Abstract
Cervical cancer cells express high-risk human papillomavirus (HPV) E6 and E7 proteins, and repression of HPV gene expression causes the cells to cease proliferation and undergo senescence. However, it is not known whether both HPV proteins are required to maintain the proliferative state of cervical cancer cells, or whether mutations that accumulate during carcinogenesis eliminate the need for one or the other of them. To address these questions, we used the bovine papillomavirus E2 protein to repress the expression of either the E6 protein or the E7 protein encoded by integrated HPV18 DNA in HeLa cervical carcinoma cells. Repression of the E7 protein activated the Rb pathway but not the p53 pathway and triggered senescence, whereas repression of the E6 protein activated the p53 pathway but not the Rb pathway and triggered both senescence and apoptosis. Telomerase activity, cyclin-dependent kinase activity, and expression of c-myc were markedly inhibited by repression of either E6 or E7. These results demonstrate that continuous expression of both the E6 and the E7 protein is required for optimal proliferation of cervical carcinoma cells and that the two viral proteins exert distinct effects on cell survival and proliferation. Therefore, strategies that inhibit the expression or activity of either viral protein are likely to inhibit the growth of HPV-associated cancers.
Cervical carcinoma is initiated by infection with a high-risk human papillomavirus (HPV), usually HPV type 16 (HPV16) or HPV18, and gene transfer studies have identified the E6 and E7 genes as the major viral oncogenes (62). The HPV E6 and the E7 proteins modulate cellular proteins that regulate the cell cycle (reviewed in references 35 and 38). The E6 protein binds to the p53 tumor suppressor protein and targets it for accelerated ubiquitin-mediated degradation. The E6 protein also stimulates telomerase activity in cultured keratinocytes. The E7 protein binds to the active, hypophosphorylated form of p105Rb and other members of the retinoblastoma (Rb) family of tumor suppressor proteins, resulting in their destabilization and loss of Rb/E2F complexes that repress transcription of genes required for cell cycle progression. The p53 and Rb pathways are interconnected: cyclin-dependent kinase (cdk)-mediated phosphorylation of p105Rb, which also disrupts Rb/E2F complexes, is inhibited by p21, a transcriptional target of p53. The E6 and E7 proteins also appear to have p53- and Rb-independent activities (35, 38).
The high-risk HPV E6 and E7 proteins have profound effects on the growth properties of primary cultured human keratinocytes. Normal human keratinocytes undergo a limited number of divisions in culture before they enter replicative senescence, an irreversible, nonproliferative state characterized by growth factor-resistant growth arrest, specific morphological changes, increased autofluorescence, and elevated senescence-associated β-galactosidase (SAβ-Gal) activity (48). Escape from replicative senescence and immortalization of cultured human keratinocytes require reactivation of telomerase and inactivation of the Rb pathway (7, 32). Coexpression of high-risk E6 and E7 genes can also induce immortalization. HPV-induced keratinocyte immortalization is inhibited by mutations that interfere with the ability of the E7 protein to inactivate p105Rb or the E6 protein to activate telomerase (32; K. Munger and D. Galloway, personal communication). Therefore, the known activities of the HPV proteins are likely to play an important role in keratinocyte immortalization, which is thought to be one of the earliest steps in carcinogenesis. However, HPV-immortalized cells are not tumorigenic, implying that additional mutations are required for malignant progression. The HPV proteins also facilitate the acquisition of mutations required for carcinogenic progression because E7 expression and E6-mediated inactivation of p53 cause increased mutagenesis and genomic instability (60).
Although irreversible cellular genetic events appear to be required for carcinogenesis, continuous expression of the HPV oncogenes is required for the proliferation of cervical carcinoma cell lines. Antisense approaches to repress HPV gene expression in cervical carcinoma cell lines typically result in a severalfold inhibition of proliferation (e.g., see reference 55). More dramatic effects are induced by papillomavirus E2 proteins, which bind to the HPV major early promoter and inhibit transcription of the E6 and E7 genes (52). Introduction of the bovine papillomavirus (BPV) E2 gene on a simian virus 40 (SV40)-based viral vector into HeLa cervical carcinoma cells represses expression of the integrated HPV18 E6 and E7 genes and can cause close to 99% inhibition of cellular DNA synthesis within 2 days (16, 26). BPV E2-mediated repression of E6 and E7 expression in HeLa cells and several other cervical carcinoma cell lines activates the p53 and Rb pathways and inhibits telomerase activity (4, 9, 16, 18, 25, 26, 59). The resulting growth arrest is durable, and the cells rapidly acquire a senescent phenotype. The BPV and HPV18 E2 genes also markedly inhibit the ability of the cells to form colonies (9, 52). In numerous experiments, the E2-induced growth arrest occurs only in cells containing HPV DNA (9, 26, 39). Furthermore, these effects on cell proliferation are mitigated by constitutive expression of the E6-E7 region and are elicited only by E2 proteins able to bind DNA and repress HPV transcription (10, 16, 17, 42, 59). Taken together, these results indicate that proliferation of cervical cancer cell lines requires continuous HPV gene expression.
In our experiments and those carried out by the Howley laboratory, the effects of the E2 protein require repression of the endogenous HPV genes. However, there are reports that in some situations papillomavirus E2 proteins can elicit HPV-independent effects, including apoptosis. Thierry and colleagues reported that transfection of the BPV or HPV18 E2 gene induces apoptosis as well as p53-dependent growth arrest. This apoptosis does not require p53 function and can occur in cells devoid of HPV DNA (4, 5). Gaston and colleagues reported that the HPV16 E2 protein activates rather than represses transcription of the HPV16 genomes in SiHa cervical cancer cells and induces rapid p53-dependent apoptosis in cells maintained in the absence of serum whether or not the cells contain HPV DNA (47, 58). Finally, expression of the HPV31 E2 protein by high-multiplicity infection with an adenovirus vector eliminates a mitotic checkpoint in epithelial cells harboring HPV31 DNA and in normal human keratinocytes (11).
The biological activities of the HPV E6 and E7 proteins have been studied extensively following their introduction into cells. However, these studies do not necessarily predict the activities of the HPV proteins in cervical carcinoma cells, because these cells presumably harbor mutations in growth-regulatory genes and frequently display features such as aneuploidy, short telomeres, and elevated p16INK4 levels that may influence the response of the cells to the HPV oncogenes. Furthermore, unlike primary cells, cervical carcinoma cells may well have adapted to the continuous expression of the HPV oncogenes during carcinogenic progression and in vitro passage, so that abrupt removal of the viral proteins may elicit unpredictable responses. Thus, it is not known which aspects of the biochemical and growth phenotypes of cervical cancer cells require expression of the E6 protein, which require the E7 protein, and which require both viral proteins. Wells et al. (59) demonstrated that cotransfecting HeLa cells with the BPV E2 gene and the HPV16 E6 or E7 gene partially inhibited E2-mediated growth inhibition. However, the biochemical responses to the E6 or the E7 gene were not determined in these experiments, and the cellular phenotype was not examined in detail. To continue our analysis of the role of the HPV oncogenes in growth control in cervical carcinoma cells, we developed a method to assess the individual contributions of the resident HPV18 E6 and E7 genes in HeLa cells. We demonstrated that E7 repression efficiently triggered senescence in HeLa cells without activating p53 or inducing p21, whereas E6 repression triggered both senescence and apoptosis without activating the retinoblastoma pathway. In contrast, high-level telomerase and cdk2 activity and c-myc expression required continuous expression of both viral proteins. Thus, individual HPV18 oncogenes play distinct roles in maintaining HeLa cell proliferation.
MATERIALS AND METHODS
Cells and viruses.
To reduce genetic variability in the starting cell population, HeLa cells were cloned by limiting dilution. The resulting HeLa/sen2 cells respond efficiently to E6-E7 repression, allowing their analysis over several weeks with minimal outgrowth of cells that escaped growth inhibition (18). Cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, penicillin-streptomycin, amphotericin B (Fungizone), and 10 mM HEPES (pH 7.3). HeLa/sen2 cells were infected with LXSN, LXSN HPV16 E6, and LXSN HPV16 E7 retroviruses obtained from Denise Galloway (Fred Hutchinson Cancer Research Center) (20), and stable cell lines were expanded from individual colonies after 2 weeks of selection in medium containing 1 mg of G418/ml, to generate the HeLa/LXSN, HeLa/16E6, and HeLa/16E7 cell lines, respectively. To construct RVY HPV18 E7, we amplified the HPV18 E7 gene (nucleotides 590 to 909) by PCR from the complete HPV18 genome and inserted it into RVY retroviral DNA, which encodes hygromycin resistance (41). RVY and RVY HPV18 E7 retroviruses were used to infect HeLa/LXSN and HeLa/16E6 cells, and stable cell lines were expanded from individual colonies after 2 weeks of selection in medium containing 0.5 mg of G418/ml and 250 μg of hygromycin B/ml, to generate HeLa/LXSN-RVY and HeLa/16E6-18E7 cell lines, respectively. Cells were maintained in medium containing 0.5 mg of G418/ml plus, if appropriate, 125 μg of hygromycin B/ml. High-titered stocks of the SV40/BPV type 1 (BPV-1) recombinant virus expressing the BPV-1 E2 protein were prepared, their titers were determined, and they were used to infect cells at a multiplicity of infection of 20 as described previously (39). Infected cells were maintained without drug selection with fresh medium every 3 days for the duration of the experiment.
Western and Northern blots.
Cells were harvested 2 days after mock infection or infection with the E2 virus and frozen. Protein extracts were prepared from frozen cells in modified EBC buffer (50 mM Tris-HCl [pH 7.5], 120 mM NaCl, 2 mM EDTA, 0.4% NP-40, 1 mM NaF, 1 mM Na orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 5 μg of leupeptin and aprotinin/ml). For the analysis of p105Rb, cells were lysed with Trizol reagent (Life Technologies Inc., Bethesda, Md.) and protein extracts were prepared as described previously (17). Total RNA was prepared from frozen cells with the RNeasy Mini kit according to the manufacturer's instructions (Qiagen, Valencia, Calif.).
For Western blots, 5 μg of total protein was resolved by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels, transferred to an Immobilon-P membrane (Millipore, Bedford, Mass.) in 12.5 mM Tris-0.1 M glycine-20% methanol transfer buffer, and blocked in 5% milk-TBST buffer (5% nonfat dry milk, 25 mM Tris-HCl [pH 8.0], 125 mM NaCl, and 0.5% Tween 20). The membranes were probed with antibodies specific for the following proteins: p53 (15801A), p16INK4 (554070), and p105Rb (14001A) from BD PharMingen (San Diego, Calif.); p21 (SC-397), c-myc (SC-40), cdk2 (SC-163), and cyclin E (SC-247) from Santa Cruz Biotechnology; and cyclin A (gift from H. Zhang, Yale University). The membranes were washed in TBST, incubated with species-specific horseradish peroxidase-conjugated donkey antibody (Jackson ImmunoResearch, West Grove, Pa.), and washed again in TBST. The membranes were then incubated with ECL+ (Amersham, Little Chalfont, United Kingdom), and the signals were detected with Hyperfilm (Amersham).
A 2.5-μg quantity of total RNA was denatured, electrophoresed on a 1% formaldehyde-agarose gel, transferred to a Nytran Supercharge membrane (Schleicher & Schuell, Keene, N.H.), UV cross-linked to the membrane with a Stratalinker (Stratagene, La Jolla, Calif.), and hybridized with random prime-labeled probes for the HPV16 or HPV18 E6-E7 region. After washing, the signal was visualized with a Storm 840 PhosphorImager (Molecular Dynamics, Inc.).
cdk2 kinase assay.
Cells were seeded at 5 × 105 per 100-mm-diameter dish and the next day mock infected or infected with E2 virus. Two days later, the cells were harvested and stored at −80°C. Frozen cell pellets were lysed in modified EBC buffer, 20 μg of extracted protein was immunoprecipitated with SC-163 anti-cdk2 antibody for 2 h, and histone H1 kinase activity was measured as previously described (25). There was no detectable activity when nonimmune immunoglobulin G was used for immunoprecipitation.
Telomerase activity.
Cells were seeded at 2 × 105 per 60-mm-diameter dish and the next day mock infected or infected with E2 virus. Two and six days later, the cells were harvested and stored at −80°C. The samples were analyzed for telomerase activity with a TRAPeze telomerase detection kit (Intergen, Purchase, N.Y.) according to the manufacturer's instructions for radioisotopic detection. The reaction products were resolved on a 10% nondenaturing polyacrylamide minigel. No activity was detectable if samples were heat inactivated prior to analysis.
Cell morphology.
Cells were seeded at 5 × 105 (HeLa/LXSN or HeLa/16E6) or 5 × 104 (HeLa/16E7) per 100-mm-diameter dish and infected with E2 virus the next day. Mock-infected cells were plated at 104 cells/plate. Ten days after infection phase-contrast photomicrographs were taken.
DNA synthesis assays.
Cells in 24-well plates were mock infected or infected with E2 virus. Two days after infection the cells were labeled with medium containing 1.5 μCi of [3H]thymidine (Amersham)/ml for 4 h. Incorporated acid-insoluble thymidine was measured as described previously (16). All assays were performed in quadruplicate.
Autofluorescence and annexin V binding.
Cells were seeded at 1 × 105 or 4 × 105 into 100-mm-diameter dishes and the next day were mock infected or infected with the E2 virus, respectively. Six days after infection, the cells were harvested by trypsinization, resuspended in fresh medium, washed once with cold phosphate-buffered saline, and resuspended in annexin binding buffer (Molecular Probes, Eugene, Oreg.). For autofluorescence, the cells were analyzed by flow cytometry as previously described except that the data were not normalized (18). For annexin V binding, the samples were then processed and analyzed by using a Vybrant apoptosis assay no. 2 kit (Molecular Probes) according to the manufacturer's instructions for flow cytometry.
SAβ-Gal assay.
Cells were seeded at 1 × 104 or 2 × 105 in 35-mm-diameter dishes and the next day were mock infected or infected with E2 virus, respectively. Ten days later, the cells were stained at pH 6.0 with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as described previously (8).
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay.
Cells in eight-well glass slides (Falcon, Franklin Lakes, N.J.) were mock infected or infected with E2 virus. Five days after infection, the cells were processed for DNA strand breaks with an in situ cell death detection kit with fluorescein (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions for adherent cells except that the cells were fixed in 4% formaldehyde (Formalde-Fresh; Fisher, Pittsburgh, Pa.). Fluorescent images of the same fields were captured in both the DAPI (4′,6′-diamidino-2-phenylindole) fluorescence channel (excitation wavelength, 350 nm; emission wavelength, 456 nm) and the fluorescein fluorescence channel (494 and 525 nm, respectively) on an Olympus AX-70 fluorescence microscope with a Sensysis camera (Photometrics, Tucson, Ariz.).
BrdU labeling.
Cells (106) in 100-mm-diameter dishes were infected with the E2 virus 2 and 6 days before processing, so that all samples could be analyzed in parallel at the same time. Cells were also mock infected 2 days before processing. The cells were labeled and processed with the bromodeoxyuridine (BrdU) flow kit (BD PharMingen) according to the manufacturer's instructions for flow cytometry.
RESULTS
Individual repression of HPV E6 and E7 expression in HeLa cells.
To determine the consequences of individually repressing the HPV oncogenes in cervical carcinoma cells, we developed a system to separately extinguish expression of the E6 or the E7 protein in HeLa cells. First, we used recombinant retroviruses to introduce constitutively expressed copies of the high-risk HPV16 E6 or E7 gene into HeLa cells. Retroviruses comprised of the empty vector (LXSN) and the vector containing the HPV16 E6 or E7 gene were used to infect a cloned strain of HeLa cells, and individual G418-resistant colonies were expanded to generate HeLa/LXSN, HeLa/16E6, and HeLa/16E7 cell lines, respectively. We then used a recombinant SV40-based viral vector to introduce the BPV E2 gene into these cells. The E2 protein specifically represses transcription of the endogenous HPV18 E6 and E7 genes in HeLa cells, whereas the retroviral long terminal repeat driving expression of the HPV16 E6 or E7 gene is not affected by the E2 protein. This approach efficiently delivers the E2 gene so that the acute biochemical and physiological response of the entire population of cells can be determined.
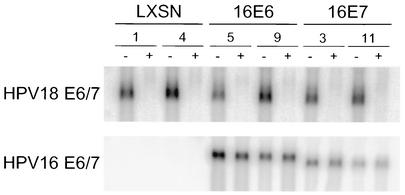
Stable cell lines established with these retroviruses were analyzed by Northern blotting to determine whether they displayed the desired pattern of HPV gene expression. As expected, expression of the endogenous HPV18 E6-E7 genes was markedly repressed by E2 expression in all of the cell lines 2 days after infection (Fig. 1, top). The same membrane was stripped and probed with the HPV16 E6-E7 region, revealing that the HPV16 genes were not repressed by the E2 protein (Fig. 1, bottom). Thus, following E2 expression, the HPV16 E6 protein was the only HPV protein expressed in HeLa/E6 cells, and the HPV16 E7 protein was the only HPV protein expressed in the HeLa/16E7 cells. In effect, these manipulations specifically repressed E7 in the HeLa/16E6 cells and E6 in the HeLa/16E7 cells.
FIG. 1.
Individual repression of HPV E6 and E7 expression. RNA was isolated from two clones each of the HeLa/LXSN, HeLa/16E6, and HeLa/16E7 cell lines (clone numbers indicated) 2 days after infection with the E2 virus (+) or mock infection (−). A 2.5-μg amount of each sample was resolved by gel electrophoresis, transferred to a membrane, and hybridized to probes specific for the HPV18 (top) or HPV16 (bottom) E6-E7 genes.
We also generated cells in which both E6 and E7 were constitutively expressed. HeLa/16E6 cells were infected with a retrovirus carrying the hygromycin resistance gene and the HPV18 E7 gene, and HeLa/16E6-18E7 cells were established from colonies resistant to both G418 and hygromycin. The E2 protein repressed expression of the endogenous HPV18 E6 and E7 genes in these cells, but expression of the exogenous E6 and E7 genes persisted (data not shown). We also infected HeLa/LXSN cells with the empty hygromycin resistance vector to generate the control cell line HeLa/LSXN-RVY. In addition, we generated HeLa/18E7 cells which constitutively expressed the HPV18 E7 gene alone. HeLa/18E7 and HeLa/16E7 cells displayed the same biochemical and biological responses to E2 expression (data not shown).
Biochemical analysis of HeLa cells following specific repression of E6 or E7 expression.
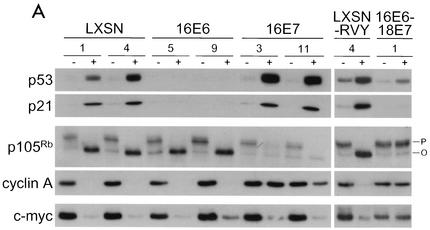
The biochemical and physiologic responses of these cells to E2 expression are summarized in Table 1. Protein extracts were prepared from cells 2 days after mock infection or infection with the E2 virus and analyzed by Western blotting to determine the status of the p53 pathway (Fig. 2A, top two panels, and data not shown). As was the case in control HeLa/LXSN cells, there was little expression of p53 or mdm2 in HeLa/16E6 or HeLa/16E7 cells in the absence of E2 expression, indicating that the p53 pathway was not activated by the HPV16 genes. The E2 protein caused a marked increase in p53, p21, and mdm2 levels in HeLa/LXSN cells and HeLa/16E7 cells due to repression of the HPV18 E6 gene in these cells and loss of E6-induced destabilization of p53. In contrast, constitutive expression of the HPV16 E6 protein in HeLa/16E6 cells prevented induction of p53, p21, and mdm2. These results demonstrated that specific repression of the E6 gene activated the p53 pathway in HeLa/16E7 cells but that the E2 protein did not activate the p53 pathway in HeLa/16E6 cells.
TABLE 1.
Biochemical and physiological responses of HeLa cells to E2 expression
| Cell line | HPV gene(s)
|
Response to E2 protein
|
||||||
|---|---|---|---|---|---|---|---|---|
| Expressed in presence of E2 | Effectively repressed by E2 | Rb pathway | p53 pathway | cdk2 activity | c-myc expression | Telomerase activity | Phenotype | |
| HeLa/LXSN | None | E6, E7 | Activated | Activated | Reduced | Repressed | Reduced | Senescence |
| HeLa/16E6 | HPV16 E6 | E7 | Activated | Inactive | Reduced | Repressed | Reduced | Senescence |
| HeLa/16E7 | HPV16 E7 | E6 | Inactive | Activated | Reduced | Repressed | Reduced | Senescence, proliferation, late apoptosis |
| HeLa/16E6-18E7 | HPV16 E6, HPV18 E7 | None | Inactive | Inactive | Not affected | Not affected | Not affected | Proliferation |
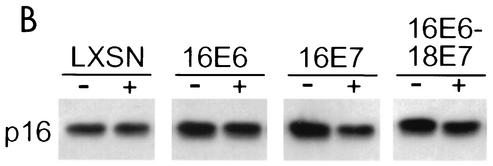
FIG. 2.
Effects of specific repression of HPV E6 and E7 genes on tumor suppressor pathways and c-myc expression. (A) Protein was extracted from two clones each of the HeLa/LXSN, HeLa/16E6, and HeLa/16E7 cell lines and LXSN-RVY-4 and 16E6-18E7-1 cells 2 days after infection with the E2 virus (+) or mock infection (−). A 5-μg amount of each sample was resolved by gel electrophoresis; transferred to a membrane; and probed with antibodies specific for p53, p21, p105Rb, cyclin A, or c-myc, as indicated. The major hyperphosphorylated and hypophosphorylated species of p105Rb are indicated by P and O, respectively. (B) Protein was extracted from HeLa/LXSN-1, HeLa/16E6-5, HeLa/16E7-3, and HeLa/16E6-18E7-1 cells 2 days after infection with the E2 virus (+) or mock infection (−). A 5-μg amount of each sample was resolved by gel electrophoresis, transferred to a membrane, and probed with antibody specific for p16INK4.
We also assessed the status of the Rb pathway (Fig. 2A, second two panels, and data not shown). In the absence of the E2 protein, all the cell lines displayed similar patterns of p105Rb expression and phosphorylation. Expression of the E2 protein caused a marked increase in the level of the hypophosphorylated, active form of p105Rb in HeLa/LXSN cells and HeLa/16E6 cells due to repression of the HPV18 E7 gene in these cells and loss of E7-induced destabilization of p105Rb. In contrast, constitutive expression of the HPV16 E7 protein in HeLa/16E7 cells prevented increased expression of hypophosphorylated p105Rb. Similarly, the E2 protein induced expression of the Rb family members p107 and p130 in the HeLa/LXSN and HeLa/16E6 cells but not in the HeLa/16E7 cells (data not shown). Importantly, the E2 protein repressed expression of the E2F-responsive cyclin A and cdc25A genes in HeLa/LXSN cells and HeLa/16E6 cells, indicating that the Rb pathway was activated in these cells, whereas E2F-responsive genes were not repressed in HeLa/16E7 cells (Fig. 2A and data not shown). The E2 protein caused a decrease in the level of hyperphosphorylated p105Rb in both HeLa/16E7 and HeLa/16E6 cells. However, as noted above, E2 expression did not induce hypophosphorylated p105Rb or repress E2F-responsive genes in HeLa/16E7 cells, indicating that the Rb pathway was not functionally activated in these cells. Thus, the activity of the Rb pathway reflects the level of hypophosphorylated p105Rb, not the level of hyperphosphorylated p105Rb, a result consistent with the assembly of the hypophosphorylated form into repressing E2F complexes. These results demonstrated that specific repression of the E7 gene activated the Rb pathway in HeLa/16E6 cells but that the E2 protein did not activate the Rb pathway in HeLa/16E7 cells.
The E2 protein caused a slight induction of p53 expression in HeLa/16E6-18E7 cells, but the induced level of p53 in these cells was comparable to the basal level in HeLa/LXSN-RVY cells, and p21 was not induced (Fig. 2A). The E2 protein did not affect the expression or phosphorylation of p105Rb or cyclin A expression in HeLa/16E6-18E7 cells. Thus, the E2 protein activated neither the p53 nor the Rb pathway in cells constitutively expressing both E6 and E7. Taken together, these results demonstrated that repression of the E6 gene in HeLa/16E7 cells caused specific activation of the p53 tumor suppressor pathway but not the Rb pathway. Conversely, repression of the E7 gene in HeLa/16E6 cells caused specific activation of the Rb pathway but not the p53 pathway.
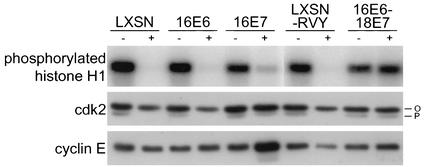
The E2-induced loss of hyperphosphorylated p105Rb in both HeLa/16E6 and HeLa/16E7 cells suggested that cdk activity was inhibited by repression of either the E6 protein or the E7 protein. To test this directly, cdk2 was immunoprecipitated from cell extracts and its ability to phosphorylate histone H1 in vitro was tested. As shown in Fig. 3 (top), repression of E7 in the HeLa/16E6 cells and to a lesser extent repression of E6 in the HeLa/16E7 cells caused a marked reduction in histone H1 kinase activity, which was eliminated if both the E6 protein and the E7 protein were constitutively expressed. To explore the basis for the reduced cdk2 activity in E2-infected cells, we examined the components of cdk2 complexes. Since the majority of cdk2 activity in HeLa cells is due to cyclin A-associated cdk2 (data not shown), the previously noted decrease in cyclin A expression following E7 repression in the HeLa/16E6 cells is likely responsible for the decreased cdk2 activity in these cells. In addition, the E2 protein caused a modest reduction in cdk2 expression when the E7 protein was repressed and little change in cyclin E expression when either E6 or E7 was repressed (Fig. 3, middle and bottom). We also note that the abundance of a minor, rapidly migrating species of cdk2, which is the activated form phosphorylated at threonine 160 by cdk-activating kinase, was markedly reduced in cell lines in which the E7 protein was repressed, presumably because these cells lack cyclin A-cdk2 complexes that stimulate cdk-activating kinase activity (13). Notably, there was no decrease in cyclin A, cyclin E, total cdk2, or phosphorylated cdk2 levels following E6 repression in HeLa/16E7 cells. We conclude that the reduction in cdk2 activity in E2-infected HeLa/16E7 cells is probably due to elevated p21. We also tested whether separate repression of E6 or E7 influenced expression of the cdk inhibitor, p16INK4, whose primary function appears to be the inhibition of p105Rb phosphorylation. As shown in Fig. 2B, expression of the E2 protein caused a modest reduction in the level of p16INK4 in HeLa/16E7 cells but had no effect on p16INK4 expression in HeLa/16E6 cells. Thus, the increase in hypophosphorylated p105Rb following E7 repression in HeLa/16E6 cells is not due to increased p16INK4 expression.
FIG. 3.
Effects of specific repression of HPV E6 and E7 genes on cdk2 activity and components. Protein was extracted from HeLa/LXSN-1, HeLa/16E6-5, HeLa/16E7-3, HeLa/LXSN-RVY-4, and HeLa/16E6-18E7-1 cells 2 days after infection with the E2 virus (+) or mock infection (−). Histone H1 kinase activity was measured following immunoprecipitation with anti-cdk2 antibody from 20 μg of protein extract (top). Each sample was resolved by gel electrophoresis, transferred to a membrane, and probed with antibodies specific for cdk2 and cyclin E (middle and bottom, respectively). Active phosphorylated cdk2 is indicated by P, while unphosphorylated cdk2 is indicated by O.
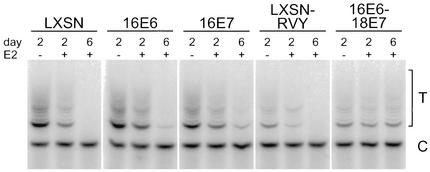
We used a TRAP assay to examine telomerase activity in extracts prepared from cells after mock infection or after infection with the E2 virus (Fig. 4). Two days after infection, telomerase activity, scored as the intensity of the ladder of bands labeled T, was reduced in both HeLa/16E6 and HeLa/16E7 cells. By 6 days after infection, telomerase activity was reduced approximately 90% in HeLa/16E6 and HeLa/16E7 cell extracts, and it was undetectable when both E6 and E7 were repressed in the HeLa/LXSN or HeLa/LXSN-RVY cells. Constitutive expression of E6 and E7 in the HeLa/16E6-18E7 cells prevented the decrease in telomerase activity. Thus, repression of either E6 or E7 was sufficient to inhibit telomerase activity. Finally, Western blot analysis demonstrated that the level of c-myc was reduced if either E6 or E7 was repressed and that constitutive expression of E6 plus E7 prevented the E2-mediated reduction in c-myc (Fig. 2A, bottom). Similarly, Northern blot analysis demonstrated that repression of either E6 or E7 caused a decrease in c-myc RNA (data not shown).
FIG. 4.
Effect of specific repression of HPV E6 and E7 genes on telomerase activity. Protein was extracted from HeLa/LXSN-1, HeLa/16E6-5, HeLa/16E7-3, HeLa/LXSN-RVY-4, and HeLa/16E6-18E7-1 cells 2 and 6 days after infection with the E2 virus (+) or 2 days after mock infection (−). Telomerase activity was measured by a TRAP assay with 100 ng of extracted protein. The lower band (C) represents the internal control used for comparisons between samples, and the upper ladder of bands (T) represents the extended products generated by telomerase activity in vitro.
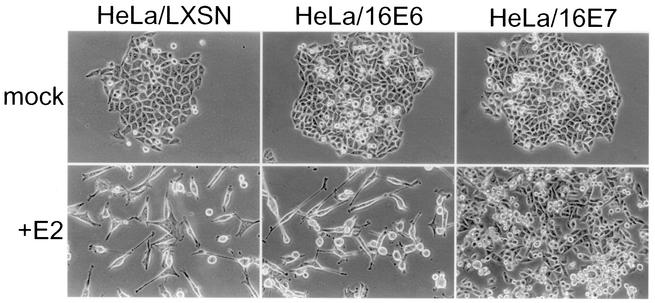
Effects of specific repression of HPV E6 or E7 expression on cellular morphology and DNA synthesis.
The appearance of the cells was assessed by phase-contrast microscopy 10 days after mock infection or infection with the E2 virus (Fig. 5). All cell lines formed compact colonies of small proliferating cells in the absence of E2 expression. The E2 protein caused the control HeLa/LXSN cells and the HeLa/16E6 cells to cease proliferation and adopt a flattened and enlarged appearance, resembling cells that had undergone replicative senescence. In contrast, most infected HeLa/16E7 cells failed to proliferate, but a significant number of proliferating colonies developed. Approximately 5 to 7 days after infection, many of the HeLa/16E7 cells rounded up and detached from the culture plate, but proliferating cells remained and eventually overgrew the culture. The E2 protein had no apparent effects on the morphology or growth of the HeLa/16E6-18E7 cells (data not shown).
FIG. 5.
Effect of specific repression of HPV E6 and E7 genes on cell morphology. Shown are phase-contrast photomicrographs at a 160× magnification of HeLa/LXSN-1, HeLa/16E6-5, and HeLa/16E7-3 cells 10 days after infection with the E2 virus or mock infection.
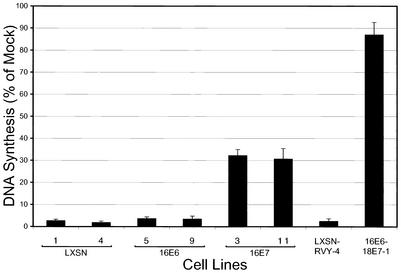
To assess the effect of repression of the individual HPV oncogenes on cell proliferation, the incorporation of tritiated thymidine into DNA was measured 2 days after infection (Fig. 6). The E2 protein dramatically inhibited DNA synthesis in HeLa/LXSN, HeLa/LXSN-RVY, and HeLa/16E6 cells. In contrast, following E2 expression, HeLa/16E7 cells displayed approximately 30% the DNA synthesis of uninfected cells. After persisting at this level for several days, DNA synthesis in the HeLa/16E7 cells was reestablished, so that by 10 days after infection, the cells were undergoing robust DNA synthesis (data not shown) (see also Fig. 9). The E2 protein caused minimal inhibition of DNA synthesis in HeLa/16E6-18E7 cells.
FIG. 6.
Effect of specific repression of HPV E6 and E7 genes on cellular DNA synthesis. Two clones each of the HeLa/LXSN, HeLa/16E6, and HeLa/16E7 cell lines and HeLa/LXSN-RVY-4 and HeLa/16E6-18E7-1 cells were assayed for incorporation of [3H]thymidine 2 days after infection with the E2 virus or mock infection. The data are expressed as percentages of incorporation by mock-infected cells for each cell line. The results of multiple experiments were averaged, and the error bars represent 1 standard deviation.
FIG. 9.
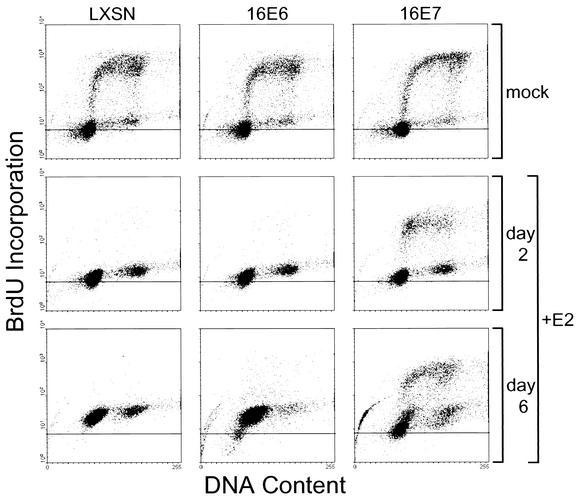
Effect of specific repression of HPV E6 and E7 genes on cell proliferation. HeLa/LXSN-1, HeLa/16E6-5, and HeLa/16E7-3 cells were pulse-labeled with BrdU 2 days after mock infection or 2 or 6 days after infection with the E2 virus as indicated, stained with fluoresceinated anti-BrdU antibody and 7-amino-actinomycin D to measure DNA content, and analyzed by flow cytometry. The horizontal line within each plot shows the peak fluorescence value for mock-infected cells.
Effect of specific repression of E6 or E7 expression on senescence.
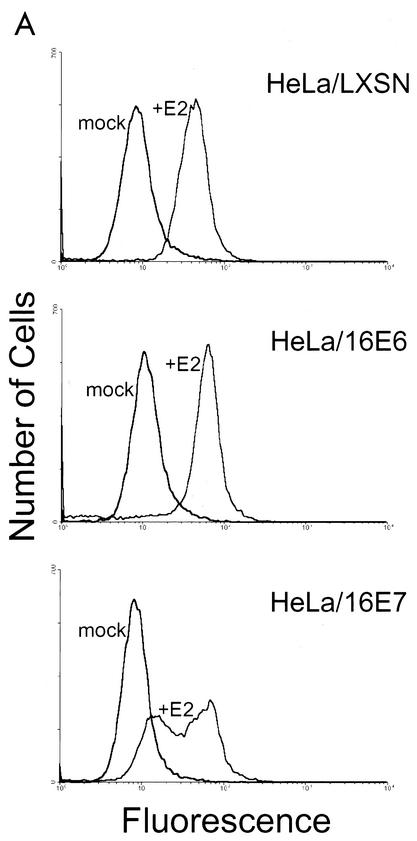
As more specific markers of senescence, autofluorescence and SAβ-Gal expression were measured in cells that were mock infected or infected with the E2 virus. After 6 days, the cells were subjected to flow cytometry to measure intrinsic fluorescence. As shown in Fig. 7A, the E2 protein induced HeLa/LXSN and HeLa/16E6 cells to undergo a dramatic and uniform shift to higher levels of fluorescence, suggesting that the cells efficiently underwent senescence. A fraction of infected HeLa/16E7 cells were highly fluorescent, but there was another population of cells that fluoresced more brightly than uninfected cells but less than the brightest population. Thus, the E2 protein did not induce the HeLa/16E7 cells to display a simple autofluorescent pattern characteristic of cells uniformly undergoing senescence. The E2 protein did not induce a significant increase in autofluorescence in HeLa/16E6-18E7 cells (data not shown).
FIG. 7.
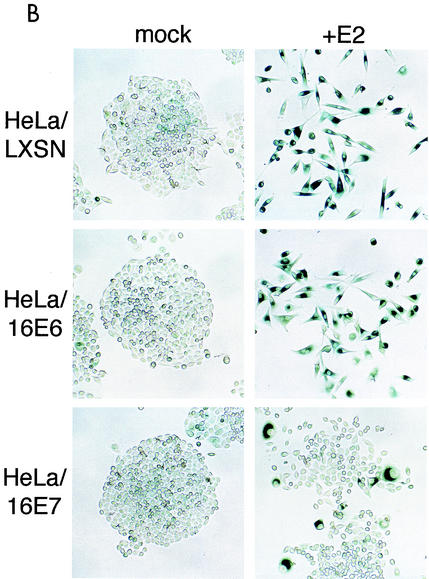
Effect of specific repression of HPV E6 and E7 expression on senescence. (A) HeLa/LXSN-1, HeLa/16E6-5, and HeLa/16E7-3 cells were assayed for autofluorescence by flow cytometry 6 days after infection with the E2 virus or mock infection. (B) HeLa/LXSN-1, HeLa/16E6-5, and HeLa/16E7-3 cells were stained for SAβ-Gal activity 10 days after infection with the E2 virus or mock infection. Cells were photographed with bright-field optics at a 132× magnification.
We measured SAβ-Gal activity by incubating cells with X-Gal at pH 6.0. As shown in Fig. 7B, the colonies arising from mock-infected HeLa/LXSN, HeLa/16E6, and HeLa/16E7 cells showed faint background staining, whereas after infection with the E2 virus almost all of the HeLa/LXSN and HeLa/16E6 cells displayed intense blue staining indicative of SAβ-Gal activity. In contrast, the infected HeLa/16E7 cell cultures contained cells displaying a flattened morphology and SAβ-Gal activity, interspersed with numerous proliferating colonies that did not stain. To estimate the fraction of HeLa/16E7 cells that underwent senescence, they were plated at limiting dilution in microtiter plates after infection, and the cells in individual wells were examined microscopically after 10 days. Approximately two-thirds of the infected HeLa/16E7 cells ceased proliferation, displayed morphological markers of senescence, and expressed SAβ-Gal (data not shown). Taken together, these results indicated that the E2 protein efficiently induced senescence in HeLa/LXSN and HeLa/16E6 cells. Most of the infected HeLa/16E7 cells underwent senescence as well, but a fraction of them continued to proliferate. At late times after infection the proliferating cells overgrew the culture, making it difficult to detect the nonreplicating senescent cells in the bulk population.
Effect of specific repression of E6 or E7 expression on apoptosis.
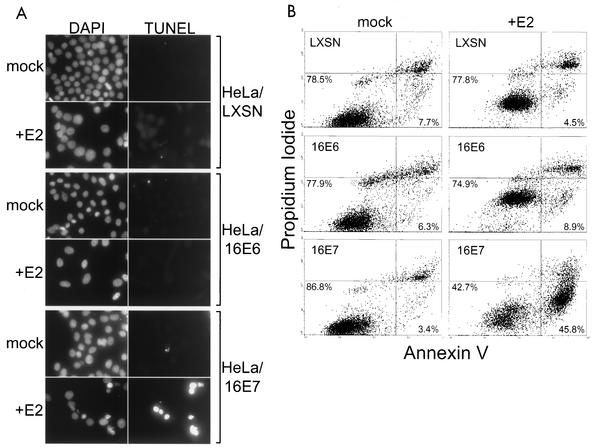
We also determined whether repression of E6 or E7 induced HeLa cells to undergo apoptosis. We carried out TUNEL assays for DNA breaks 5 days after mock infection or infection with the E2 virus. As shown in Fig. 8A, there were very few TUNEL-positive cells in uninfected samples or in the infected HeLa/LXSN or HeLa/16E6 cells. In contrast, a significant fraction of the infected HeLa/16E7 cells were TUNEL positive and displayed alterations in nuclear morphology consistent with apoptosis.
FIG. 8.
Effect of specific repression of HPV E6 and E7 genes on apoptosis. (A) HeLa/LXSN-1, HeLa/16E6-5, and HeLa/16E7-3 cells were assayed for the presence of DNA strand breaks by TUNEL assay 5 days after infection with the E2 virus or mock infection. Nuclei were stained by incubating the cells with DAPI. Cells were photographed at a 300× magnification in the DAPI and fluorescein isothiocyanate fluorescence channels. (B) Unfixed HeLa/LXSN-1, HeLa/16E6-5, and HeLa/16E7-3 cells were assayed by flow cytometry for annexin V binding and propidium iodide staining 6 days after infection with the E2 virus or mock infection. The percentages of cells in the proliferating-senescing quadrant (lower left) and the apoptotic quadrant (lower right) are shown.
To confirm that apoptosis was occurring in cultures of E2-expressing HeLa/16E7 cells, nonpermeabilized cells were stained with fluorescent annexin V and propidium iodide at various times after infection and analyzed by flow cytometry (Fig. 8B). In this analysis, proliferating and senescing cells take up neither stain and are visualized in the lower left quadrant of a two-dimensional plot. Early apoptotic cells are present in the lower right quadrant because they display annexin staining but are impermeable to propidium iodide. Within the first 3 days after introduction of the E2 gene, none of the cultures displayed a significant number of apoptotic cells (data not shown). Six days after infection, few infected HeLa/LXSN or HeLa/16E6 cells displayed evidence of apoptosis. However, in the lower left quadrant of the scans, the senescent cells in these cultures shifted to higher fluorescence in both channels due to increased autofluorescence. Strikingly, infected HeLa/16E7 cells displayed a greater-than-13-fold increase in the number of apoptotic cells in comparison to uninfected cells. By 9 days after infection, there was a slight increase in the number of apoptotic HeLa/16E6 cells, but the fraction was considerably lower than was the case for the HeLa/16E7 cells (data not shown). The E2 protein did not induce apoptosis in HeLa/16E6-18E7 cells, which constitutively expressed both E6 and E7 (data not shown). Thus, approximately 1 week after specific repression of the E6 gene, a substantial fraction of the HeLa/16E7 cells underwent apoptosis.
Other laboratories have reported that DNA binding-defective E2 mutants induced apoptosis in HeLa cells. Therefore, we tested the activity of E2 mutants defective for DNA binding (mutant C340R) or transcriptional regulation (mutant Q15H). These mutants are severely impaired in their ability to repress HPV E6-E7 expression and to inhibit proliferation of cervical carcinoma cell lines (17). Neither mutant induced significant growth inhibition, autofluorescence, or annexin staining in HeLa/LXSN, HeLa/16E6, or HeLa/16E7 cells (data not shown), indicating that the mutants did not induce senescence or apoptosis.
Cellular responses to individual repression of the HPV E6 and E7 genes.
To examine cell cycle dynamics in more detail, we carried out flow cytometry on cells labeled with BrdU and stained for DNA content (Fig. 9). Actively proliferating, uninfected cells displayed a standard profile consisting of discrete populations of unlabeled cells with G1 and G2 DNA content, together with a diffuse arc of BrdU-labeled, S-phase cells with intermediate DNA content. Within 2 days, the E2 protein caused the redistribution of the S-phase HeLa/LXSN and HeLa/16E6 cells into the G1 and G2 populations. The arrested HeLa/LXSN and HeLa/16E6 cells displayed increased fluorescence in both channels that was apparent at 2 days and more dramatic at 6 days after infection, due to the autofluorescence of the senescent cells in the culture. Six days after infection, the HeLa/16E6 cultures consisted of a majority senescent population with increased autofluorescence and a small minority population of actively proliferating cells that are the progeny of the rare cells that escaped growth inhibition.
The infected HeLa/16E7 cells displayed a markedly different profile. At 2 days after infection, there was a modest increase in autofluorescence due to senescence as seen in the other two cell lines, but a significant number of cells remained in S phase. At 6 days after infection, the nonreplicating senescent cells were largely overgrown by cells that displayed a proliferating profile. In addition, a distinct new minority population of apoptotic cells appeared as a tight arc with primarily sub-G1 DNA content. Taken together, these results indicated that repression of E7 alone in the HeLa/16E6 cells resulted in the rapid and uniform attainment of the senescent phenotype in virtually all of the cells in the culture. Repression of E6 alone in the HeLa/16E7 cells caused most of the cells to undergo senescence, but the remaining cells proliferated and overgrew the culture. By 6 days after infection, a wave of apoptosis occurred, but cells that escaped apoptosis continued to proliferate.
The proliferating HeLa/16E7 cells that outgrew following E2 expression expressed abundant HPV18 E6 and E7 mRNA (data not shown), presumably because the nonreplicating SV40 vector and its encoded E2 protein were diluted out by cell proliferation. These proliferating cells were indistinguishable from HeLa/16E7 cells that had not been infected with the E2 virus. Specifically, they expressed low levels of p53 and p21, displayed high telomerase activity, exhibited low levels of apoptosis, and did not display elevated autofluorescence (data not shown). Reinfection of these proliferating cells with the E2 virus again caused an approximately 70% drop in DNA synthesis and the delayed induction of apoptosis (data not shown). Thus, expression of the E2 protein did not select a population of HeLa/16E7 cells that was resistant to growth inhibition.
DISCUSSION
Introduction of the high-risk HPV E6 and E7 genes into normal cultured cells impairs cell cycle control and induces genetic instability, but the viral proteins do not directly induce tumorigenesis (35, 38). Therefore, it is likely that cellular genetic changes occurring subsequent to HPV gene expression are required for the malignant phenotype. Nevertheless, repression of the HPV genes inhibits proliferation of cervical carcinoma cells, indicating that these secondary genetic changes are not sufficient to maintain the malignant state in the absence of the viral proteins. To determine the roles played by individual viral proteins in maintaining the biochemical properties and growth state of cervical carcinoma cells, we used the BPV E2 protein to repress the endogenous HPV18 E6 and E7 genes in HeLa cells that constitutively expressed an exogenous E6 or E7 gene. The expression level of the exogenous E6 and E7 genes following E2 expression was similar to the expression of the endogenous HPV18 genes in the absence of the E2 protein (Fig. 1 and unpublished results). The E2 protein, in effect, repressed the E7 gene alone in the HeLa/16E6 cells and the E6 gene alone in the HeLa/16E7 cells. All of the biochemical and phenotypic effects caused by E2 expression were prevented by the combined constitutive expression of the E6 and the E7 genes. Therefore, although we have not ruled out the possibility that the E2 protein itself exerts direct effects on cells, the effects studied here required repression of the HPV oncogenes and were not merely fortuitous cellular responses to the E2 protein. In contrast, in other systems E2 proteins appear to exert HPV-independent effects (5, 11, 58).
Biochemical effects of E6 and E7 repression in cervical cancer cells.
We and others previously showed that several biochemical abnormalities in cervical carcinoma cells can be reversed by repression of HPV expression. One of the goals of the experiments reported here was to determine which biochemical properties of HeLa cells can be reversed by E6 repression, which can be reversed by E7 repression, and which can by reversed by repression of both viral proteins. Our results demonstrated that repression of HPV E7 expression in the HeLa/16E6 cells activated the Rb pathway but not the p53 pathway and that repression of HPV E6 expression in the HeLa/16E7 cells activated the p53 pathway but not the Rb pathway (Table 1). Thus, the p53 and Rb pathways remain under the specific control of the E6 and the E7 proteins, respectively, in HeLa cells, and these pathways appear intact and largely distinct. We conclude that the genetic changes accumulated by HeLa cells during malignant progression and in vitro passage did not uncouple these tumor suppressor pathways from the viral proteins that perturbed them during the earliest stages of carcinogenesis.
Many of the biochemical phenotypes observed following repression of E6 or E7 can be accounted for by the direct effects of the E6 and E7 proteins on the p53 and the Rb pathways. For example, p53-mediated induction of cdk inhibitors such as p21 was likely responsible for cdk inhibition when the E6 protein was repressed in the HeLa/16E7 cells, since there were no detectable changes in cyclin E, cyclin A, or cdk2 in these cells. Similarly, Rb-mediated repression of cyclin A and the cdk-activating cdc25A phosphatase was predicted to inhibit cdk activity when the E7 protein was repressed in the HeLa/16E6 cells. We also note that cells express abundant hyperphosphorylated p105Rb when both E6 and E7 are expressed, for example in cells not exposed to the E2 protein. This presumably reflects the inability of the E7 protein to efficiently bind and destabilize the hyperphosphorylated form of p105Rb (1, 27). When E6 is specifically repressed in the E2-infected HeLa/16E7 cells, p105Rb, which is now hypophosphorylated due to inhibition of cdk activity, is subject to E7-induced degradation.
Other biochemical responses suggest that the activities of isolated viral genes in primary cells do not necessarily predict the response of cervical carcinoma cells to the removal of the viral proteins. For example, cdk activity is reduced in E2-expressing HeLa/16E7 cells, which constitutively express the E7 protein, despite the ability of the E7 protein to counteract p21-mediated cdk inhibition in other settings (12, 28, 46). Also, telomerase activity was reduced if either E6 or E7 was repressed in HeLa cells, even though the E6 protein but not the E7 protein is sufficient to induce hTERT transcription and telomerase activity in primary keratinocytes (33, 43, 45, 50, 54). The reduction in telomerase activity following E2 expression in cervical cancer cells is due to decreased levels of hTERT mRNA (34). We hypothesize that repression of hTERT expression is not a direct result of loss of the E6 or the E7 protein but rather an indirect response to modulation of other cell cycle regulators, such as the reduction in c-myc expression or the induction of p53 or p105Rb, all of which have been reported elsewhere to reduce hTERT expression in other systems (29, 40, 57). Similarly, introduction of high-risk HPV E7 into normal keratinocytes induces expression of p16INK4, an effect that is thought to be due to inactivation of the Rb pathway by the E7 protein (30, 36). However, repression of the E7 protein has little, if any, effect on p16INK4 expression in HeLa/16E6 cells, despite the rapid activation of the Rb pathway and repression of E2F-responsive genes. Thus, the E7 protein does not appear to directly regulate p16INK4 expression in HeLa cells, possibly because events that occurred during carcinogenic progression uncoupled p16INK4 expression from E7. Furthermore, the modest decline in p16INK4 following E6 repression is unlikely to have physiological significance, because p105Rb is virtually absent from the E2-infected HeLa/16E7 cells.
The regulation of c-myc expression is complex. For example, c-myc transcription can be regulated both by the activity of the Rb pathway and by the growth state of the cell. There are published reports that the HPV E6 protein can increase c-myc transcription in a p53-dependent fashion (31) as well as stimulate c-myc degradation (19). However, in most recent studies, introduction of the HPV oncogenes has no consistent effect on c-myc expression (14, 43, 45, 54). Our results demonstrate that repression of either E6 or E7 inhibits c-myc expression in HeLa cells, but it is not known if this is a direct response to the viral proteins and the pathways they control or if it merely reflects the rate of cell proliferation.
Effect of E6 and E7 repression on senescence of cervical cancer cells.
Repression of the E7 gene alone caused virtually all of the HeLa/16E6 cells to rapidly senesce. Repression of the E6 gene alone also caused the majority of HeLa/16E7 cells to senesce, although a significant number of the cells continued to proliferate. The strikingly different biochemical profiles of the HeLa/16E6 and HeLa/16E7 cells soon after E2 expression suggested that at least two biochemical pathways can induce senescence in HeLa cells. This conclusion is consistent with numerous studies implicating Rb and p53 signaling and telomerase function in senescence. For example, either p53 signaling or Rb signaling is sufficient to impose a senescence block in normal fibroblasts (21, 49), and overexpression of p105Rb, p53, or p21 can induce senescence in various cancer cells (e.g., see references 51, 59, and 61).
Senescence induced by E7 repression in the HeLa/16E6 cells appears to be due to activation of the Rb pathway. p105Rb mutants defective for binding the E7 protein can induce senescence in HeLa cells, but wild-type p105Rb cannot (6), and loss of Rb function, commonly mediated by p16INK4 inactivation, is required for keratinocyte immortalization (7, 32). Furthermore, the ability of the E7 protein to counteract E2-induced senescence appears to require Rb binding activity (59; A. Psyrri, R. DeFilippis, and D. DiMaio, unpublished results). Thus, the endogenous Rb pathway can signal senescence in HeLa cells once the interaction with HPV18 E7 is eliminated.
Although p21 can mediate senescence in some cell types, such as fibroblasts, senescence in HeLa cells does not require p21 induction. First, senescence occurred efficiently when the E7 protein was repressed in HeLa/16E6 cells even though p53 was not activated and p21 was not induced. This result is consistent with the ability of the E2 protein to induce p21-independent senescence in HT-3 cells, a cervical carcinoma cell line containing HPV30 DNA and a dominant-negative p53 allele (18, 39). Second, expression of a dominant-negative p53 gene in HeLa cells blocked E2-mediated induction of p21, but the cells still efficiently underwent senescence (L. Manuelidis and D. DiMaio, unpublished results). In contrast, Wells et al. reported that an antisense p21 oligonucleotide prevented E2-induced senescence in HeLa cells (59). However, there are numerous technical differences between the experiments conducted by Wells et al. and the ones reported here which may affect the cellular response to growth-inhibitory signals. For example, Wells et al. examined individual cells that had been microinjected with an E2 expression plasmid and an antisense p21 morpholino oligonucleotide, incubated for 5 days in low-serum medium at 32°C, and then shifted to 10% serum and 37°C, whereas we analyzed the entire population of cells after infection with the E2 virus and continuous incubation in 10% serum at 37°C.
The mechanism of senescence induction when the E6 protein is repressed in the HeLa/16E7 cells is unknown. p21 was induced and a substantial number of senescent cells were generated when the E6 protein was repressed. However, the continued expression of E2F-responsive genes such as cyclin A and cdc25A in these cells indicated that the Rb pathway was not physiologically activated by E6 repression, even though cdk2 activity was inhibited and p105Rb phosphorylation was decreased. Taken together, our results suggest that E7 repression can trigger senescence in HeLa cells by activating the Rb pathway and that E6 repression can trigger senescence, albeit less efficiently, by activating an Rb-independent pathway. Although inhibition of telomerase can induce senescence in various cells, the reduction of telomerase activity is unlikely to be responsible for induced senescence in HeLa cells, since the E2 protein efficiently induces senescence in HeLa cells engineered to express greatly elevated telomerase activity and containing extended telomeres (15).
Effect of E6 and E7 repression on apoptosis of cervical cancer cells.
E6 repression caused a substantial fraction of HeLa/16E7 cells to undergo apoptosis after a delay of several days. Similarly, expression of a small peptide that binds and presumably inactivates the E6 protein induces apoptosis in a cervical carcinoma cell line (2). Repression of E6 alone may induce apoptosis by activating the p53 pathway in the absence of Rb activation. Apoptosis can be induced in HeLa cells by treatment with agents that activate p53 (3, 23) or by ectopic overexpression of p53 or p21 (22, 53), and the ability of p105Rb to prevent apoptosis has been documented in other systems (e.g., see reference 37). Alternatively, the HPV E7 protein can stimulate apoptosis in a variety of settings, whereas the E6 protein can protect cells from apoptosis (e.g., see references 24, 44, and 60). Therefore, the apoptosis that occurred upon E6 repression may primarily be a consequence of the proapoptotic activity of the E7 protein when it is not counteracted by the E6 protein. It is also possible that, as is the case for other systems (56), senescence can protect cells from a default apoptotic response triggered by unbalanced oncogene expression. When E7 was repressed, few cells escaped senescence and hence little apoptosis occurred. In contrast, when E6 alone was repressed, enough cells escaped senescence that a significant number of apoptotic cells eventually emerged.
In our experiments, apoptosis was induced only when E6 alone was repressed, only at late times after repression, and only by the wild-type E2 protein. Other laboratories reported that DNA binding-defective E2 mutants as well as wild-type E2 proteins induced apoptosis as early as 20 h after introduction into cells with or without HPV DNA (4, 5, 47, 58). Unlike our experiments, these experiments employed conditions such as lipid-based transfection protocols, physical isolation of cells expressing the highest level of the transfected genes, serum-free culture conditions, or induction of E2 expression by treatment of the cells with heavy metals that may have facilitated HPV-independent apoptosis in response to the E2 protein.
Summary.
HPV E6 and E7 genes, which initiate carcinogenesis, are also required in malignant cervical carcinoma cells. Our results demonstrate that the E6 and E7 proteins play distinct roles in maintaining proliferation and survival of HeLa cells and imply that multiple pathways can impose senescence in these cells. Because endogenous senescence or apoptosis pathways are activated if expression of either the E6 or the E7 gene is repressed, therapies directed against either viral protein may be beneficial. In addition, because separate repression of HPV E6 and E7 eliminates redundant pathways and unmasks responses such as apoptosis that do not occur when the genes are repressed together, this approach will facilitate further dissection of the dormant growth-inhibitory pathways in cervical cancer cells.
Acknowledgments
We thank D. Galloway for retroviruses expressing HPV16 E6 and E7, L. Manuelidis and S. Horner for assistance with some of the experiments, and J. Zulkeski for assistance in preparing the manuscript.
E.C.G. was supported in part by an Institutional Research Grant from the American Cancer Society (IRG 58-012-45). This work was supported by grants from the NCI (CA16038 and CA37157).
REFERENCES
- 1.Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteosome pathway. Cancer Res. 56:4620-4624. [PubMed] [Google Scholar]
- 2.Butz, K., C. Denk, A. Ullmann, M. Scheffner, and F. Hoppe-Seyler. 2000. Induction of apoptosis in human papillomavirus-positive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc. Natl. Acad. Sci. USA 97:6693-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butz, K., C. Geisen, A. Ullmann, D. Spitkovsky, and F. Hoppe-Seyler. 1996. Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int. J. Cancer 68:506-513. [DOI] [PubMed] [Google Scholar]
- 4.Desaintes, C., C. Demeret, S. Goyat, M. Yaniv, and F. Thierry. 1997. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 16:504-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desaintes, C., S. Goyat, S. Garbay, M. Yaniv, and F. Thierry. 1999. Papillomavirus E2 induces p53-independent apoptosis in HeLa cells. Oncogene 18:4538-4545. [DOI] [PubMed] [Google Scholar]
- 6.Dick, F. A., E. Sailhamer, and N. J. Dyson. 2000. Mutagenesis of the pRB pocket reveals that cell cycle arrest functions are separable from binding to viral oncoproteins. Mol. Cell. Biol. 20:3715-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, R. A. Weinberg, D. N. Louis, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimri, G. P., X. Lee, G. Basile, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowhanick, J. J., A. A. McBride, and P. M. Howley. 1995. Suppression of cellular proliferation by the papillomavirus E2 protein. J. Virol. 69:7791-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis, D. A., S. I. Schmid, and P. M. Howley. 2000. Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J. Virol. 74:2679-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frattini, M. G., S. D. Hurst, H. B. Lim, S. Swaminathan, and L. A. Laimins. 1997. Abrogation of a mitotic checkpoint by E2 proteins from oncogenic human papillomaviruses correlates with increased turnover of the p53 tumor suppressor protein. EMBO J. 16:318-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrett, S., W. A. Barton, R. Knights, P. Jin, D. O. Morgan, and R. P. Fisher. 2001. Reciprocal activation by cyclin-dependent kinases 2 and 7 is directed by substrate specificity determinants outside the T loop. Mol. Cell. Biol. 21:88-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewin, L., and D. A. Galloway. 2001. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J. Virol. 75:7198-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin, E. C., and D. DiMaio. 2001. Induced senescence in HeLa cervical carcinoma cells containing elevated telomerase activity and extended telomeres. Cell Growth Differ. 12:525-534. [PubMed] [Google Scholar]
- 16.Goodwin, E. C., and D. DiMaio. 2000. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 97:12513-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin, E. C., L. K. Naeger, D. E. Breiding, E. J. Androphy, and D. DiMaio. 1998. Transactivation-competent bovine papillomavirus E2 protein is specifically required for efficient repression of human papillomavirus oncogene expression and for acute growth inhibition of cervical carcinoma cell lines. J. Virol. 72:3925-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin, E. C., E. Yang, C.-J. Lee, H.-W. Lee, D. DiMaio, and E.-S. Hwang. 2000. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 97:10978-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross-Mesilaty, S., E. Reinstein, B. Bercovich, K. E. Tobias, A. L. Schwartz, C. Kahana, and A. Ciechanover. 1998. Basal and human papillomavirus E6 oncoprotein-induced degradation of Myc proteins by the ubiquitin pathway. Proc. Natl. Acad. Sci. USA 95:8058-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara, E., H. Tsuri, S. Shinozaki, and K. Oda. 1991. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of lifespan in human diploid fibroblasts. Biochem. Biophys. Res. Commun. 179:528-534. [DOI] [PubMed] [Google Scholar]
- 22.Haupt, Y., S. Rowan, and M. Oren. 1995. p53-mediated apoptosis in HeLa cells can be overcome by excess pRB. Oncogene 10:1563-1571. [PubMed] [Google Scholar]
- 23.Hietanen, S., S. Lain, E. Krausz, C. Blattner, and D. P. Lane. 2000. Activation of p53 in cervical carcinoma cells by small molecules. Proc. Natl. Acad. Sci. USA 97:8501-8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howes, K. A., N. Ransom, D. S. Papermaster, J. G. Lasudry, D. M. Albert, and J. J. Windle. 1994. Apoptosis or retinoblastoma: alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53. Genes Dev. 8:1300-1310. (Erratum, 8:1738.) [DOI] [PubMed] [Google Scholar]
- 25.Hwang, E.-S., L. K. Naeger, and D. DiMaio. 1996. Activation of the endogenous p53 growth inhibitory pathway in HeLa cervical carcinoma cells by expression of the bovine papillomavirus E2 gene. Oncogene 12:795-803. [PubMed] [Google Scholar]
- 26.Hwang, E.-S., D. J. Riese II, J. Settleman, L. A. Nilson, J. Honig, S. Flynn, and D. DiMaio. 1993. Inhibition of cervical carcinoma cell line proliferation by introduction of a bovine papillomavirus regulatory gene. J. Virol. 67:3720-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai, Y., Y. Matsushima, T. Sugimura, and M. Terada. 1991. Purification and characterization of human papillomavirus type 16 E7 protein with preferential binding capacity to the underphosphorylated form of retinoblastoma gene product. J. Virol. 65:4966-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanaya, T., S. Kyo, K. Hamada, M. Takakura, Y. Kitagawa, H. Harada, and M. Inoue. 2000. Adenoviral expression of p53 represses telomerase activity through down-regulation of human telomerase reverse transcriptase transcription. Clin. Cancer Res. 6:1239-1247. [PubMed] [Google Scholar]
- 30.Khleif, S. N., J. DeGregori, C. L. Yee, G. A. Otterson, F. J. Kaye, J. R. Nevins, and P. M. Howley. 1996. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc. Natl. Acad. Sci. USA 93:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinoshita, T., H. Shirasawa, Y. Shino, H. Moriya, L. Desbarats, M. Eilers, and B. Simizu. 1997. Transactivation of prothymosin α and c-myc promoters by human papillomavirus type 16 E6 protein. Virology 232:53-61. [DOI] [PubMed] [Google Scholar]
- 32.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Kleingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 33.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 34.Lee, C. J., E. J. Suh, H. T. Kang, J. S. Im, S. J. Um, J. S. Park, and E. S. Hwang. 2002. Induction of senescence-like state and suppression of telomerase activity through inhibition of HPV E6/E7 gene expression in cells immortalized by HPV16 DNA. Exp. Cell Res. 277:173-182. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani, F., and L. Banks. 2001. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874-7887. [DOI] [PubMed] [Google Scholar]
- 36.Martin, L. G., G. W. Demers, and D. A. Galloway. 1998. Disruption of the G1/S transition in human papillomavirus type 16 E7-expressing human cells is associated with altered regulation of cyclin E. J. Virol. 72:975-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgenbesser, S. D., B. O. Williams, T. Jacks, and R. A. DePinho. 1994. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371:72-74. [DOI] [PubMed] [Google Scholar]
- 38.Munger, K., J. R. Basile, S. Duensing, A. Eichten, S. L. Gonzalez, M. Grace, and V. L. Zacny. 2001. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20:7888-7898. [DOI] [PubMed] [Google Scholar]
- 39.Naeger, L. K., E. C. Goodwin, E.-S. Hwang, R. A. DeFilippis, H. Zhang, and D. DiMaio. 1999. Bovine papillomavirus E2 protein activates a complex growth-inhibitory program in p53-negative HT-3 cervical carcinoma cells that includes repression of cyclin A and cdc25A phosphatase genes and accumulation of hypophosphorylated retinoblastoma protein. Cell Growth Differ. 10:413-422. [PubMed] [Google Scholar]
- 40.Nguyen, D. C., and D. L. Crowe. 1999. Intact functional domains of the retinoblastoma gene product (pRb) are required for downregulation of telomerase activity. Biochim. Biophys. Acta 1445:207-215. [DOI] [PubMed] [Google Scholar]
- 41.Nilson, L. A., and D. DiMaio. 1993. Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein. Mol. Cell. Biol. 13:4137-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura, A., T. Ono, A. Ishimoto, J. J. Dowhanick, M. A. Frizzell, P. M. Howley, and H. Sakai. 2000. Mechanisms of human papillomavirus E2-mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J. Virol. 74:3752-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh, S. T., S. Kyo, and L. A. Laimins. 2001. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 75:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan, H., and A. E. Griep. 1994. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 8:1285-1299. [DOI] [PubMed] [Google Scholar]
- 45.Pei, X. F., L. Sherman, Y. H. Sun, and R. Schlegel. 1998. HPV-16 E7 protein bypasses keratinocyte growth inhibition by serum and calcium. Carcinogenesis 19:1481-1486. [DOI] [PubMed] [Google Scholar]
- 46.Ruesch, M. N., and L. A. Laimins. 1997. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J. Virol. 71:5570-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Perez, A.-M., S. Soriano, A. R. Clarke, and K. Gaston. 1997. Disruption of the human papillomavirus type 16 E2 gene protects cervical carcinoma cells from E2F-induced apoptosis. J. Gen. Virol. 78:3009-3018. [DOI] [PubMed] [Google Scholar]
- 48.Sedivy, J. M. 1998. Can ends justify the means?: Telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc. Natl. Acad. Sci. USA 95:9078-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shay, J. W., O. M. Pereira-Smith, and W. E. Wright. 1991. A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 196:33-39. [DOI] [PubMed] [Google Scholar]
- 50.Stoppler, H., D. P. Hartmann, L. Sherman, and R. Schlegel. 1997. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J. Biol. Chem. 272:13332-13337. [DOI] [PubMed] [Google Scholar]
- 51.Sugrue, M. M., D. Y. Shin, S. W. Lee, and S. A. Aaronson. 1997. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl. Acad. Sci. USA 97:9648-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thierry, F., and M. Yaniv. 1987. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 6:3391-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsao, Y.-P., S.-J. Huang, J.-L. Chang, J.-T. Hsieh, R.-C. Pong, and S.-L. Chen. 1999. Adenovirus-mediated p21(WAF1/SDII/CIP1) gene transfer induces apoptosis of human cervical cancer cell lines. J. Virol. 73:4983-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veldman, T., I. Horikawa, J. C. Barrett, and R. Schlegel. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 75:4467-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Knebel Doeberitz, M., T. Oltersdorf, E. Schwarz, and L. Gissmann. 1988. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 48:3780-3786. [PubMed] [Google Scholar]
- 56.Wang, E. 1995. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 55:2284-2292. [PubMed] [Google Scholar]
- 57.Wang, J., L.-Y. Xie, S. Allan, D. Beach, and G. J. Hannon. 1998. Myc activates telomerase. Genes Dev. 12:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster, K., J. Parish, M. Pandya, P. L. Stern, A. R. Clarke, and K. Gaston. 2000. The human papillomavirus (HPV) 16 E2 protein induces apoptosis in the absence of other HPV proteins and via a p53-dependent pathway. J. Biol. Chem. 275:87-94. [DOI] [PubMed] [Google Scholar]
- 59.Wells, S. I., D. A. Francis, A. Y. Karpova, J. J. Dowhanick, J. D. Benson, and P. M. Howley. 2000. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21CIP-dependent pathways. EMBO J. 19:5762-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White, A. E., E. M. Livanos, and T. D. Tlsty. 1994. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev. 8:666-677. [DOI] [PubMed] [Google Scholar]
- 61.Xu, H.-J., Y. Zhou, W. Ji, et al. 1997. Reexpression of the retinoblastoma protein in tumor cells induces senescence and telomerase inhibition. Oncogene 15:2589-2596. [DOI] [PubMed] [Google Scholar]
- 62.zur Hausen, H. 1999. Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin. Cancer Biol. 9:405-411. [DOI] [PubMed] [Google Scholar]