Abstract
The transmission across the gut of suckling rats of several types of anti-Brucella abortus agglutinins separated from a bovine immune serum by electrophoresis, gel-filtration and chromatography has been investigated.
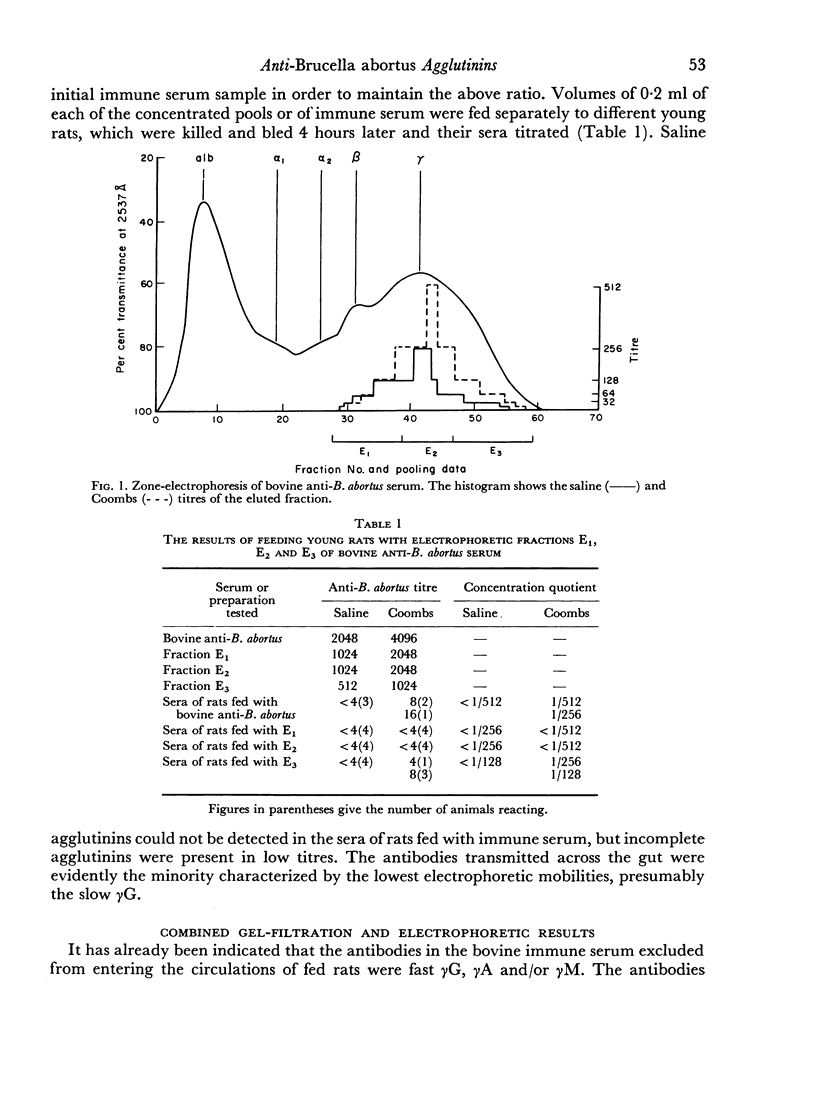
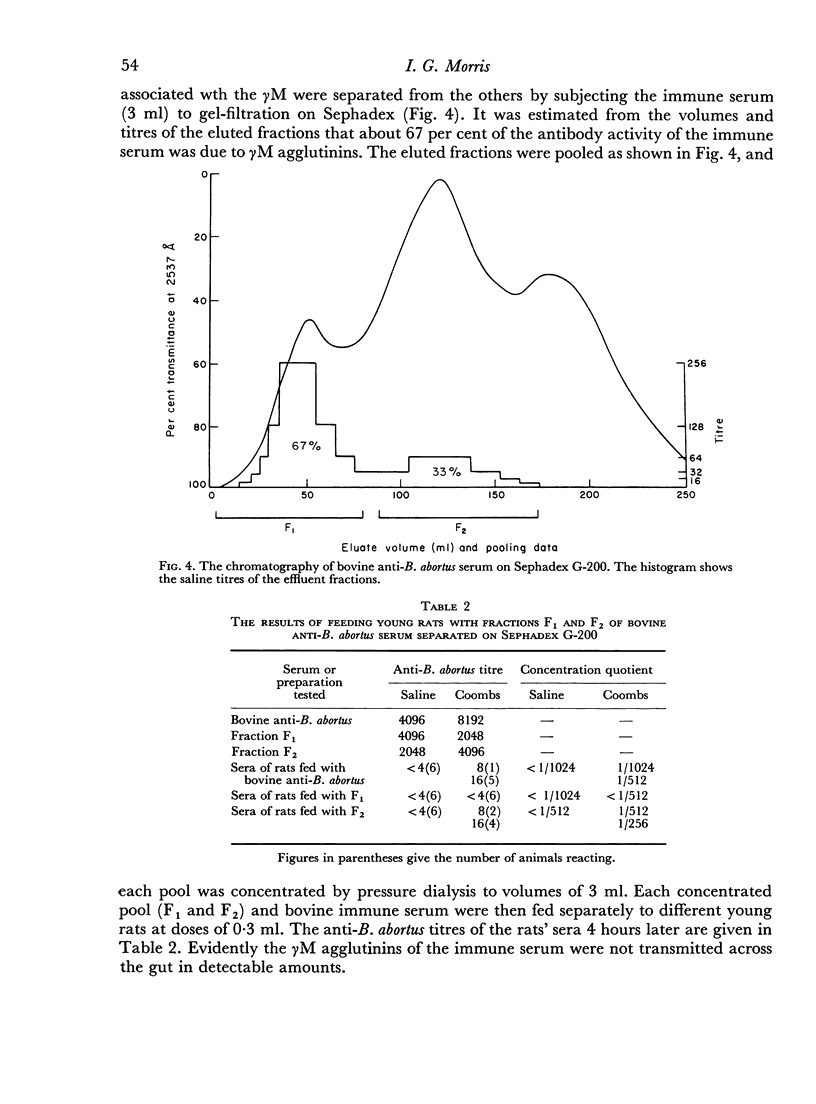
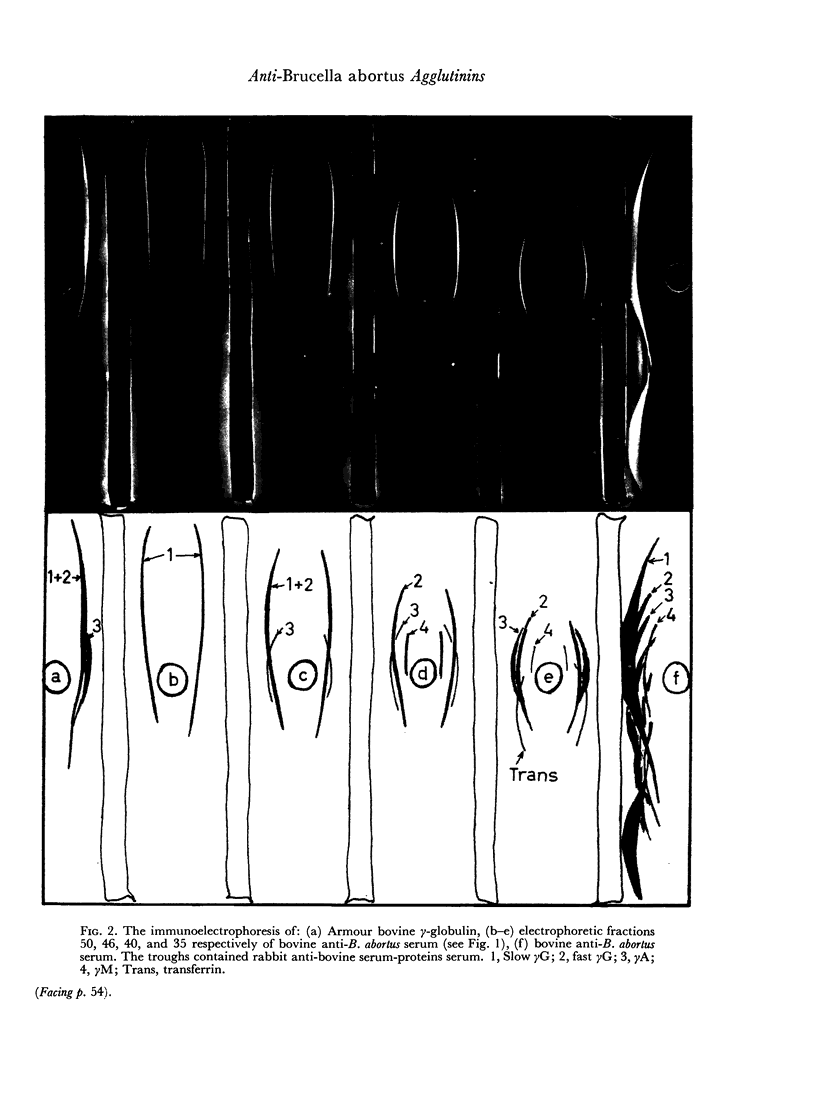
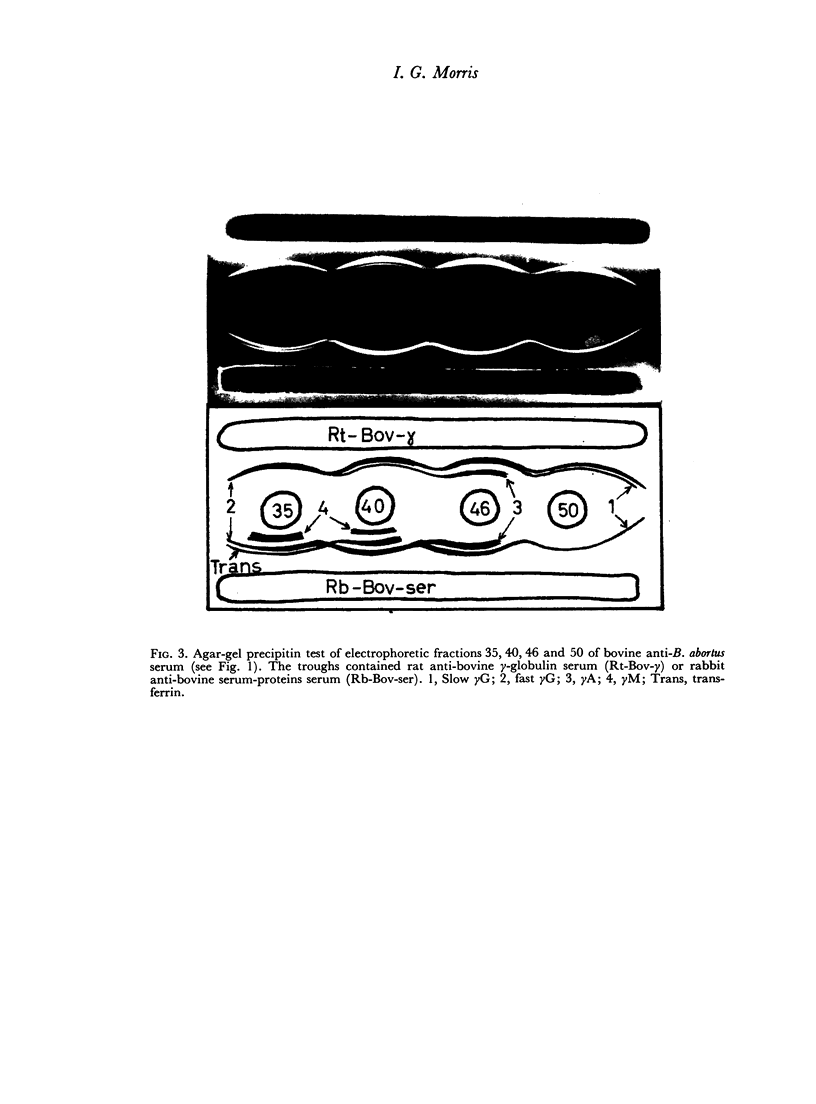
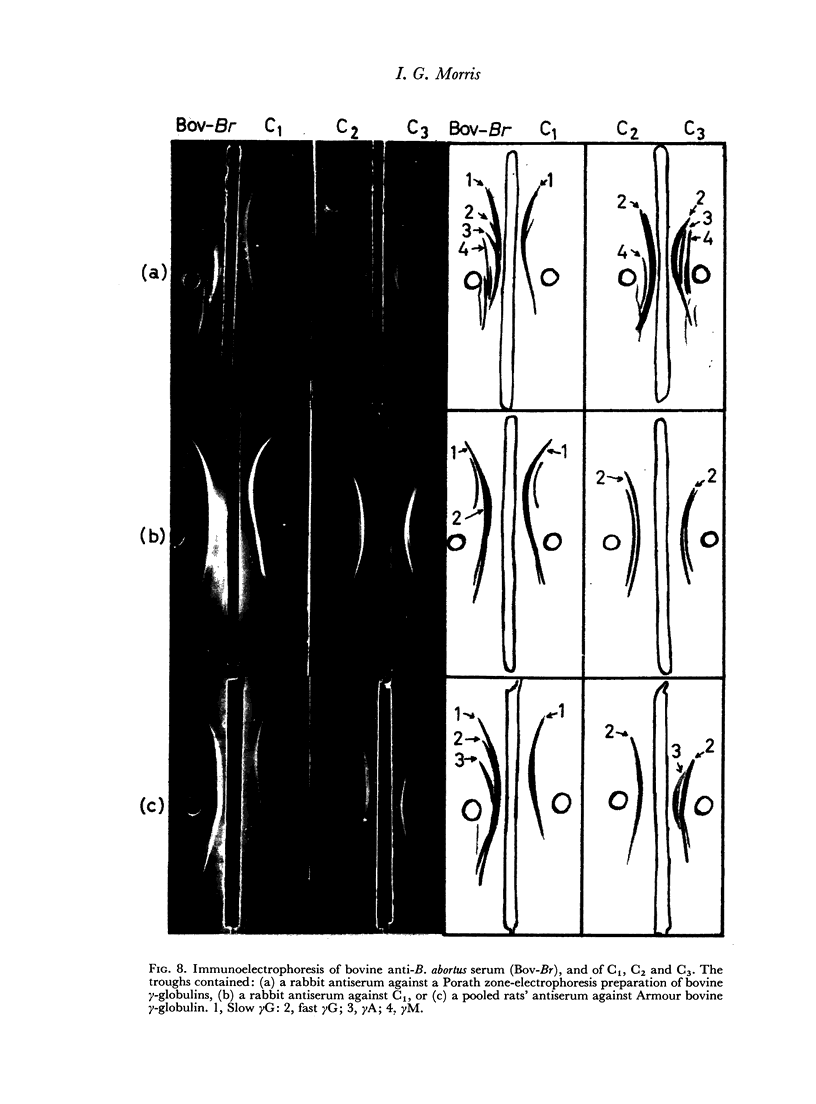
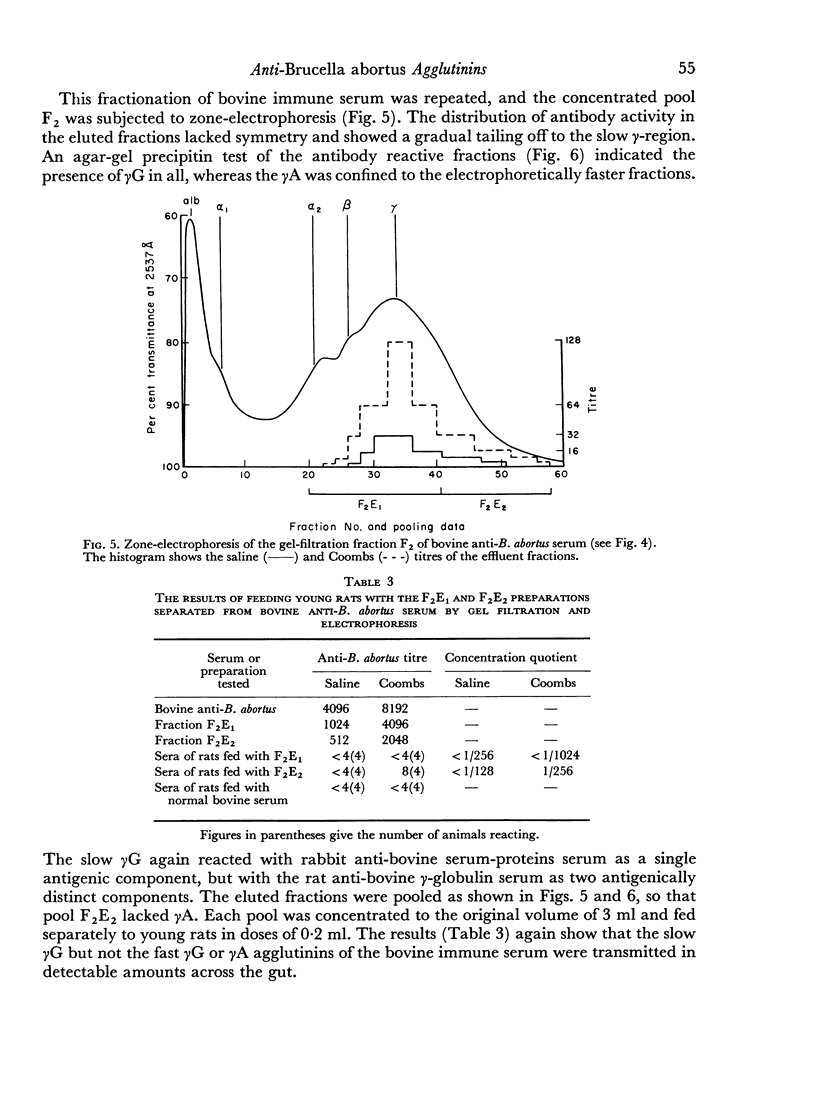
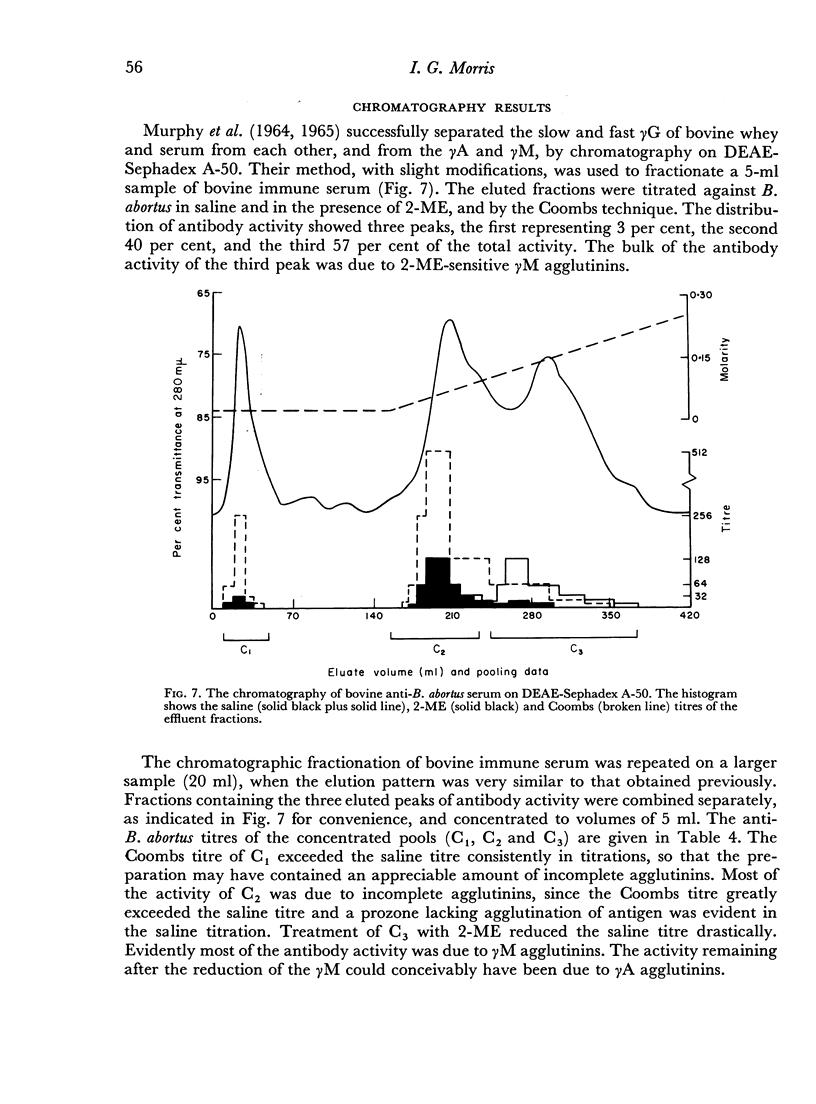
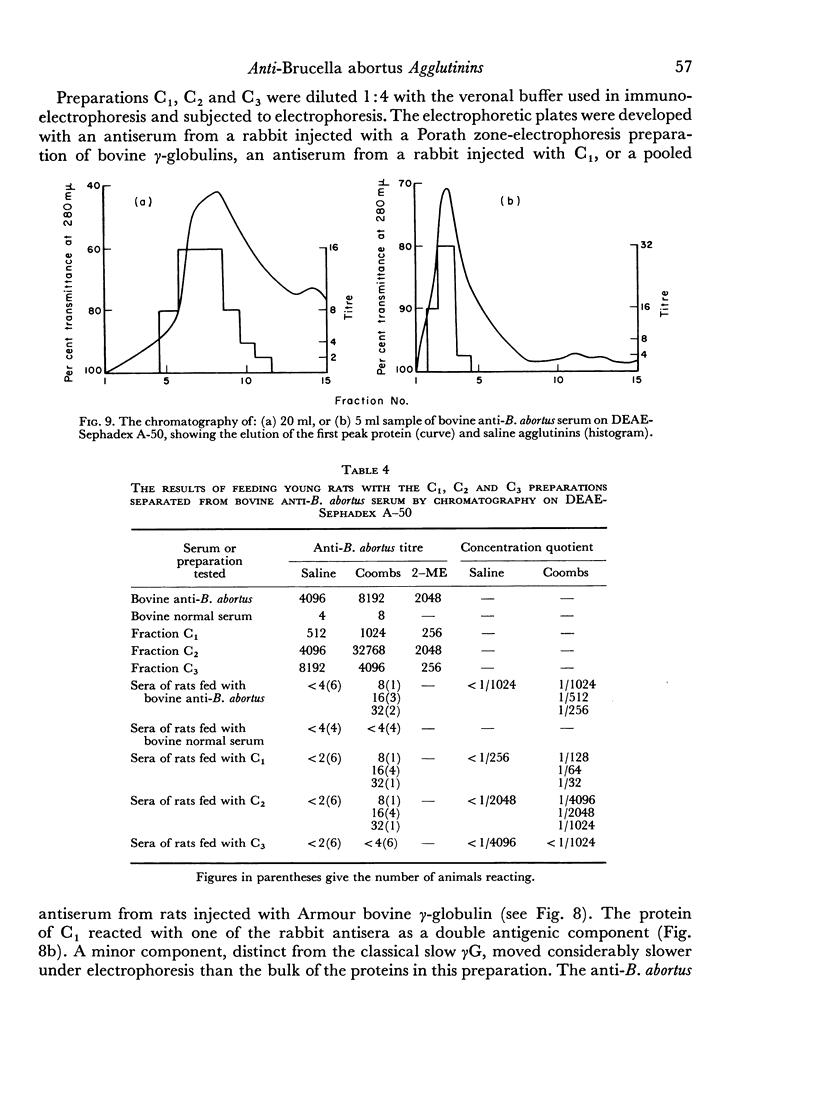
When the immune serum was subjected to immunoelectrophoresis a minor component distinct from the main classes of immunoglobulins was detected in the γ-region. The mean electrophoretic mobility of this component was considerably lower than that of slow γG. The anti-B. abortus agglutinins of the immune serum were tentatively identified with some of the immunoglobulins because of similarities in their electrophoretic and chromatographic behaviour. Sixty to 70 per cent of the antibody activity was due to γM agglutinins. Most of the remaining activity was due to fast γG agglutinins, and less than 5 per cent was due to more slowly migrating γ-globulins. The latter could be the slow γG, the minor slow component, or both.
Chromatographic preparations of the above three types of antibodies were fed to young rats which were killed and bled 4 hours later and their sera titrated for anti-B. abortus agglutinins. The γM agglutinins were not transmitted across the gut in detectable amounts. Transmission of the fast γG agglutinins was of a very low order. The slow γG or the minor slow component agglutinins were transmitted readily and were detected in the circulation as incomplete agglutinins. It was not possible to determine whether the incomplete agglutinins appearing in the circulation of fed rats were transmitted preferentially from the preparation administered or whether complete agglutinins suffered a change into the incomplete type during transmission.
The transmission of the agglutinins is discussed in relation to their physicochemical and immunochemical properties.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALUND O., OSEBOLD J. W., MURPHY F. A. ISOLATION AND CHARACTERIZATION OF OVINE GAMMA GLOBULINS. Arch Biochem Biophys. 1965 Jan;109:142–149. doi: 10.1016/0003-9861(65)90299-7. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., TERRY R. J. The absorption of 131I-labelled homologous and heterologous serum proteins fed orally to young rats. Biochem J. 1957 Aug;66(4):579–583. doi: 10.1042/bj0660579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENACERRAF B., OVARY Z., BLOCH K. J., FRANKLIN E. C. Properties of guinea pig 7S antibodies. I. Electrophoretic separation of two types of guinea pig 7S antibodies. J Exp Med. 1963 Jun 1;117:937–949. doi: 10.1084/jem.117.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAMBELL F. W., HALLIDAY R., MORRIS I. G. Interference by human and bovine serum and serum protein fractions with the absorption of antibodies by suckling rats and mice. Proc R Soc Lond B Biol Sci. 1958 Jul 1;149(934):1–11. doi: 10.1098/rspb.1958.0046. [DOI] [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., WUNDERLICH J., MISHELL R. THE IMMUNOGLOBULINS OF MICE. I. FOUR MAJOR CLASSES OF IMMUNOGLOBULINS: 7S GAMMA-2-, 7S GAMMA-1-, GAMMA-1A (BETA-2A)-, AND 18S GAMMA-1M-GLOBULINS. J Exp Med. 1964 Aug 1;120:223–242. doi: 10.1084/jem.120.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK C. W., MILLER W. E., Jr, DORWARD B., LOSPALLUTO J. The formation of macroglobulin antibodies. II. Studies on neonatal infants and older children. J Clin Invest. 1962 Jul;41:1422–1428. doi: 10.1172/JCI104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman J. B. Immunoglobulins. Annu Rev Biochem. 1966;35:835–872. doi: 10.1146/annurev.bi.35.070166.004155. [DOI] [PubMed] [Google Scholar]
- JONES L. M. Further studies of the pathogenicity and immunogenicity of mucoid variants of Brucella abortus for guinea pigs. J Infect Dis. 1953 Jan-Feb;92(1):26–32. doi: 10.1093/infdis/92.1.26. [DOI] [PubMed] [Google Scholar]
- LICHTER E. A., DRAY S. IMMUNOELECTROPHORETIC CHARACTERIZATION OF HUMAN SERUM PROTEINS WITH PRIMATE ANTISERA. J Immunol. 1964 Jan;92:91–99. [PubMed] [Google Scholar]
- MORRIS I. G. THE TRANSMISSION OF ANTIBODIES AND NORMAL GAMMA-GLOBULINS ACROSS THE YOUNG MOUSE GUT. Proc R Soc Lond B Biol Sci. 1964 May 19;160:276–292. doi: 10.1098/rspb.1964.0040. [DOI] [PubMed] [Google Scholar]
- MURPHY F. A., AALUND O., OSEBOLD J. W., CARROLL E. J. GAMMA GLOBULINS OF BOVINE LACTEAL SECRETIONS. Arch Biochem Biophys. 1964 Nov;108:230–239. doi: 10.1016/0003-9861(64)90380-7. [DOI] [PubMed] [Google Scholar]
- Morris I. G. The transmission of anti-Brucella abortus agglutinins across the gut in young rats. Proc R Soc Lond B Biol Sci. 1965 Nov 23;163(992):402–416. doi: 10.1098/rspb.1965.0075. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Osebold J. W., Aalund O. Physical heterogeneity of bovine gamma-globulins: characterization of gamma-M and gamma-G globulins. Arch Biochem Biophys. 1965 Oct;112(1):126–136. doi: 10.1016/0003-9861(65)90020-2. [DOI] [PubMed] [Google Scholar]
- PORATH J. Methodological studies of zone-electrophoresis in vertical columns. I. Fractionation in cellulose powder columns of substances of low molecular weight exemplified by amino acids and related compounds. Biochim Biophys Acta. 1956 Oct;22(1):151–175. doi: 10.1016/0006-3002(56)90234-7. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN A. M., THORBECKE G. J., KRANER K. L., LUKES R. J. FETAL RESPONSE TO ANTIGENIC STIMULUS. III. GAMMA-GLOBULIN PRODUCTION IN NORMAL AND STIMULATED FETAL LAMBS. J Immunol. 1963 Sep;91:384–395. [PubMed] [Google Scholar]
- Weir R. C., Porter R. R., Givol D. Comparison of the C-terminal amino-acid sequence of two horse immunoglobulins IgG and IgG(T). Nature. 1966 Oct 8;212(5058):205–206. doi: 10.1038/212205a0. [DOI] [PubMed] [Google Scholar]






