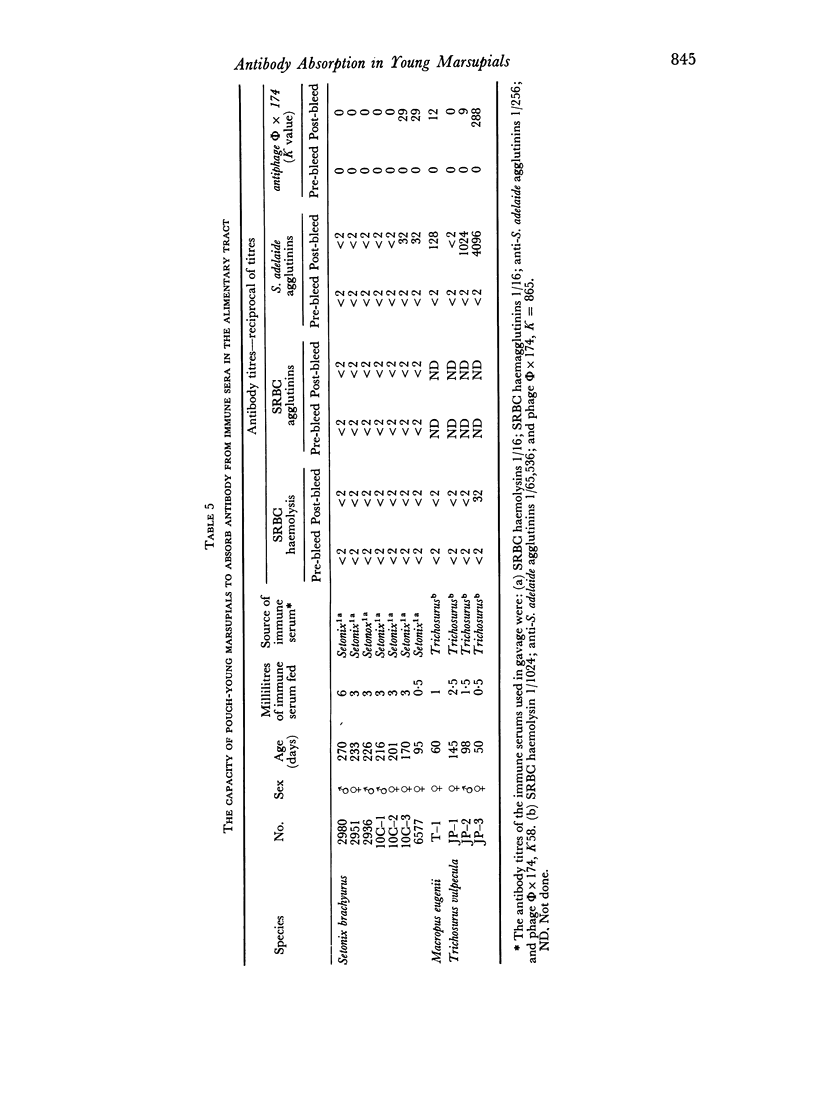
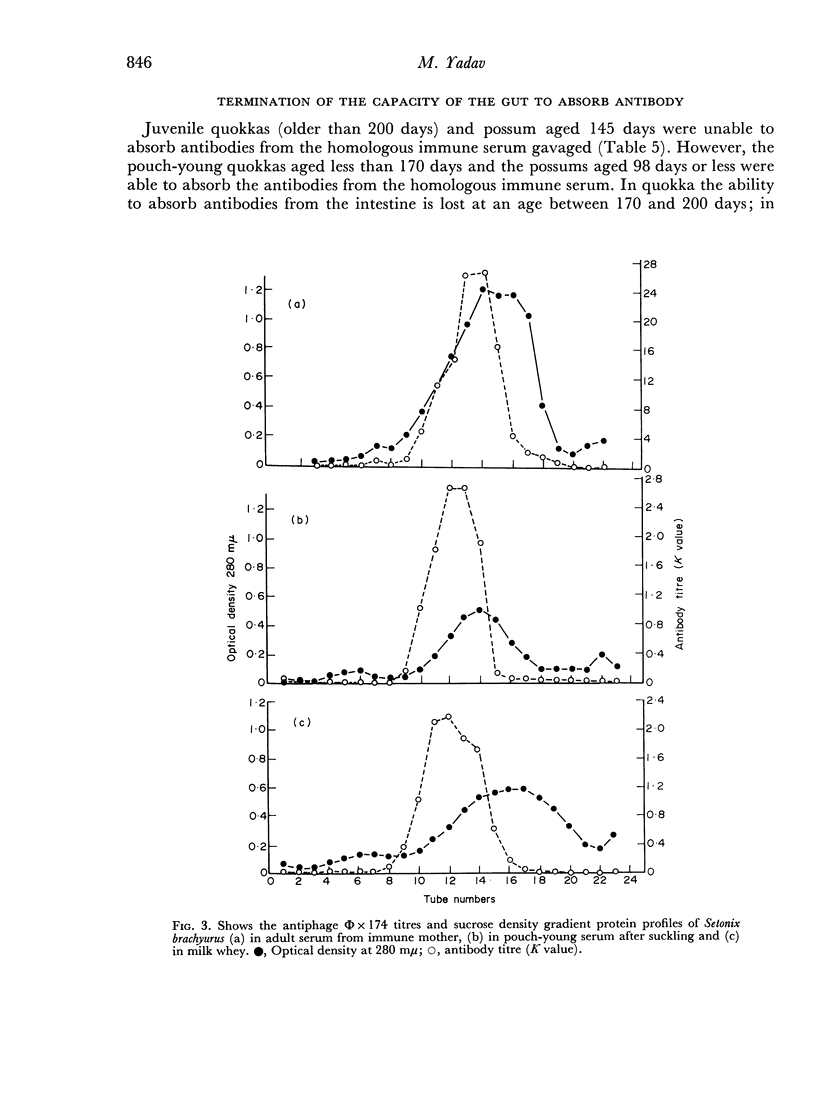
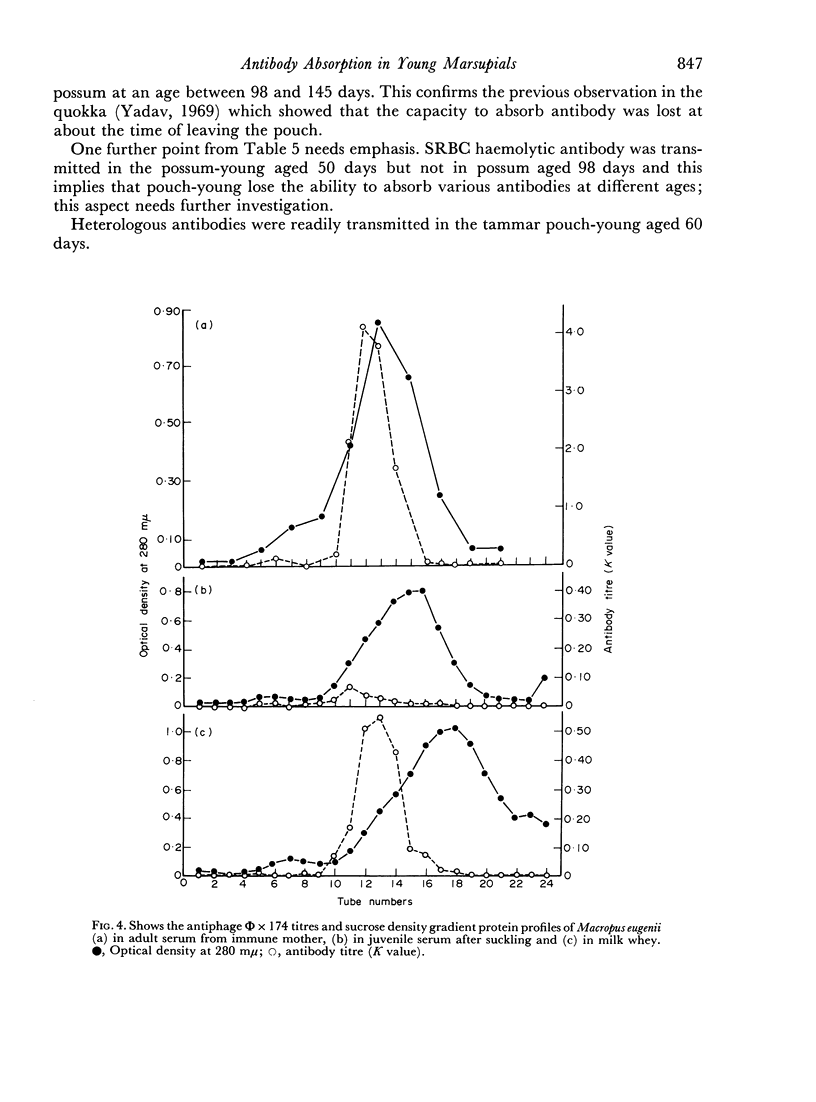
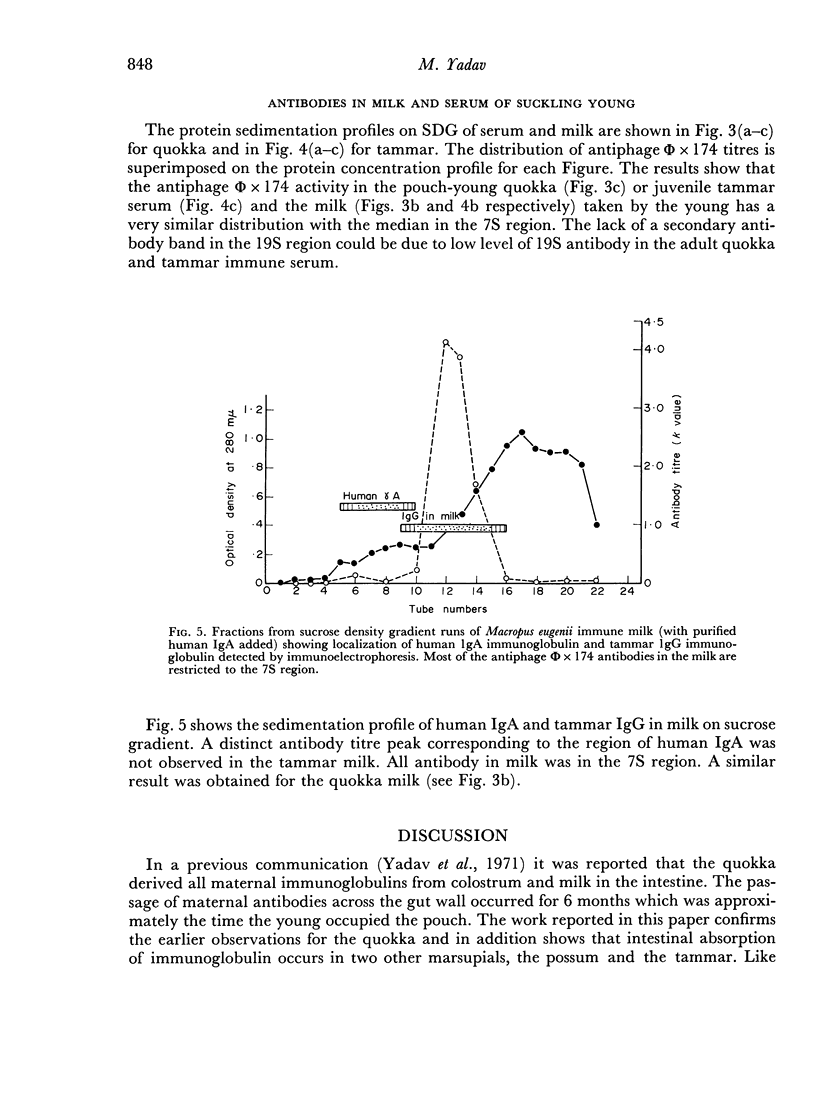
Mother Setonix, immunized against Salmonella adelaide flagella and Bacteriophage Φ × 174, transmitted the antibodies in milk to their young. In sucrose density gradient runs, the antibody activity in milk whey and in serum of pouch-young, of Setonix and Macropus was found to be in the 7S region only; antibody in the 11S and 19S regions was not detected. Chromatographic preparations of IgM antibodies were fed to pouch-young Setonix which were later bled and their serum titrated for anti-S. adelaide agglutinins and antiphage Φ × 174 activity. The IgM antibodies were not transmitted across the gut in detectable amounts.
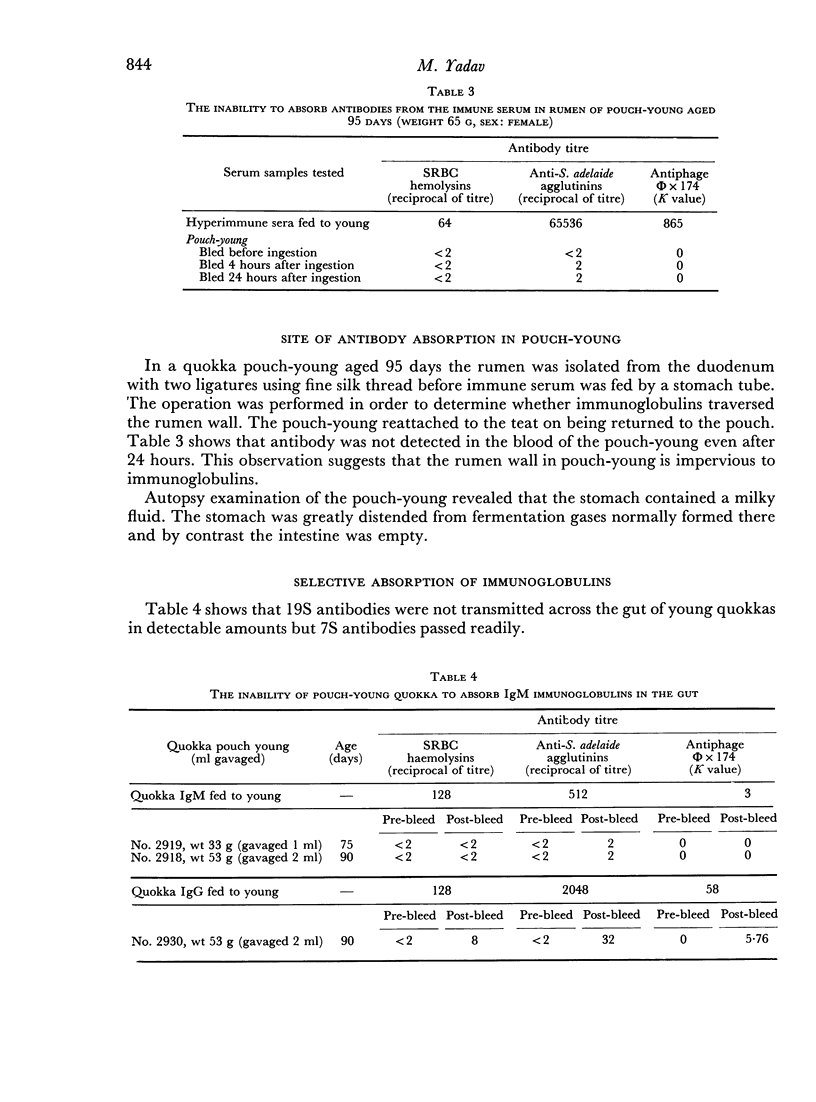
Antibodies were present in the blood of pouch-young Setonix within 15–60 minutes of gavage (feeding by stomach tube) of immune serum. In Setonix the capacity to absorb antibodies in the intestine was lost at an age between 170 and 200 days and in Trichosurus it was lost at an age between 98 and 145 days. At these ages the pouch-young were able to leave the marsupium for varying lengths of time. Antibodies did not traverse the rumen wall in a young Setonix whose rumen was isolated from the intestine with ligatures before immune serum was gavaged.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienenstock J., Bloch K. J. Immunoglobulins of the hamster. I. Antibody activity in four immunoglobulin classes. J Immunol. 1970 May;104(5):1220–1227. [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIDAY R. The absorption of antibodies from immune sera by the gut of the young rat. Proc R Soc Lond B Biol Sci. 1955 Mar 15;143(912):408–413. doi: 10.1098/rspb.1955.0020. [DOI] [PubMed] [Google Scholar]
- KALMUTZ S. E. Antibody production in the opossum embryo. Nature. 1962 Mar 3;193:851–853. doi: 10.1038/193851a0. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Bradley S. G., Watson D. W. Ontogeny of the immune response. I. Development of immunoglobulins in germfree and conventional colostrum-deprived piglets. J Immunol. 1966 Jul;97(1):52–63. [PubMed] [Google Scholar]
- Klaus G. G., Bennett A., Jones E. W. A quantitative study of the transfer of colostral immunoglobulins to the newborn calf. Immunology. 1969 Mar;16(3):293–299. [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Campiche M. A. Early stages of intestinal absorption of specific antibiodies in the newborn. An ultrastructural, cytochemical, and immunological study in the pig, rat, and rabbit. J Cell Biol. 1969 Aug;42(2):345–365. doi: 10.1083/jcb.42.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECCE J. G., MORGAN D. O. Effect of dietary regimen on cessation of intestinal absorption of large molecules (closure) in the neonatal pig and lamb. J Nutr. 1962 Nov;78:263–268. doi: 10.1093/jn/78.3.263. [DOI] [PubMed] [Google Scholar]
- LOCKE R. F., MYERS W. L., SEGRE D. THE IMMUNOLOGIC BEHAVIOR OF BABY PIGS. IV. INTESTINAL ABSORPTION AND PERSISTANCE OF 6.6S AND 18S ANTIBODIES OF OVINE ORIGIN AND THEIR ROLE IN THE IMMUNOLOGIC COMPETENCE OF BABY PIGS. J Immunol. 1964 Oct;93:576–583. [PubMed] [Google Scholar]
- Morris I. G. The selective transmission of bovine gamma-globulins across the gut of suckling rodents. Immunology. 1969 Jul;17(1):139–149. [PMC free article] [PubMed] [Google Scholar]
- Morris I. G. The transmission of bovine anti-Brucella abortus agglutinins across the gut of suckling rats. Immunology. 1967 Jul;13(1):49–61. [PMC free article] [PubMed] [Google Scholar]
- Porter P., Noakes D. E., Allen W. D. Secretory IgA and antibodies to Escherichia coli in porcine colostrum and milk and their significance in the alimentary tract of the young pig. Immunology. 1970 Feb;18(2):245–257. [PMC free article] [PubMed] [Google Scholar]
- Porter P. Porcine colostral IgA and IgM antibodies to Escherichia coli and their intestinal absorption by the neonatal piglet. Immunology. 1969 Oct;17(4):617–626. [PMC free article] [PubMed] [Google Scholar]
- ROWLANDS D. T., Jr, LAVIA M. F., BLOCK M. H. THE BLOOD FORMING TISSUES AND BLOOD OF THE NEWBORN OPOSSUM (DIDELPHYS VIRGINIANA). II. ONTOGENESIS OF ANTIBODY FORMATION TO FLAGELLA OF SALMONELLA TYPHI. J Immunol. 1964 Jul;93:157–164. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Stechschulte D. J., Austen K. F. Immunoglobulins of rat colostrum. J Immunol. 1970 May;104(5):1052–1062. [PubMed] [Google Scholar]
- Waring H., Moir R. J., Tyndale-Biscoe C. H. Comparative physiology of marsupials. Adv Comp Physiol Biochem. 1966;2:237–376. doi: 10.1016/b978-0-12-395511-1.50009-3. [DOI] [PubMed] [Google Scholar]
- Yadav M., Papadimitriou J. M. The ultrastructure of the neonatal thymus of a marsupial, Setonix brachyurus. Aust J Exp Biol Med Sci. 1969 Dec;47(6):653–668. doi: 10.1038/icb.1969.163. [DOI] [PubMed] [Google Scholar]


