Abstract
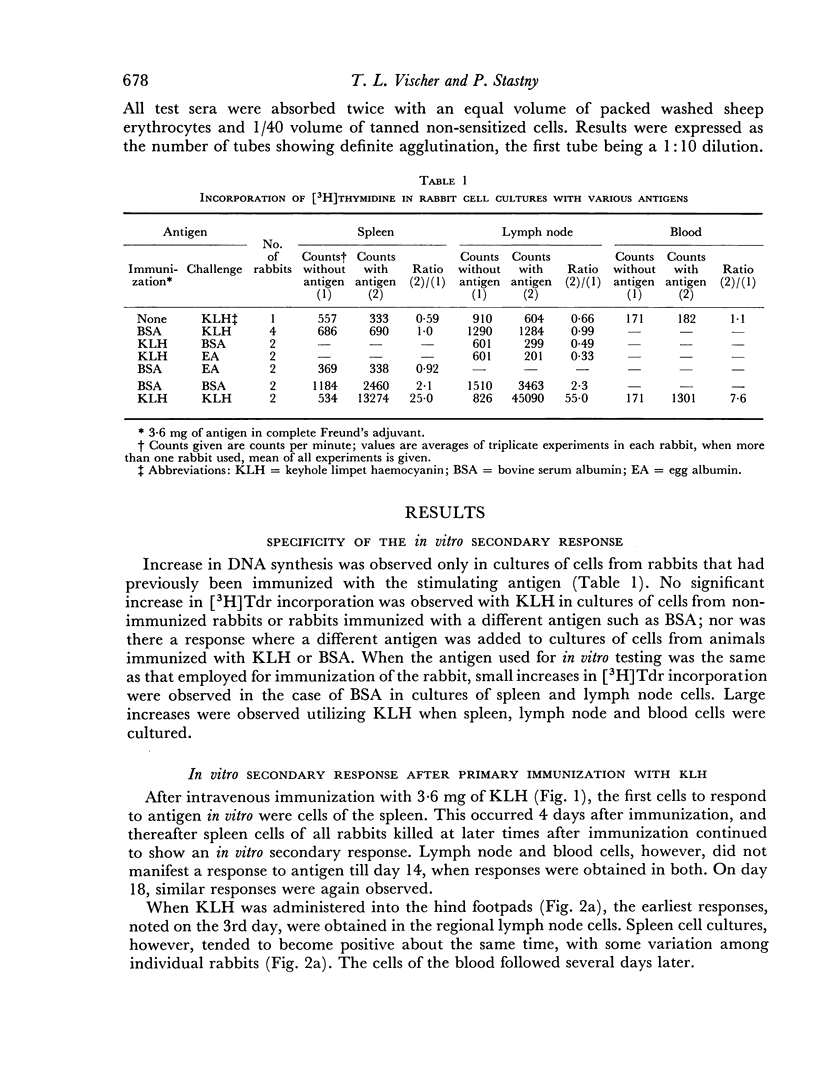
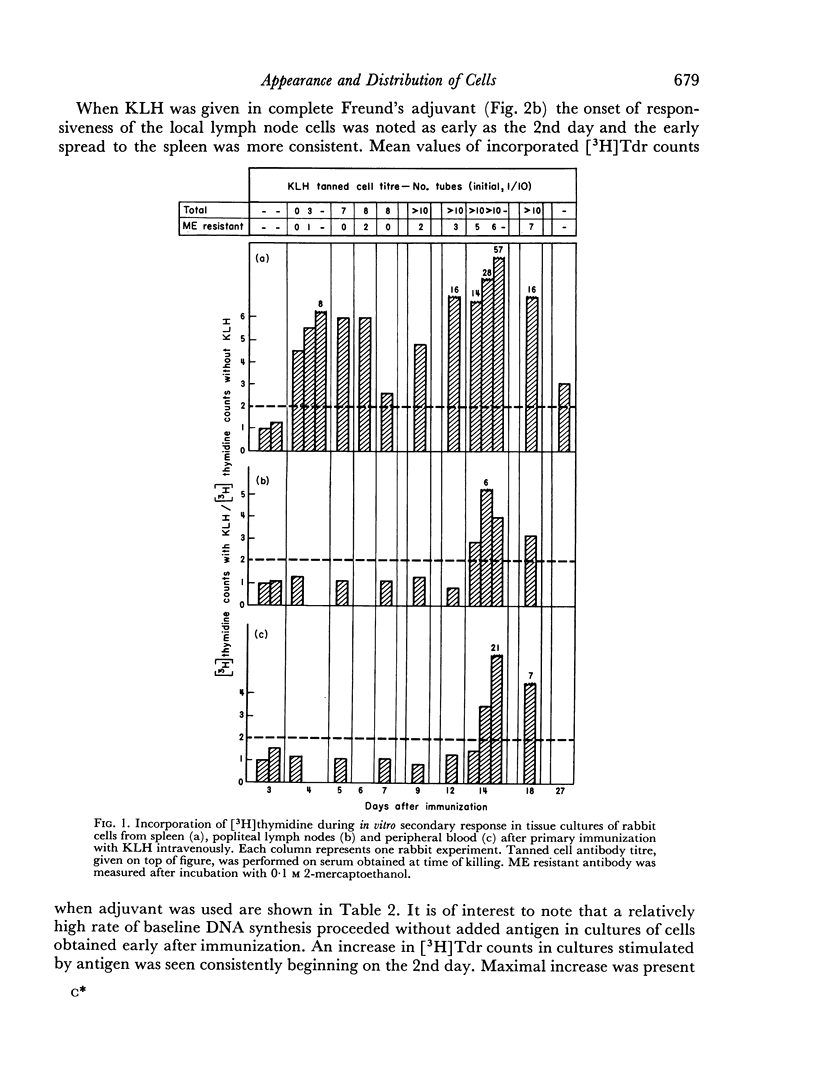
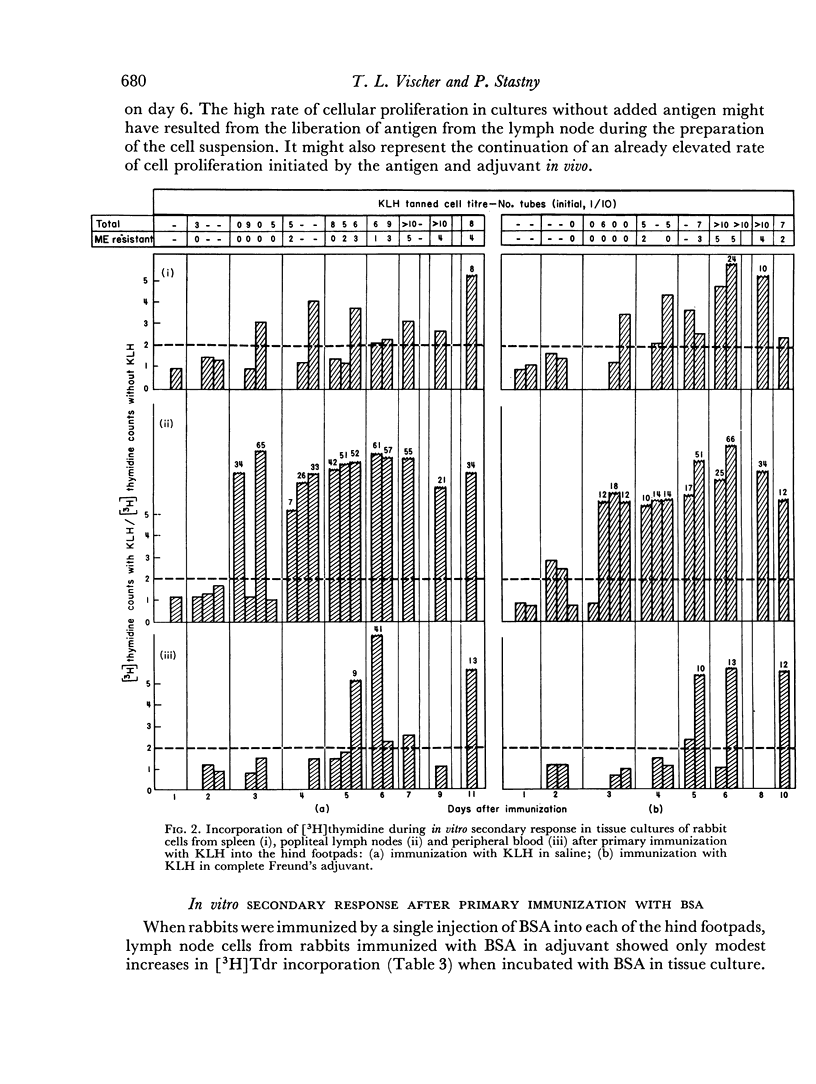
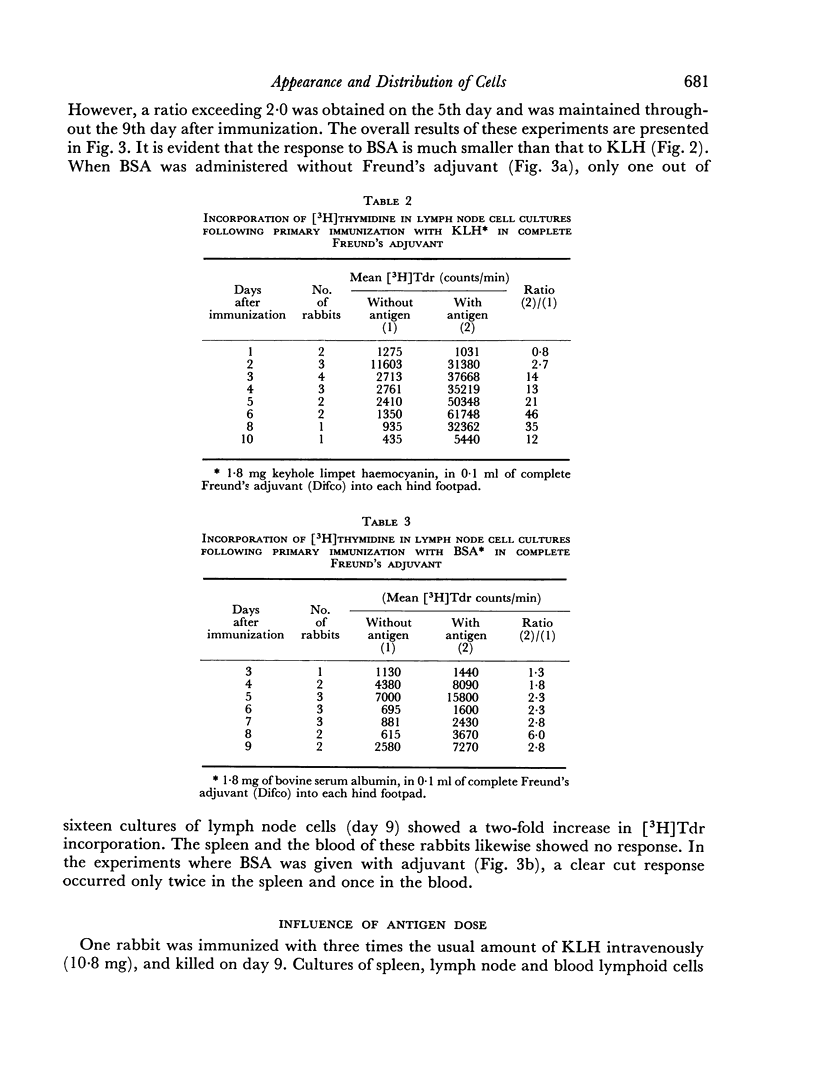
Immunological memory was studied by measurement of tritiated thymidine incorporation in tissue culture. After primary immunization with keyhole limpet haemocyanin (KLH) secondary responsiveness could be detected as early as the 2nd day after immunization with Freund's adjuvant into the footpads and on the 4th day after injection of KLH intravenously. In each case immunological memory developed first in the area of the injection, that is, the popliteal lymph nodes after footpad immunization and the spleen after intravenous injection. The secondary response could also be detected in the lymphoid cells of the blood. Cell suspensions enriched in small lymphocytes showed a similar reactivity. Cells from the thymus, however, did not develop immunological memory. Rabbits immunized with BSA showed a relatively weaker response which was clearly detectable only when Freund's adjuvant was used for immunization.
The results suggest that a response essentially of a secondary type may play an important role in what is usually considered the primary immune response.
Full text
PDF


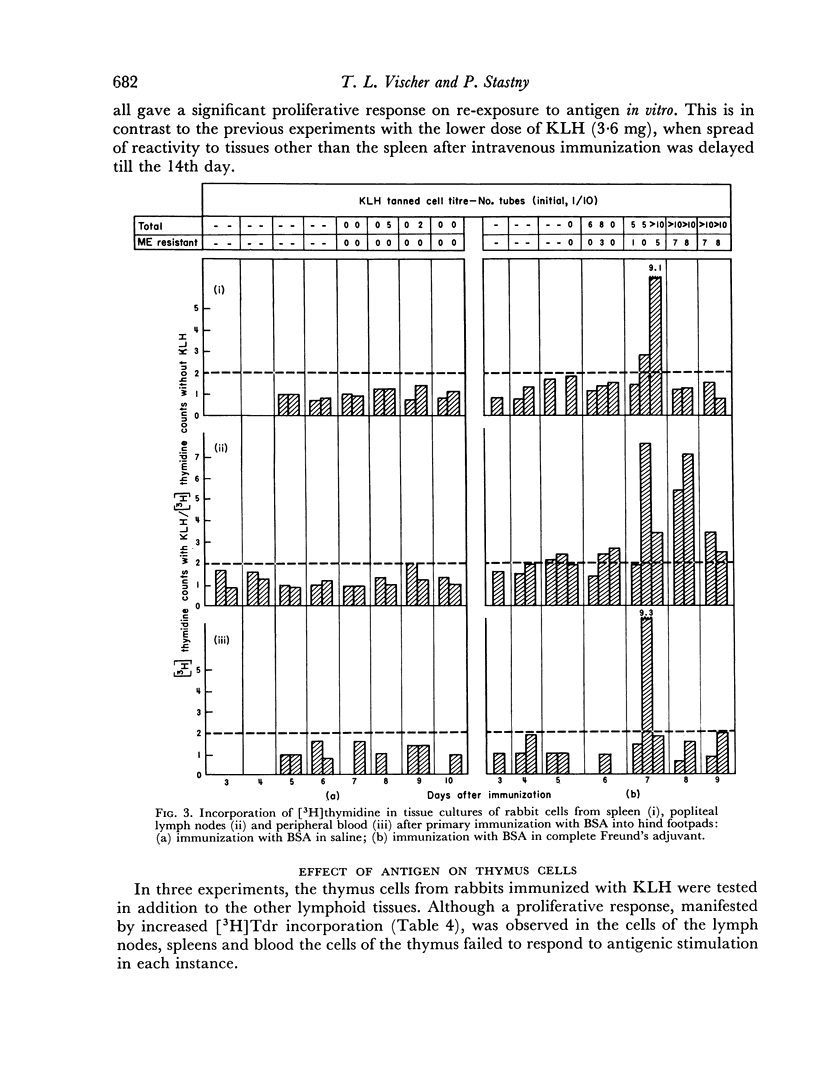
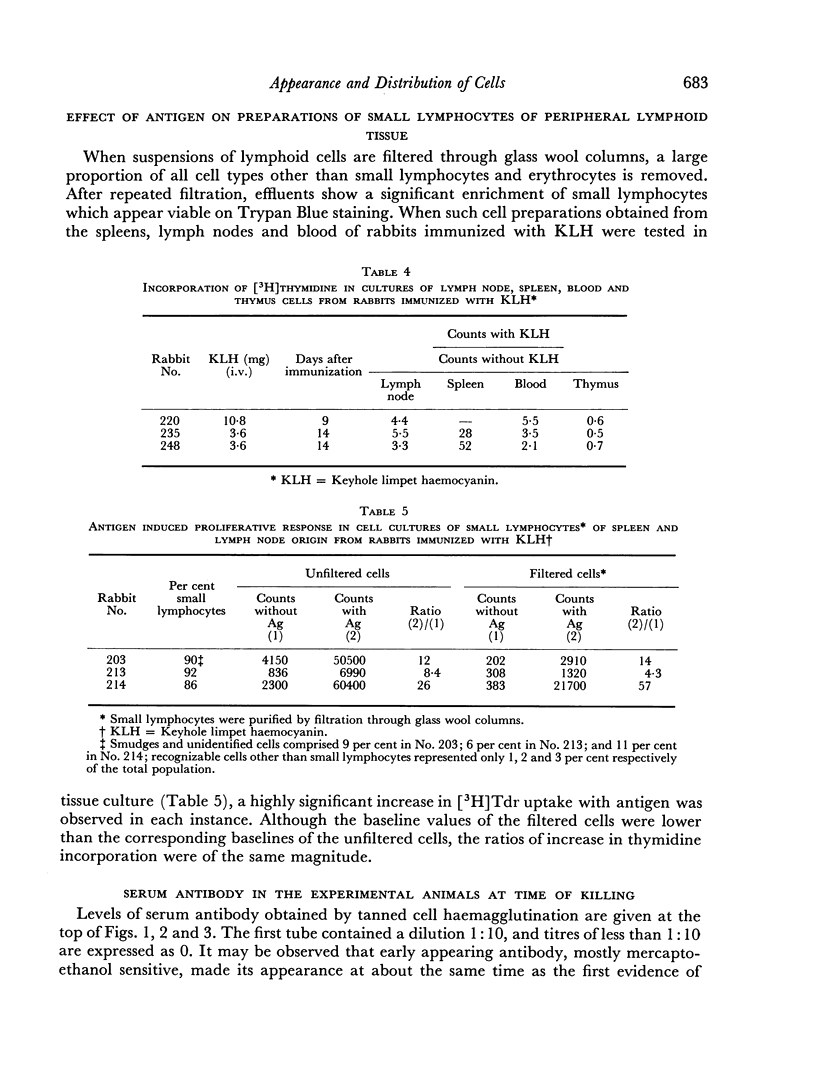




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., WHITE R. G. Sites of antibody production in the guinea-pig; the relation between in vitro synthesis of anti-ovalbumin and gamma-globulin and distribution of antibody-containing plasma cells. Br J Exp Pathol. 1956 Feb;37(1):61–74. [PMC free article] [PubMed] [Google Scholar]
- COHEN E. P., TALMAGE D. W. ONSET AND DURATION OF DNA SYNTHESIS IN ANTIBODY FORMING CELLS AFTER ANTIGEN. J Exp Med. 1965 Jan 1;121:125–132. doi: 10.1084/jem.121.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., WEIGLE W. O., ROBERTS J. C. Comparison of antibody responses associated with the transfer of rabbit lymph-node, peritoneal exudate, and thymus cells. J Immunol. 1957 Jan;78(1):56–62. [PubMed] [Google Scholar]
- FISHMAN M. Antibody formation in vitro. J Exp Med. 1961 Dec 1;114:837–856. doi: 10.1084/jem.114.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD C. E., MICKLEM H. S. The thymus and lymph-nodes in radiation chimaeras. Lancet. 1963 Feb 16;1(7277):359–362. doi: 10.1016/s0140-6736(63)91385-0. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., McGREGOR D. D., COWEN D. M. Initiation of immune responses by small lymphocytes. Nature. 1962 Nov 17;196:651–655. doi: 10.1038/196651a0. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. The fate of parental strain small lymphocytes in F1 hybrid rats. Ann N Y Acad Sci. 1962 Oct 24;99:432–455. doi: 10.1111/j.1749-6632.1962.tb45326.x. [DOI] [PubMed] [Google Scholar]
- Globerson A., Auerbach R. Primary immune reactions in organ cultures. Science. 1965 Aug 27;149(3687):991–993. doi: 10.1126/science.149.3687.991. [DOI] [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. EFFECT OF X-IRRADIATION OF THE POPLITEAL LYMPH-NODE ON ITS OUTPUT OF LYMPHOCYTES AND IMMUNOLOGICAL RESPONSIVENESS. Lancet. 1964 May 16;1(7342):1077–1080. doi: 10.1016/s0140-6736(64)91274-7. [DOI] [PubMed] [Google Scholar]
- HIRSCHHORN K., BACH F., KOLODNY R. L., FIRSCHEIN I. L., HASHEM N. IMMUNE RESPONSE AND MITOSIS OF HUMAN PERIPHERAL BLOOD LYMPHOCYTES IN VITRO. Science. 1963 Nov 29;142(3596):1185–1187. doi: 10.1126/science.142.3596.1185. [DOI] [PubMed] [Google Scholar]
- HIRSCHHORN K., SCHREIBMAN R. R., VERBO S., GRUSKIN R. H. THE ACTION OF STREPTOLYSIN S ON PERIPHERAL LYMPHOCYTES OF NORMAL SUBJECTS AND PATIENTS WITH ACUTE RHEUMATIC FEVER. Proc Natl Acad Sci U S A. 1964 Nov;52:1151–1157. doi: 10.1073/pnas.52.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING N. R., HUSBAND E. M. SPECIFIC AND NON-SPECIFIC STIMULATION OF PERIPHERAL LYMPHOCYTES. Lancet. 1964 Feb 15;1(7329):363–365. doi: 10.1016/s0140-6736(64)92102-6. [DOI] [PubMed] [Google Scholar]
- LING N. R., SPICER E., JAMES K., WILLIAMSON N. THE ACTIVATION OF HUMAN PERIPHERAL LYMPHOCYTES BY PRODUCTS OF STAPHYLOCOCCI. Br J Haematol. 1965 Jul;11:421–431. doi: 10.1111/j.1365-2141.1965.tb06604.x. [DOI] [PubMed] [Google Scholar]
- MAKELA O., NOSSAL G. J. Autoradiographic studies on the immune response. II. DNA synthesis amongst single antibody-producing cells. J Exp Med. 1962 Jan 1;115:231–244. doi: 10.1084/jem.115.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAELIDES M. C., COONS A. H. Studies on antibody production. V. The secondary response in vitro. J Exp Med. 1963 Jun 1;117:1035–1051. doi: 10.1084/jem.117.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSSAL G. J., MAKELA O. Autoradiographic studies on the immune response.I. The kinetics of plasma cell proliferation. J Exp Med. 1962 Jan 1;115:209–230. doi: 10.1084/jem.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- ORLOV A. S., ORLOVA E. I. [A simple method for the quantitative determination of desoxyribonucleic acid in animal tissues]. Biokhimiia. 1961 Sep-Oct;26:834–839. [PubMed] [Google Scholar]
- PORTER K. A., COOPER E. H. Transformation of adult allogeneic small lymphocytes after transfusion into newborn rats. J Exp Med. 1962 May 1;115:997–1008. doi: 10.1084/jem.115.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES E. D., SINGER S. J. A preliminary study of the properties of proteins in some nonaqueous solvents. Arch Biochem Biophys. 1956 Jul;63(1):144–159. doi: 10.1016/0003-9861(56)90018-2. [DOI] [PubMed] [Google Scholar]
- RICHARDSON M., DUTTON R. W. ANTIBODY SYNTHESIZING CELLS: APPEARANCE AFTER SECONDARY ANTIGENIC STIMULATION IN VITRO. Science. 1964 Oct 30;146(3644):655–656. doi: 10.1126/science.146.3644.655. [DOI] [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]
- Saunders G. C., King D. W. Antibody synthesis initiated in vitro by paired explants of spleen and thymus. Science. 1966 Mar 18;151(3716):1390–1391. doi: 10.1126/science.151.3716.1390. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J., KEUNING F. J. Antibody formation in vitro by haemopoietic organs after subcutaneous and intravenous immunization. J Immunol. 1953 Feb;70(2):129–134. [PubMed] [Google Scholar]
- Tao T. W., Uhr J. W. Primary-type antibody response in vitro. Science. 1966 Mar 4;151(3714):1096–1098. doi: 10.1126/science.151.3714.1096. [DOI] [PubMed] [Google Scholar]
- URSO P., MAKINODAN T. THE ROLES OF CELLULAR DIVISION AND MATURATION IN THE FORMATION OF PRECIPITATING ANTIBODY. J Immunol. 1963 Jun;90:897–907. [PubMed] [Google Scholar]
- WHITE R. G., COONS A. H., CONNOLLY J. M. Studies on antibody production. III. The alum granuloma. J Exp Med. 1955 Jul 1;102(1):73–82. doi: 10.1084/jem.102.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]


