Abstract
Transient CD154 blockade at the onset of Theiler's murine encephalomyelitis virus-induced demyelinating disease ameliorated disease progression for 80 days, reduced immune cell infiltration, and transiently increased viral loads in the central nervous system. Peripheral antiviral and autoimmune T-cell responses were normal, and disease severity returned to control levels by day 120.
Multiple sclerosis (MS) is a T-cell-mediated autoimmune demyelinating disease of the central nervous system (CNS) (32). While much is known of the disease pathology of MS, very little is clear about its etiology (26). Epidemiological studies provide strong evidence for an environmental trigger, most likely viral (4, 16, 26). CNS pathology may result from bystander damage mediated by T cells targeting a virus that persists in the CNS and subsequent epitope spreading resulting from the release of sequestered myelin antigens secondary to virus-specific T-cell-initiated myelin damage (20, 21).
Theiler's murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD) is a virally induced MS model in which chronic TMEV infection of the CNS in susceptible strains of mice leads to a chronic-progressive form of paralytic disease (25, 26, 30). Inflammation is initiated by recruitment of CD4+ T cells and macrophages in response to persistent low-level viral infection in the CNS (13-15, 22).
The CD154-CD40 ligand pair interaction (31) has been demonstrated in active CNS lesions of patients with multiple sclerosis (5). Members of our group and others have demonstrated that CD154 blockade is an effective long-term way to treat both the induction of and ongoing relapsing-remitting experimental autoimmune encephalomyelitis (EAE) (5, 7-9, 11). Previous studies of virally induced disease have demonstrated expression of CD40 in the CNS of TMEV-IDD mice (27) and that therapeutic blockade of CD154 can ameliorate clinical disease in the short term (3). In this paper, we address the long-term effects and mechanisms of CD154 blockade in TMEV-IDD.
CD154 blockade results in a transient amelioration of clinical TMEV-IDD and reduced immune cell infiltration into the CNS.
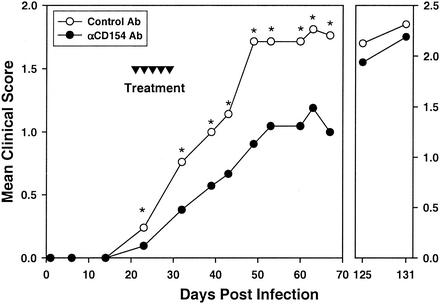
Mice, inoculated with TMEV in the right cerebral hemisphere as previously described (17, 18), were monitored for clinical disease for approximately 140 days. Starting at the time of clinical disease onset (day 21), mice were given five treatments every other day with 200 ìg of control hamster immunoglobulin G (IgG) or blocking anti-CD154 antibody (Ab) (MR1) (9). Anti-CD154-treated mice demonstrated a significantly reduced severity of clinical disease immediately upon treatment with anti-CD154 Ab, compared to control Ab-treated mice. This amelioration continued until at least 70 days postinfection (Fig. 1, left panel). At all time points this reduction was statistically significant (P < 0.05), and it was most apparent by day 50 postinfection. Anti-CD154-treated mice over this period demonstrated an approximately 35 to 40% reduction in the severity of clinical disease, although the incidence of disease was 100% in both groups. Some mice were monitored for an additional 60 to 70 days (Fig. 1, right panel). By 125 days postinfection, the mean clinical disease severity for anti-CD154-treated mice was no longer significantly different from that of control-treated mice (Fig. 1, right panel).
FIG. 1.
Short-term anti-CD154 treatment at disease onset results in transient reduction of severity of TMEV-induced demyelinating disease. Mice were infected intracerebrally with 9 × 107 PFU of TMEV on day zero and administered a total of five treatments with either control hamster IgG Ab (○) or anti-CD154 Ab (•) at the indicated time points (▾). Clinical disease was evaluated on an incremental six-point severity scale until day 68 for a total of 21 mice from three separate experiments (left panel). An additional 14 mice from two of these experiments were monitored for clinical disease until day 135 (right panel). Statistically significant difference as determined by a Mann-Whitney nonparametric test between control- and anti-CD154-treated mice is denoted by an asterisk above the day on which differences were observed (P < 0.05).
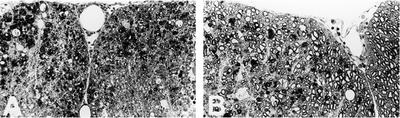
Spinal cord sections were taken from mice 75 days postinfection, and histopathologic scores were determined as previously described (9). Sections taken from anti-CD154-treated mice at this time point, where clinical disease was reduced, demonstrated significantly less inflammatory cell infiltration than that for control Ab-treated animals and very little demyelination (Fig. 2 and Table 1). This supports the argument that reduction in disease is due to inhibition of T-cell effector function within the CNS or modulation of Th1 cell differentiation in the periphery with similar downstream effects (1, 6, 8, 10, 11, 24).
FIG. 2.
Anti-CD154-treated mice demonstrate reduced immune cell infiltration and demyelination. Lumbar spinal cord sections from representative animals of the treatment groups described in Fig. 1 were examined for CNS histopathology. (A) Section of spinal cord from a mouse treated with hamster control IgG from days 21 to 29 and sacrificed on day 75. Inflammatory cell infiltrates are identified by dark filled spots throughout the spinal cord section, while extensive demyelination was seen by the absence of myelinated axons (ring structures with dark circumferences). (B) Section of spinal cord from a mouse treated with anti-CD154. Details of the degree of inflammation and demyelination are given in Table 1. One-micrometer-thick Epon-embedded sections stained with toluidine blue. Original magnification, ×220. Images are taken from two mice in the same experiment and are representative of a total of six mice from each group over two experiments, as noted in Table 1.
TABLE 1.
Summary of histology of spinal cord sections from mice treated from day 21 post-TMEV inoculation
| Experiment No.a | Hamster Ig controls
|
Anti-CD154 treated
|
||
|---|---|---|---|---|
| Disease scoreb | Histopathology scorec | Disease score | Histopathology score | |
| 1A | 4 | +++ | 1 | ++ |
| 1B | 3 | +++ | 1 | + |
| 1C | 3 | +++ | 2 | ++ |
| 2A | 2 | +++ | 1 | ++ |
| 2B | 2 | +++ | 1 | + |
| 2C | 2 | +++ | 2 | +++ |
Lumbar spinal cord sections from mice treated from days 21 to 29, every other day for five treatments, with either hamster Ig control or anti-CD154 MR1 antibody, were assessed for CNS histopathological changes 75 days after inoculation with TMEV.
Peak disease score severity at time of spinal cord removal.
Ten sections were examined for each mouse and scored for disease on the following scale: 0, no disease; ±, meningitis; +, focal infiltration and demyelination; ++, multiple infiltrates and demyelination; +++, confluent infiltrates and demyelination.
Peripheral virus- and myelin-antigen-specific Th1 responses in vivo are not affected by anti-CD154 treatment.
To determine whether CD154 blockade affected T-cell differentiation in the periphery or whether this reduced infiltration in the CNS could be ascribed to effector function within the CNS alone, delayed-type hypersensitivity (DTH) responses were evaluated, as a measure of in vivo peripheral Th1-cell differentiation and effector function, 45 days after treatment (day 74 postinfection), to ensure clearance of the MR1 Ab. DTH responses to both viral antigen, VP270-86 peptide (WTTSQEAFSHIRIPLPH), and immunodominant myelin antigens, PLP139-151 (HSLGKWLGHPDKF) and MBP84-104 (VHFFKNIVTPRTPSQGKG), were determined as previously described (9).
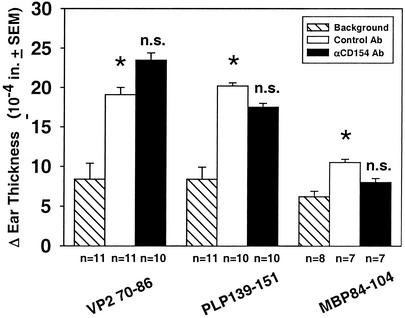
Anti-CD154 Ab results in reduced DTH to immunizing antigens, provided treatment is given at the time of immunization (8, 9, 11). DTH responses to VP270-86 were comparable between control- and anti-CD154-treated mice (Fig. 3), reflecting the lack of effect of delayed Ab treatment (day 21 postinfection) on antiviral responses. Curiously, CD154 blockade did not affect development of DTH responses to the myelin autoantigen PLP139-151 or MBP84-104 (Fig. 3). This indicates that although the response to myelin antigens cannot be detected by DTH until over 50 days postinoculation (22), epitope spreading has probably already begun by day 21 when CNS damage begins.
FIG. 3.
Day 75 DTH responses to viral and myelin antigens are not affected by short-term anti-CD154 treatment. DTH responses to viral (VP270-86) and myelin (PLP139-151 and MBP84-104) protein epitopes were determined in hamster control-treated and anti-CD154 Ab-treated mice 75 days after intracerebral infection with TMEV. Hatched bars represent naïve background responses from uninfected, untreated mice; clear bars represent responses from hamster control IgG-treated, TMEV-infected mice; and filled bars represent responses from anti-CD154-treated, TMEV-infected mice. Data represent pooled individual results from three separate grouped experiments. Numbers of mice in each group are identified below the x axis. Asterisks denote a significant DTH response in control-treated inoculated mice versus naïve mice (P < 0.05), while n.s. denotes no significant difference between anti-CD154 and control Ab-treated, TMEV-infected mice.
Diminished clinical severity in TMEV-IDD is associated with a transient increased viral load in the CNS.
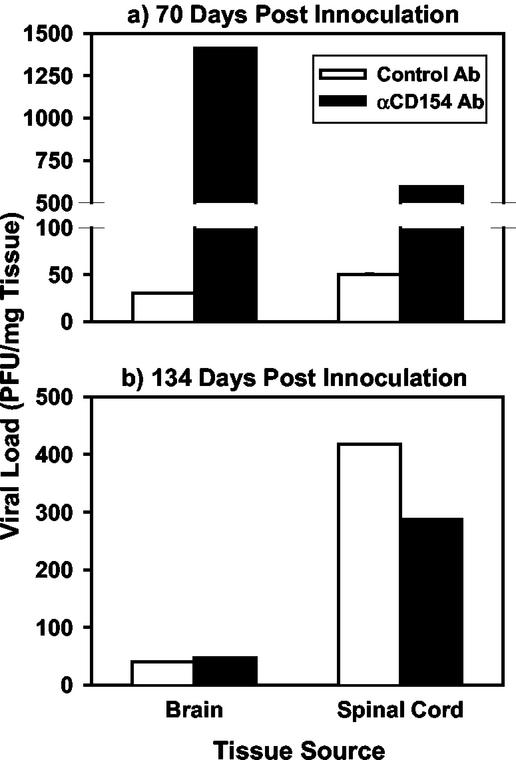
The data clearly demonstrate that while clinical disease severity (Fig. 1) and histopathology scores (Fig. 2 and Table 1) were significantly reduced in anti-CD154 versus control Ab-treated mice, Th1 activation (Fig. 3) is not affected in the long term by transient CD154 blockade at the onset of disease. This suggests that perhaps Th1 effector function, but not the continued development of peripheral Th1 responses, is blocked within the CNS, similar to what we have previously reported in the EAE model (10). Since the TMEV-IDD model is dependent on persistent infection of macrophages infiltrating into the CNS (12, 19, 29), we determined the effect of anti-CD154 treatment on viral activity in the CNS using a viral plaque assay (23). Anti-CD154 treatment suppressed antiviral effector immune responses in the CNS as illustrated by the finding that at 75 days postinfection, a time point where inflammation, demyelination, and clinical disease were suppressed by anti-CD154 treatment, the viral load present in both the brains (40-fold increased) and spinal cords (12-fold increased) of treated mice was markedly higher than that of controls (Fig. 4a). However, at a later time point, 134 days after inoculation (105 days after the end of Ab therapy), viral loads in both the brains and spinal cords of anti-CD154 treated mice had returned to control levels (Fig. 4b). This may be explained by the fact that as the therapeutic anti-CD154 Ab is cleared, Th1 cells may then resume their effector activity in the CNS and both help to control viral replication (Fig. 4b) and contribute to demyelination as a consequence of their inflammatory activity (Fig. 1).
FIG. 4.
Short-term anti-CD154 treatment results in a transient increase in TMEV viral loads in the CNS. Mice from Fig. 1 were sacrificed on either day 70 (a) or day 134 (b), and viral titers in the CNS were determined from spinal cords and brains. Clear bars represent control Ab-treated, TMEV-infected mice, while filled bars represent anti-CD154-treated mice. Three organs per group per experiment were collected, pooled, and used for viral titration assays, which were done in triplicate. Plaque counts were then adjusted to the gram weight of each tissue harvested and were expressed in PFU/milligram of harvested tissue. Data are representative of two separate experiments.
Progress of clinical disease is sustained in anti-CD154-treated mice, albeit at a significantly slower rate than that of control-treated mice (Fig. 1). Direct viral cytopathic effects on oligodendrocytes, as reflected by the high CNS viral titers, were most probably the cause of low-level demyelination observed in the CNS of mice recently treated with anti-CD154, since little or no effector Th1 cells are present at this time (Fig. 2). We and others have demonstrated the critical nature of the CD154-CD40 ligand pair interaction in the initiation of both CD4- and CD8-T-cell effector function in the target organ (1, 2, 10). Thus, inhibition of T-cell function within the CNS would allow viral replication to continue unchecked, as demonstrated in a lymphocytic choriomeningitis virus model (28). In contrast, since viral loads are significantly lower in control Ab-treated mice, with higher levels of clinical disease, it is likely that the T-cell-mediated inflammatory response to both viral and myelin antigens within the CNS is the major cause for demyelination and disease.
In summary, we demonstrate that CD154 blockade transiently inhibits T-cell effector function in the CNS of mice with ongoing TMEV-IDD. However, due to the significant long-term effect of anti-CD154 Ab treatment on suppression of antiviral immune responses in the CNS, these data suggest caution in the use of this immunotherapeutic regimen for the treatment of chronic-progressive forms of MS, which may be associated with a persistent CNS virus infection, and perhaps of relapsing forms where disease exacerbation is triggered by flaring of a viral infection.
Acknowledgments
This work was supported by U.S. Public Health Service National Institutes of Health research grants U19 AI/DK-51973, NS30871, and NS34819 and National Multiple Sclerosis Society grant RG3289-A-5.
REFERENCES
- 1.Becher, B., B. G. Durell, A. V. Miga, W. F. Hickey, and R. J. Noelle. 2001. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J. Exp. Med. 193:967-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, P., D. F. Tough, D. Eto, A. Tishon, I. S. Grewal, J. Sprent, R. A. Flavell, and M. B. Oldstone. 1998. CD40 ligand-mediated interactions are involved in the generation of memory CD8+ cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J. Virol. 72:7440-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drescher, K. M., L. J. Zoecklein, K. D. Pavelko, C. Rivera-Quinones, D. Hollenbaugh, and M. Rodriguez. 2000. CD40L is critical for protection from demyelinating disease and development of spontaneous remyelination in a mouse model of multiple sclerosis. Brain Pathol. 10:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebers, G. C., A. D. Sadovnick, and N. J. Risch. 1995. A genetic basis for familial aggregation in multiple sclerosis. Nature 377:150-151. [DOI] [PubMed] [Google Scholar]
- 5.Gerritse, K., J. D. Laman, R. J. Noelle, A. Aruffo, J. A. Ledbetter, W. J. Boersma, and E. Claassen. 1996. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 93:2499-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 7.Grewal, I. S., H. G. Foellmer, K. D. Grewal, J. Xu, F. Hardardottir, J. L. Baron, C. A. Janeway, Jr., and R. A. Flavell. 1996. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science 273:1864-1867. [DOI] [PubMed] [Google Scholar]
- 8.Howard, L. M., M. C. Dal Canto, and S. D. Miller. 2002. Transient anti-CD154-mediated immunotherapy of ongoing relapsing experimental autoimmune encephalomyelitis induces long-term inhibition of disease relapses. J. Neuroimmunol. 129:58-65. [DOI] [PubMed] [Google Scholar]
- 9.Howard, L. M., A. Miga, C. L. Vanderlugt, M. C. Dal Canto, J. D. Laman, R. J. Noelle, and S. D. Miller. 1999. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J. Clin. Investig. 103:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard, L. M., and S. D. Miller. 2001. Autoimmune intervention by CD154 blockade prevents T cell retention and effector function in the target organ. J. Immunol. 166:1547-1553. [DOI] [PubMed] [Google Scholar]
- 11.Howard, L. M., S. Ostrovidov, C. E. Smith, M. C. Dal Canto, and S. D. Miller. 2002. Normal Th1 development following long-term therapeutic blockade of CD154-CD40 in experimental autoimmune encephalomyelitis. J. Clin. Investig. 109:233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jelachich, M. L., and H. L. Lipton. 1999. Restricted Theiler's murine encephalomyelitis virus infection in murine macrophages induces apoptosis. J. Gen. Virol. 80:1701-1705. [DOI] [PubMed] [Google Scholar]
- 13.Katz-Levy, Y., K. L. Neville, A. M. Girvin, C. L. Vanderlugt, J. G. Pope, L. J. Tan, and S. D. Miller. 1999. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice. J. Clin. Investig. 104:599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, B. S., M. A. Lyman, B. S. Kang, H. K. Kang, H. G. Lee, M. Mohindru, and J. P. Palma. 2001. Pathogenesis of virus-induced immune-mediated demyelination. Immunol. Res. 24:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knobler, R. L., M. Rodriguez, P. W. Lampert, and M. B. Oldstone. 1983. Virologic models of chronic relapsing demyelinating disease. Acta Neuropathol. Suppl. 9:31-37. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke, J. F. 1993. Epidemiologic evidence for multiple sclerosis as an infection. Clin. Microbiol. Rev. 6:382-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipton, H. L., and M. C. Dal Canto. 1976. Chronic neurologic disease in Theiler's virus infection of SJL/J mice. J. Neurol. Sci. 30:201-207. [DOI] [PubMed] [Google Scholar]
- 18.Lipton, H. L., and R. Melvold. 1984. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J. Immunol. 132:1821-1825. [PubMed] [Google Scholar]
- 19.Lipton, H. L., G. Twaddle, and M. L. Jelachich. 1995. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 69:2525-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McRae, B. L., C. L. Vanderlugt, M. C. Dal Canto, and S. D. Miller. 1995. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 182:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, S. D., and W. J. Karpus. 1994. The immunopathogenesis and regulation of T-cell mediated demyelinating diseases. Immunol. Today 15:356-361. [DOI] [PubMed] [Google Scholar]
- 22.Miller, S. D., C. L. Vanderlugt, W. S. Begolka, W. Pao, R. L. Yauch, K. L. Neville, Y. Katz-Levy, A. Carrizosa, and B. S. Kim. 1997. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3:1133-1136. [DOI] [PubMed] [Google Scholar]
- 23.Neville, K. L., M. C. Dal Canto, J. A. Bluestone, and S. D. Miller. 2000. CD28 costimulatory blockade exacerbates disease severity and accelerates epitope spreading in a virus-induced autoimmune disease. J. Virol. 74:8349-8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieland, J. D., Y. F. Graus, Y. E. Dortmans, B. L. J. M. Kremers, and A. M. Kruisbeek. 1998. CD40 and CD70 co-stimulate a potent in vivo antitumor T cell response. J. Immunother. 21:225-236. [DOI] [PubMed] [Google Scholar]
- 25.Olson, J. K., J. L. Croxford, M. Calenoff, M. C. Dal Canto, and S. D. Miller. 2001. A virus-induced molecular mimicry model of multiple sclerosis. J. Clin. Investig. 108:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson, J. K., J. L. Croxford, and S. D. Miller. 2001. Virus-induced autoimmunity: potential role of viruses in initiation, perpetuation, and progression of T cell-mediated autoimmune diseases. Viral Immunol. 14:227-250. [DOI] [PubMed] [Google Scholar]
- 27.Olson, J. K., A. M. Girvin, and S. D. Miller. 2001. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J. Virol. 75:9780-9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomsen, A. R., A. Nansen, J. P. Christensen, S. O. Andreasen, and O. Marker. 1998. CD40 ligand is pivotal to efficient control of virus replication in mice infected with lymphocytic choriomeningitis virus. J. Immunol. 161:4583-4590. [PubMed] [Google Scholar]
- 29.Trottier, M., P. Kallio, W. Wang, and H. L. Lipton. 2001. High numbers of viral RNA copies in the central nervous system of mice during persistent infection with Theiler's virus. J. Virol. 75:7420-7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsunoda, I., and R. S. Fujinami. 1996. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J. Neuropathol. Exp. Neurol. 55:673-686. [DOI] [PubMed] [Google Scholar]
- 31.Van Kooten, C., and J. Banchereau. 1997. Functional role of CD40 and its ligand. Int. Arch. Allergy Immunol. 113:393-399. [DOI] [PubMed] [Google Scholar]
- 32.Wekerle, H. 1991. Immunopathogenesis of multiple sclerosis. Acta Neurol. 13:197-204. [PubMed] [Google Scholar]