Abstract
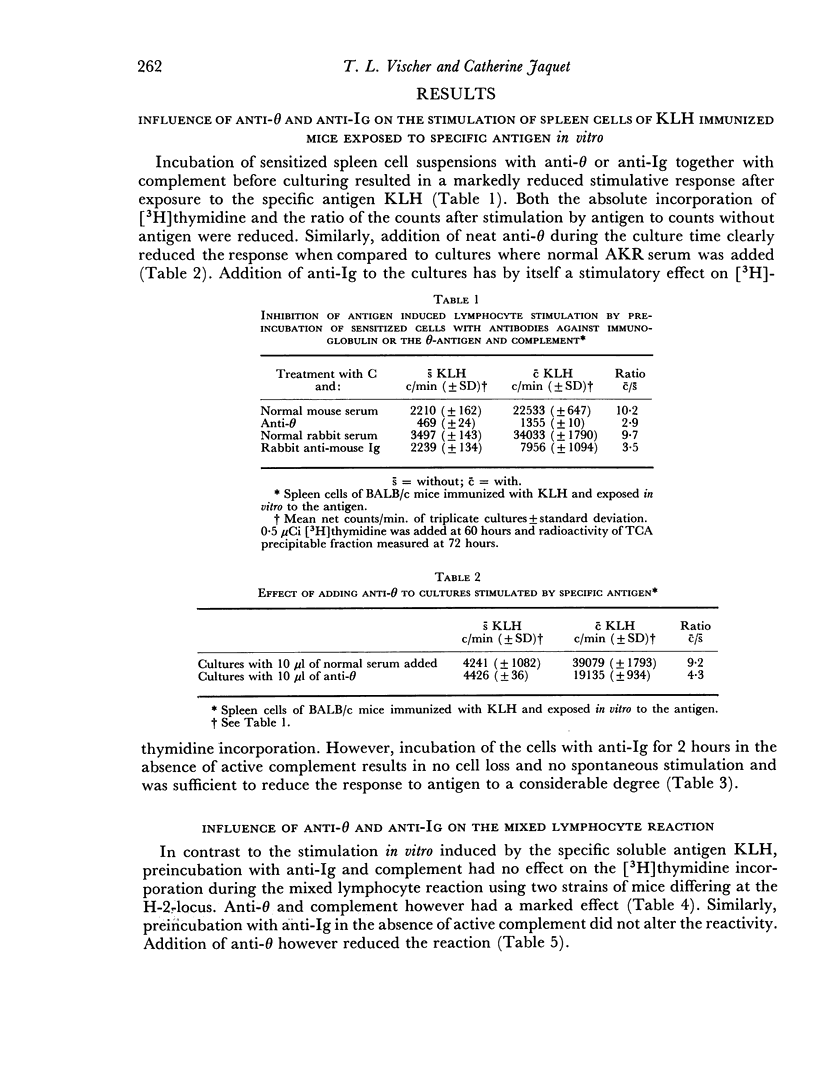
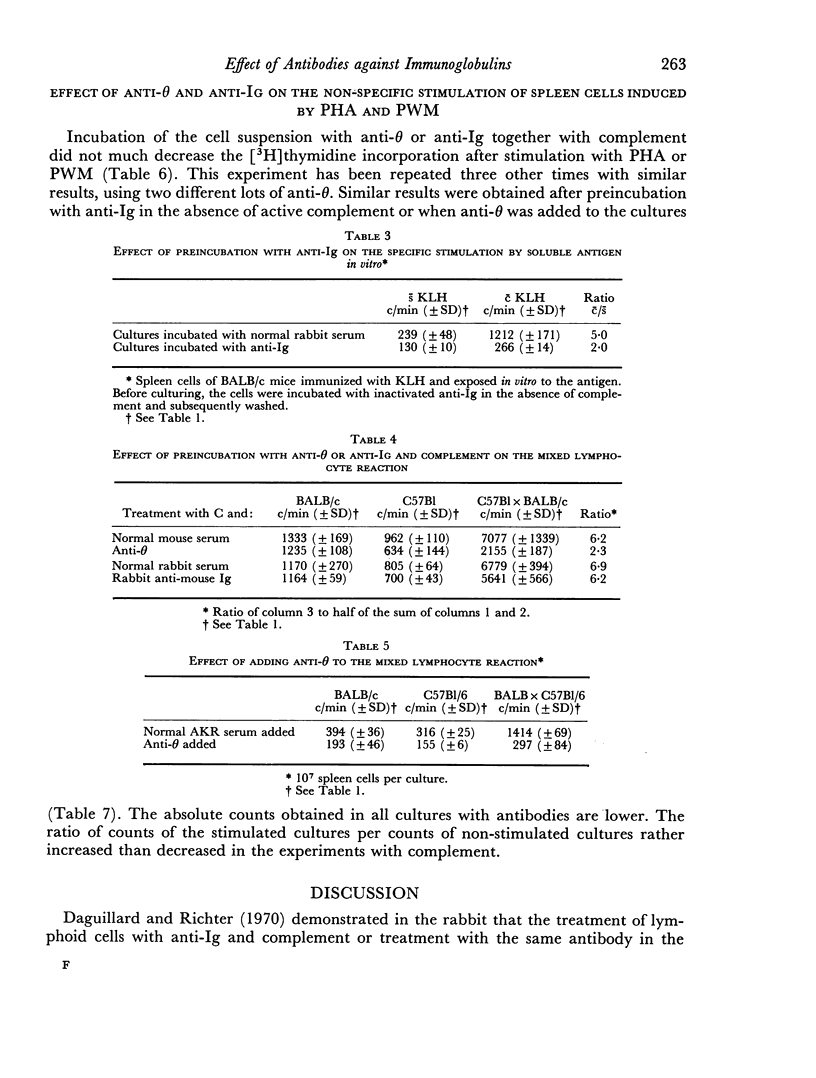
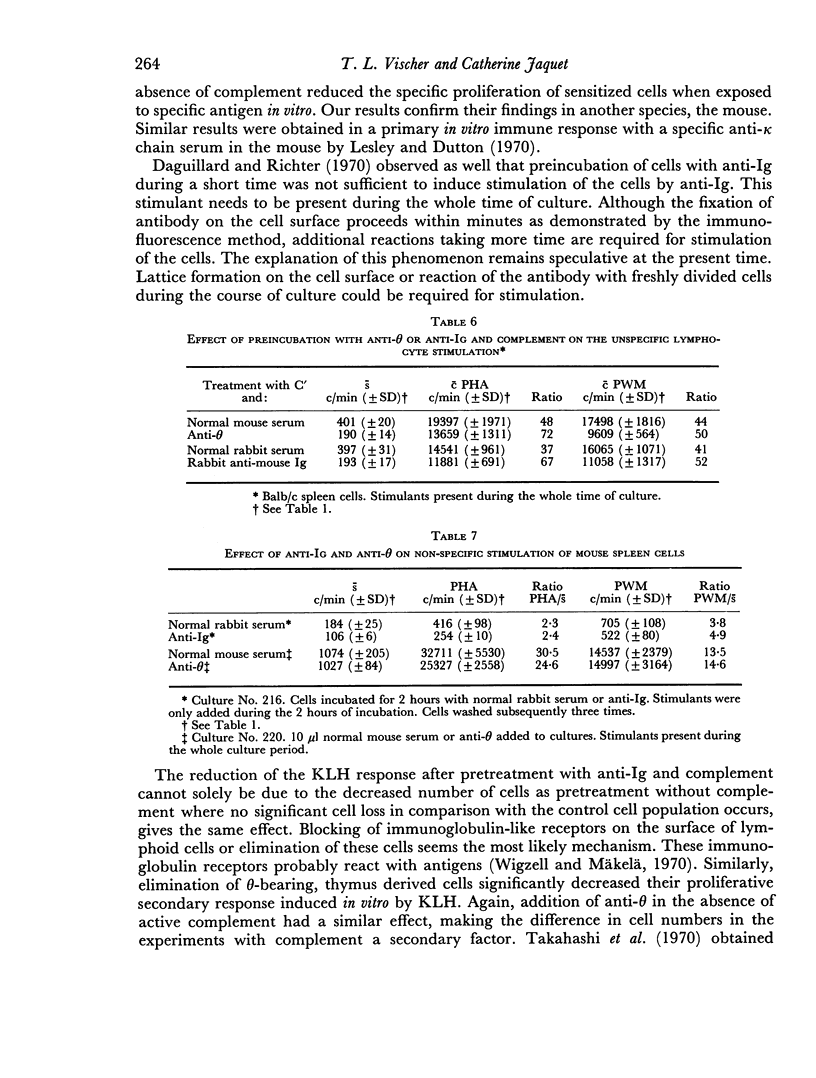
Normal mouse spleen cells were stimulated in culture by phytohaemagglutinin (PHA), pokeweed mitogen (PWM), allogeneic cells; keyhole limpet haemocyanin (KLH) sensitized cells by the specific antigen. The stimulation of the cells was measured by [3H]thymidine incorporation into the TCA precipitable fraction of the cultures. In this system, the effect of treating the cells with an antibody against the theta antigen and an antibody against immunoglobulin, with or without complement, was investigated. Treatment of the cells with antisera and complement or without complement gave similar results. The secondary in vitro stimulation with soluble antigen KLH could be markedly reduced with both anti-θ and anti-immunoglobulin serum. The response to allogeneic cells in the mixed lymphocyte reaction was only reduced by anti-θ serum and not by anti-immunoglobulin serum. No definite effect could be demonstrated by either antibody on the non-specific stimulation by PHA or PWM.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colley D. G., Wu A. Y., Waksman B. H. Cellular differentiation in the thymus. 3. Surface properties of rat thymus and lymph node cells separated on density gradients. J Exp Med. 1970 Dec 1;132(6):1107–1121. doi: 10.1084/jem.132.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., McConnell I., Munro A. Immunoglobulin determinants on mouse lymphocytes from blood, lymph nodes, bone marrow and thymus. Int Arch Allergy Appl Immunol. 1970;39(2-3):280–291. doi: 10.1159/000230354. [DOI] [PubMed] [Google Scholar]
- Daguillard F., Richter M. Cells involved in the immune response. XVI. The response of immune rabbit cells to phytohemagglutinin, antigen, and goat anti-rabbit immunoglobulin antiserum. J Exp Med. 1970 Jan 1;131(1):119–131. doi: 10.1084/jem.131.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallily R., Garvey J. S. Primary stimulation of rats and mice with hemocyanin in soluton and adsorbed on bentonite. J Immunol. 1968 Nov;101(5):924–929. [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Blocking of the lymphocyte receptor site for cell mediated hypersensitivity and transplantation reactions by anti-light chain sera. Nature. 1969 May 31;222(5196):885–886. doi: 10.1038/222885a0. [DOI] [PubMed] [Google Scholar]
- Imrie R. C., Mueller G. C. Release of a lymphocyte growth promoter in leucocyte cultures. Nature. 1968 Sep 21;219(5160):1277–1279. doi: 10.1038/2191277a0. [DOI] [PubMed] [Google Scholar]
- Larralde C. In vitro mitotic activity of unsensitized lymphocytes in the presence of antigen and sensitized lymphocytes. Proc Soc Exp Biol Med. 1970 Apr;133(4):1175–1177. doi: 10.3181/00379727-133-34649. [DOI] [PubMed] [Google Scholar]
- Lesley J., Dutton R. W. Antigen receptor molecules: inhibition by antiserum against kappa light chains. Science. 1970 Jul 31;169(3944):487–488. doi: 10.1126/science.169.3944.487. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970 Jun 27;226(5252):1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Rieke W. O. Lymphocytes from thymectomized rats: immunologic, proliferative, and metabolic properties. Science. 1966 Apr 22;152(3721):535–538. doi: 10.1126/science.152.3721.535. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Inhibition of in vitro immune response by treatment of spleen cell suspensions with anti-theta serum. Nature. 1970 Jun 27;226(5252):1258–1259. doi: 10.1038/2261258a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Carswell E. A., Thorbecke G. J. Surface antigens of immunocompetent cells. I. Effect of theta and PC.1 alloantisera on the ability of spleen cells to transfer immune responses. J Exp Med. 1970 Dec 1;132(6):1181–1190. doi: 10.1084/jem.132.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi T., Adler W. H., Smith R. T. Cellular recognition in vitro by mouse lymphocytes. Effects of neonatal thymectomy and thymus graft restoration on alloantigen and PHA stimulation of whole and gradient-separated subpopulations of spleen cells. J Exp Med. 1971 Jan 1;133(1):63–80. doi: 10.1084/jem.133.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L., Stastny P. Time of appearance and distribution of cells capable of secondary immune response following primary immunization. Immunology. 1967 Jun;12(6):675–687. [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Mäkelä O. Separation of normal and immune lymphoid cells by antigen-coated coated columns. Antigen-binding characteristics of membrane antibodies as analyzed by hapten-protein antigens. J Exp Med. 1970 Jul 1;132(1):110–126. doi: 10.1084/jem.132.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]


