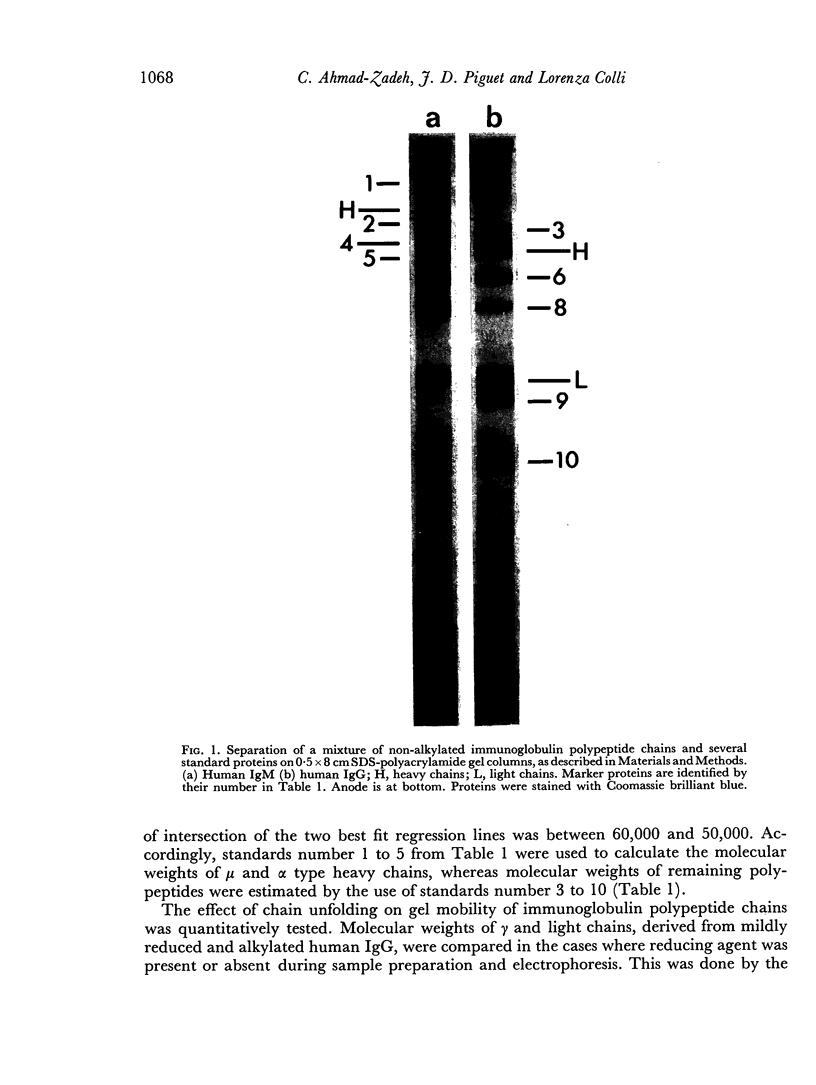
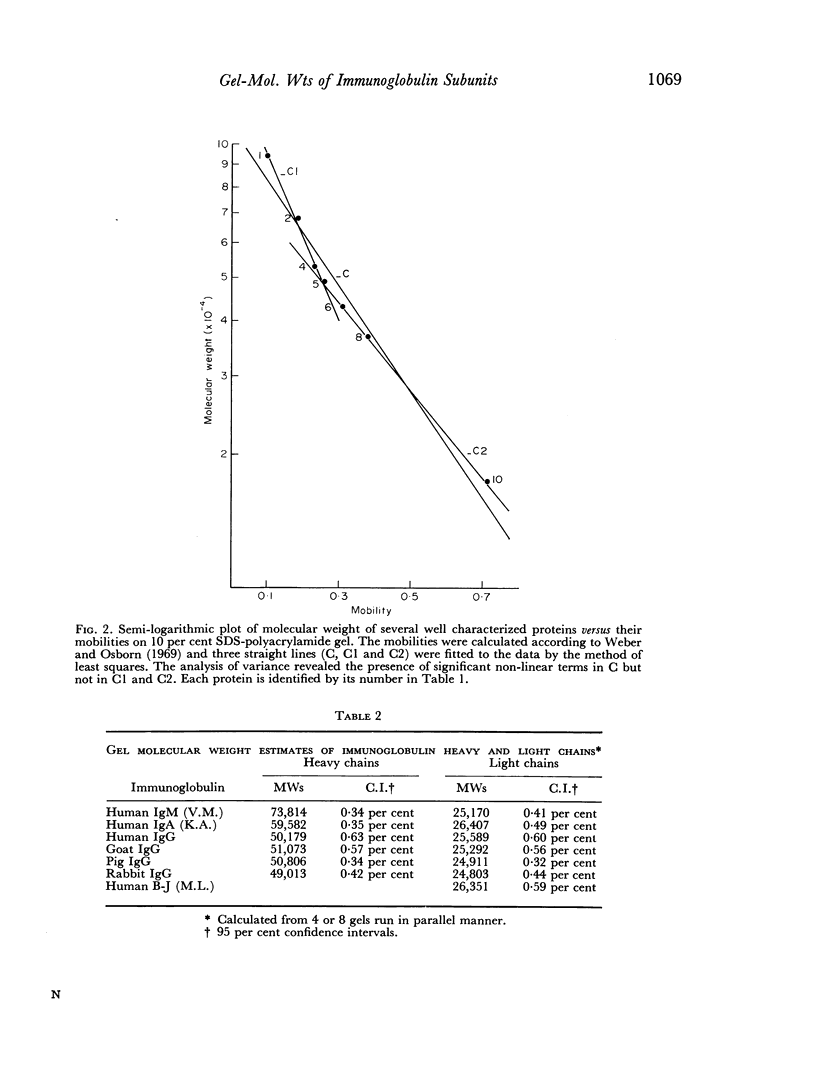
Abstract
Molecular weights of immunoglobulin polypeptide chains can be accurately determined by dodecyl sulphate polyacrylamide gel electrophoresis. Non-alkylated immunoglobulin preparations can be used. Depending on the mobility of the polypeptide chain, a relatively narrow range of standard size should be used to calibrate the molecular weight plots; this is concluded from an evaluation of the molecular weight-mobility relationship of dodecyl sulphate-complexes of proteins in 10 per cent polyacrylamide gel.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Black L. W. Ahmad-Zadeh C,+AHMADAAZADEH C: Internal proteins of bacteriophage T4D: their characterization and relation to head structure and assembly. J Mol Biol. 1971 Apr 14;57(1):71–92. doi: 10.1016/0022-2836(71)90120-3. [DOI] [PubMed] [Google Scholar]
- Buxbaum J., Franklin E. C., Scharff M. D. Immunoglobulin M heavy chain disease: intracellular origin of the mu chain fragment. Science. 1970 Aug 21;169(3947):770–773. doi: 10.1126/science.169.3947.770. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Dorrington K. J., Tanford C. Molecular size and conformation of immunoglobulins. Adv Immunol. 1970;12:333–381. doi: 10.1016/s0065-2776(08)60173-x. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A., Dourmashkin R. R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970 Apr;18(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS A. J., KEMP P. G., Jr, VANNIER W. E., GOODMAN H. C. PURIFICATION OF HUMAN SERUM GAMMA-GLOBULIN FOR IMMUNOLOGIC STUDIES: GAMMA-GLOBULIN FRAGMENTATION AFTER SULFATE PRECIPITATION AND PROLONGED DIALYSIS. J Immunol. 1964 Jul;93:24–34. [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Delius H., Ahmad-Zadeh C., Bickle T. A., Pearson P., Tissières A. Ribosomal proteins of E. Coli: stoichiometry and implications for ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:25–38. doi: 10.1101/sqb.1969.034.01.007. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]