Abstract
The number of antibody producing cells, i.e. rosette forming cells (RFC) has been studied in the spleen of mice injected intravenously with a full immunizing dose of sheep RBC.
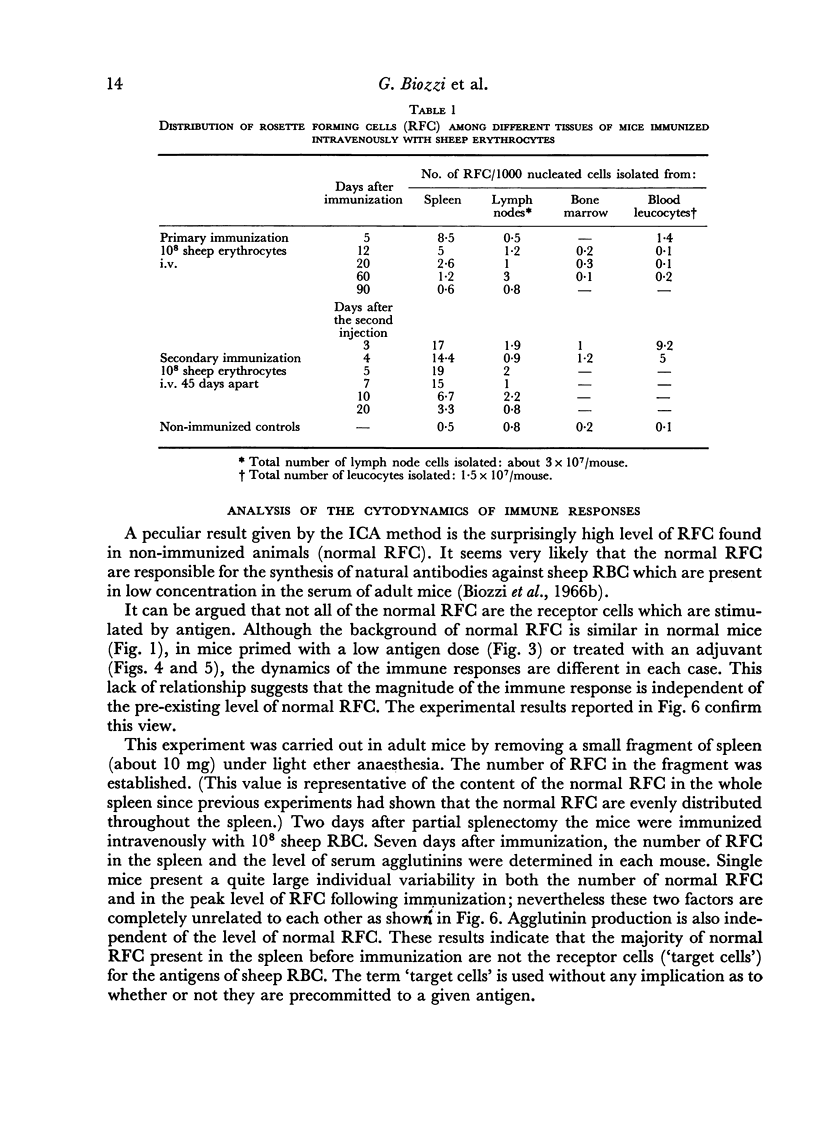
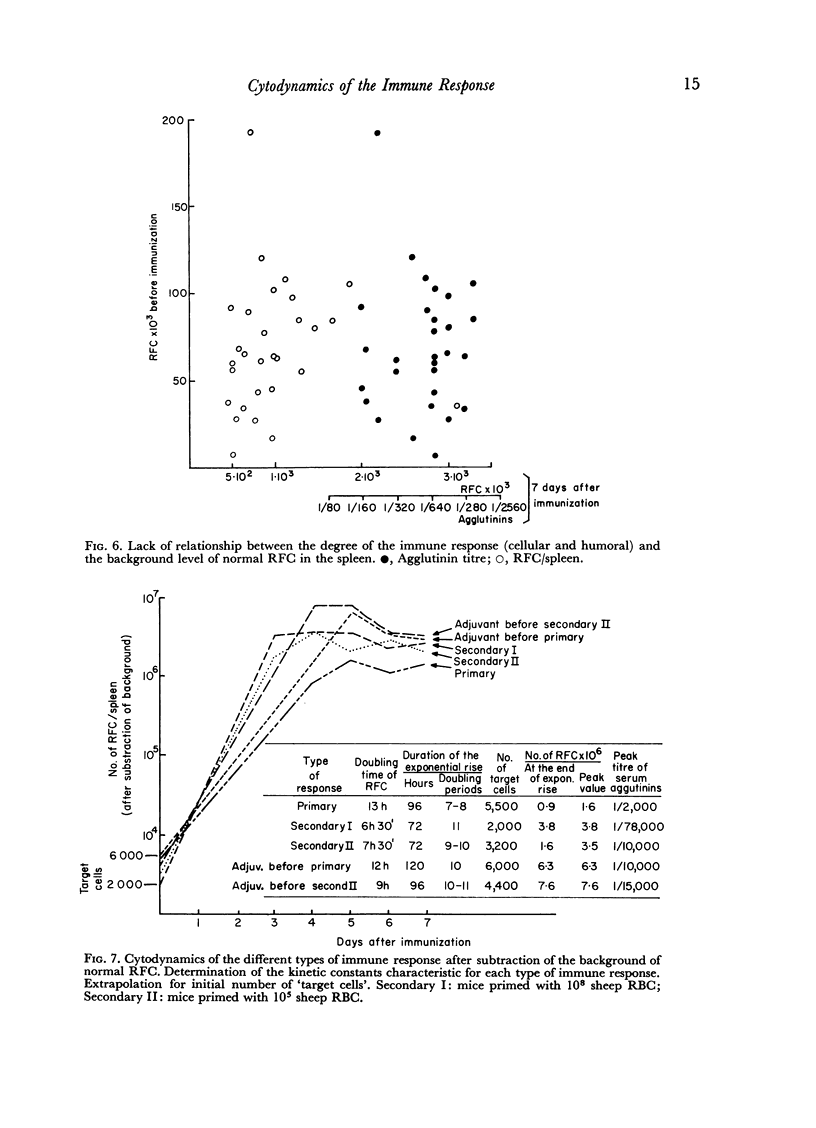
The spleen of a non-immunized mouse contains a background of about 70,000 RFC (normal RFC), which do not appear to be the `target cells' for antigens of sheep RBC. Our findings suggest that the spleen of a mouse contains about 4000 `target cells' which initiate the immune response to sheep RBC.
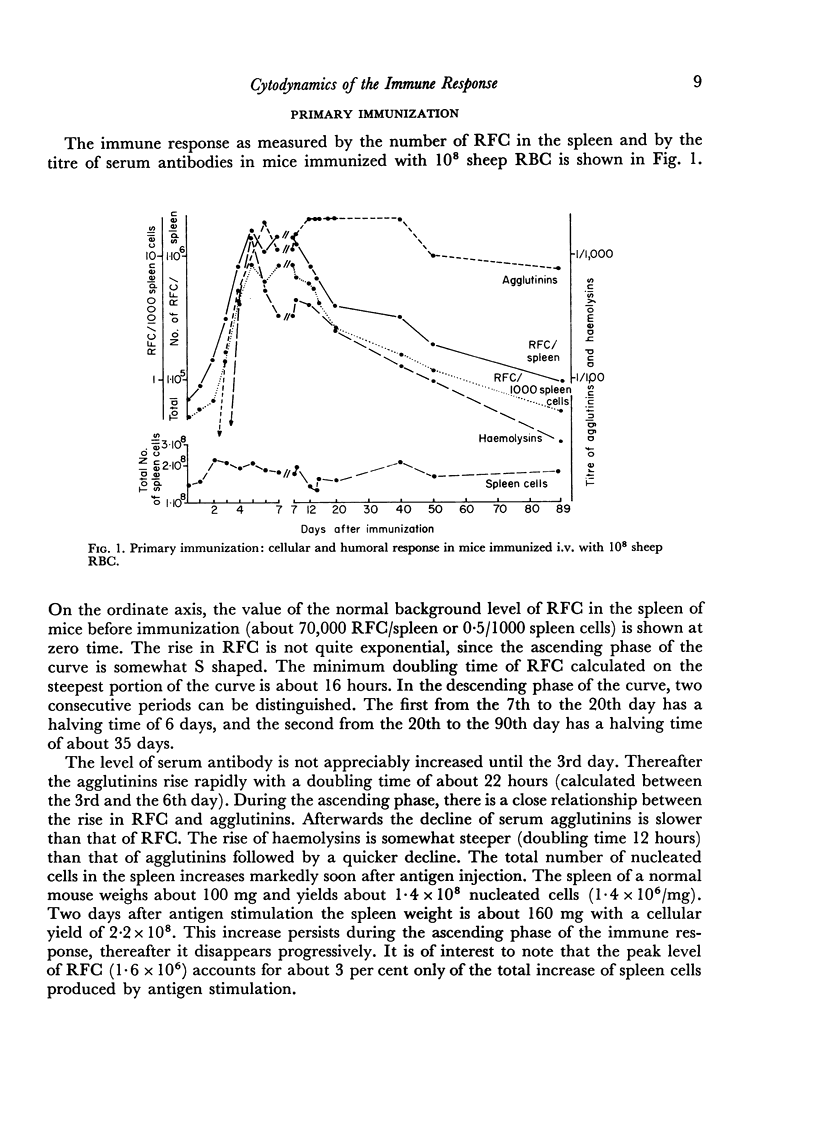
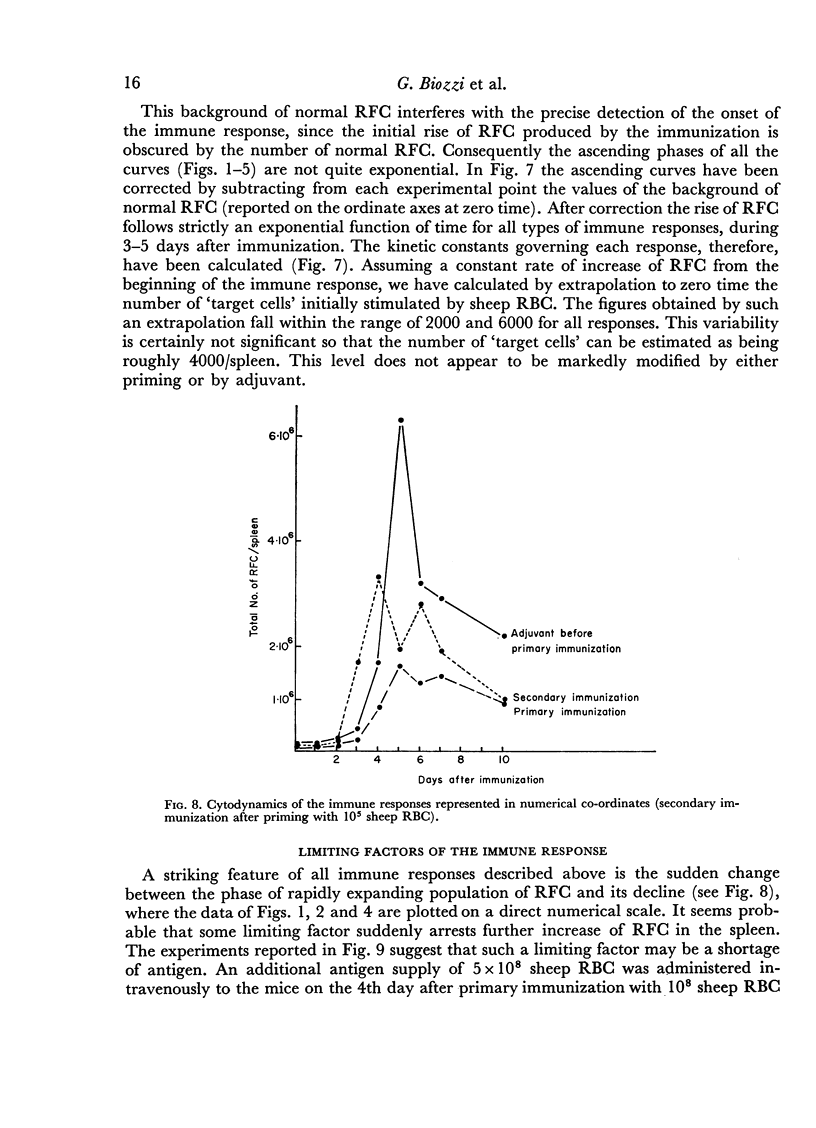
In the primary response, the rise of RFC is exponential for about 96 hours with a doubling time of 13 hours involving seven to eight consecutive doubling periods. The peak value of RFC is 1·6 × 106 per spleen.
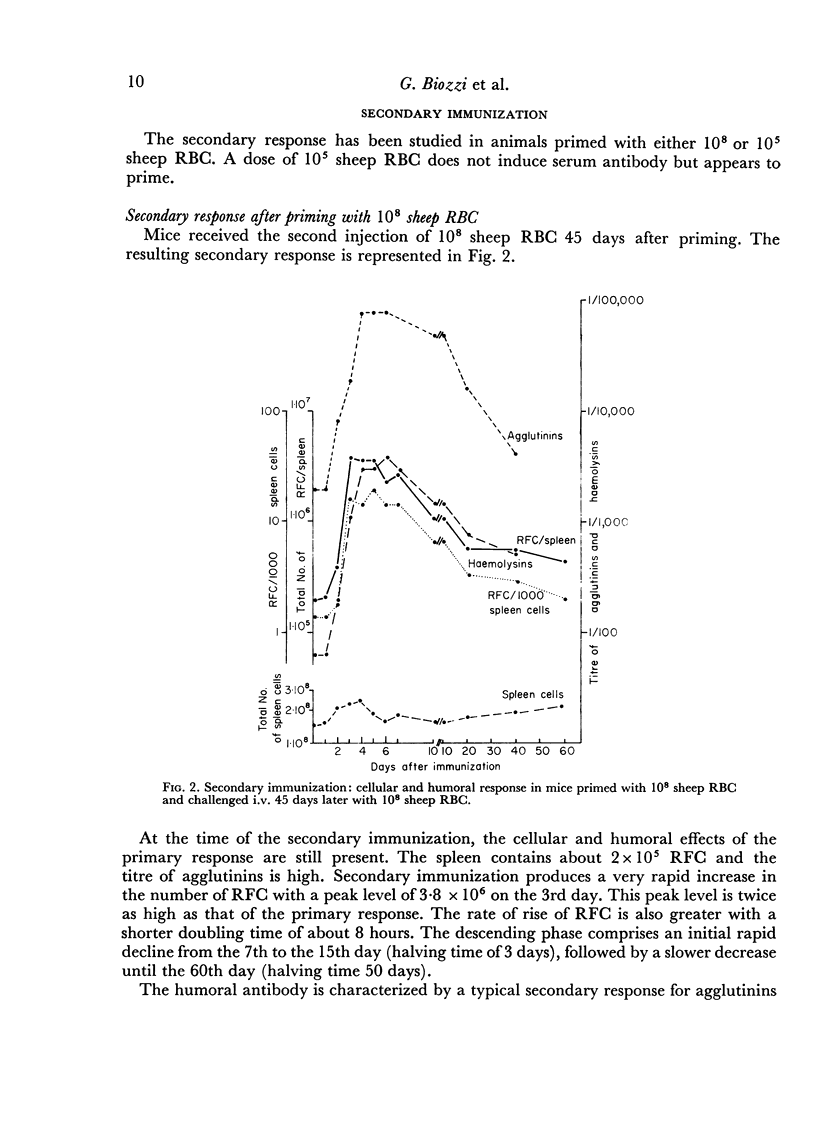
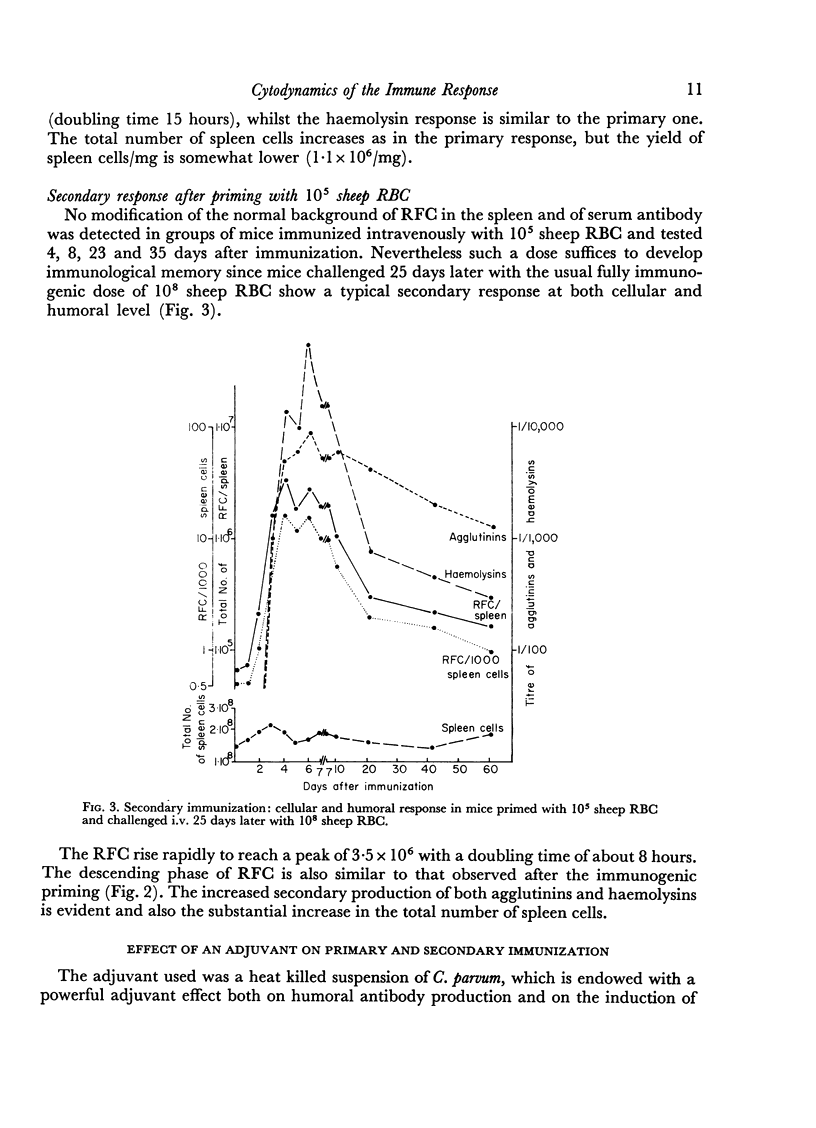
In the secondary response, the doubling time of RFC is 6–7 hours. The exponential rise lasts 72 hours and includes nine to eleven doubling periods leading to a peak of 3·5 × 106 RFC/spleen.
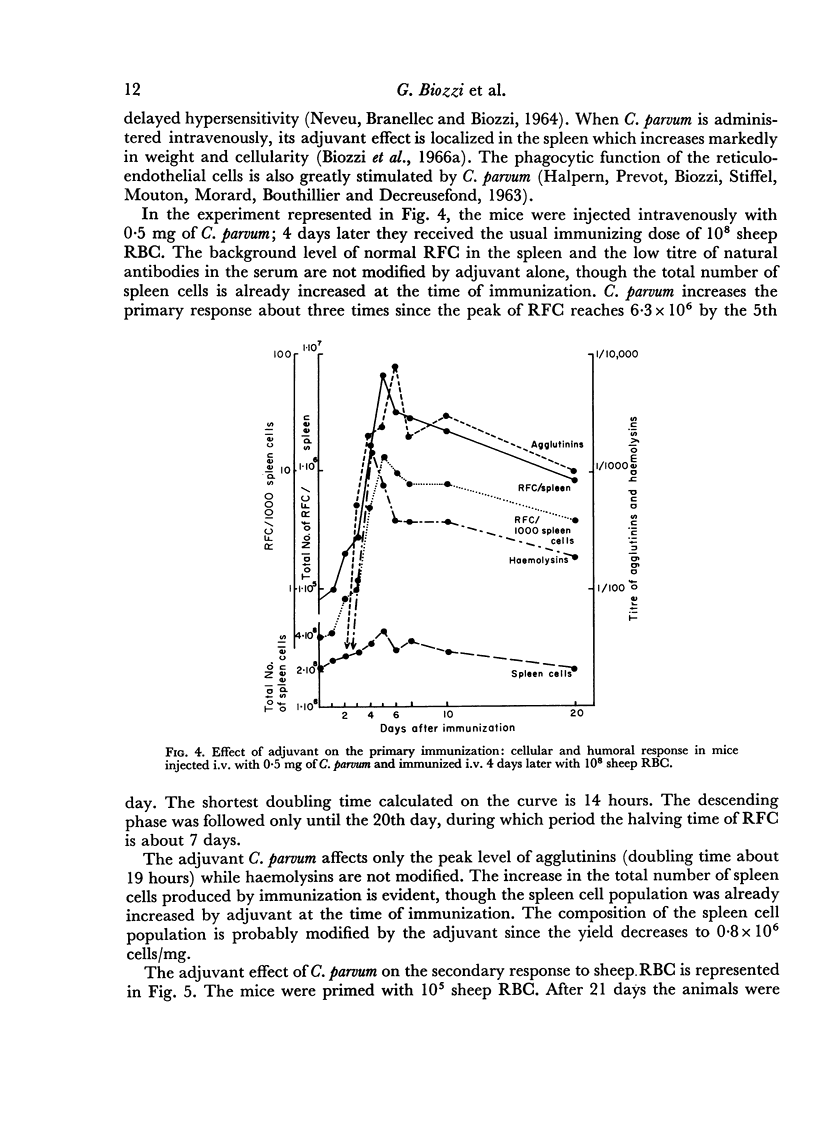
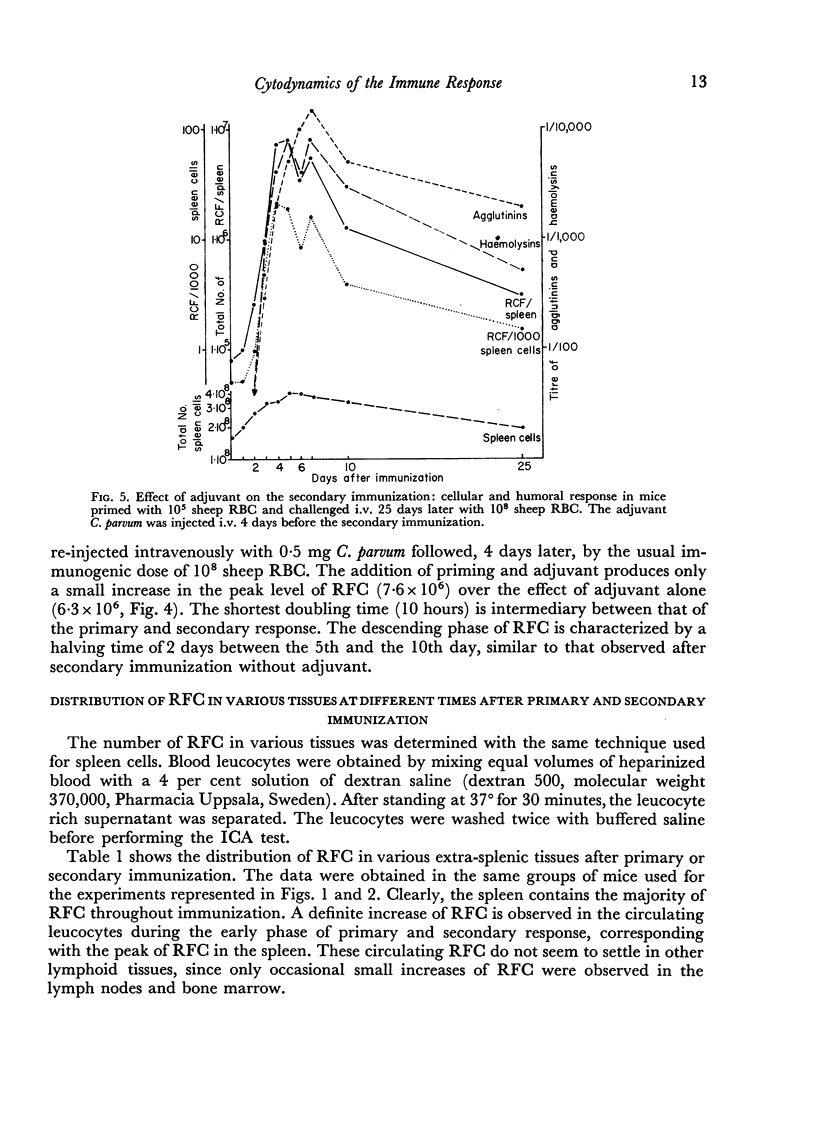
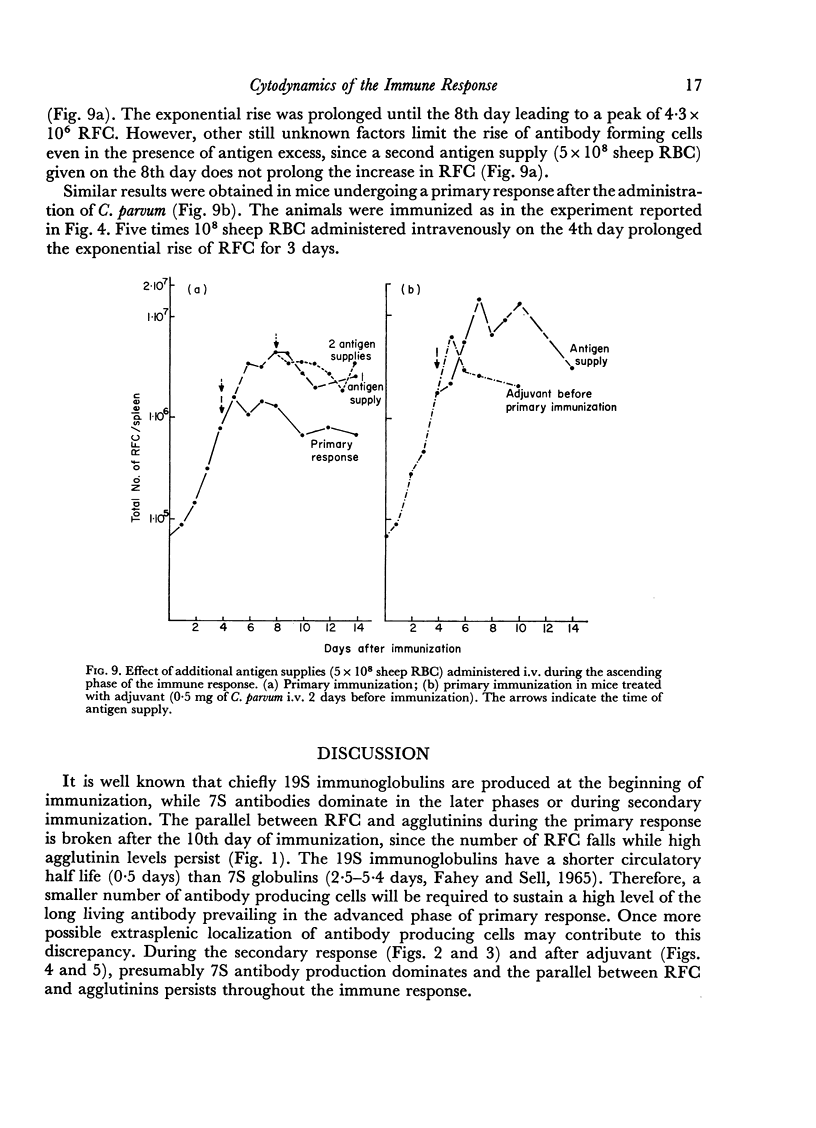
Adjuvant in the primary response leaves the doubling time of RFC unaltered but prolongs the exponential rise until the 120th hour leading to a peak of 6·3 × 106 RFC/spleen after ten doubling periods. The effects of priming and adjuvant are not fully additive.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham A. J., Smith J. B., Mercer E. H. Antibody formation by single cells from lymph nodes and efferent lymph of sheep. J Exp Med. 1966 Oct 1;124(4):701–714. doi: 10.1084/jem.124.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich F. M. The immune response to heterologous red cells in mice. Immunology. 1966 Apr;10(4):365–376. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- Hege J. S., Cole L. J. Antibody plaque-forming cells: kinetics of primary and secondary responses. J Immunol. 1966 Apr;96(4):559–569. [PubMed] [Google Scholar]
- INGRAHAM J. S., BUSSARD A. APPLICATION OF A LOCALIZED HEMOLYSIN REACTION FOR SPECIFIC DETECTION OF INDIVIDUAL ANTIBODY-FORMING CELLS. J Exp Med. 1964 Apr 1;119:667–684. doi: 10.1084/jem.119.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- KENNEDY J. C., TILL J. E., SIMINOVITCH L., MCCULLOCH E. A. RADIOSENSITIVITY OF THE IMMUNE RESPONSE TO SHEEP RED CELLS IN THE MOUSE, AS MEASURED BY THE HEMOLYTIC PLAQUE METHOD. J Immunol. 1965 May;94:715–722. [PubMed] [Google Scholar]
- Kennedy J. C., Till J. E., Siminovitch L., McCulloch E. A. The proliferative capacity of antigen-sensitive precursors of hemolytic plaque-forming cells. J Immunol. 1966 Jun;96(6):973–980. [PubMed] [Google Scholar]
- Landy M., Baker P. J. Cytodynamics of the distinctive immune response produced in regional lymph nodes by Salmonella somatic polysaccharide. J Immunol. 1966 Nov;97(5):670–679. [PubMed] [Google Scholar]
- Landy M., Sanderson R. P., Jackson A. L. Humoral and cellular aspects of the immune response to the somatic antigen of Salmonella enteritidis. J Exp Med. 1965 Sep 1;122(3):483–504. doi: 10.1084/jem.122.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKELA O., NOSSAL G. J. Autoradiographic studies on the immune response. II. DNA synthesis amongst single antibody-producing cells. J Exp Med. 1962 Jan 1;115:231–244. doi: 10.1084/jem.115.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Albright J. F. Proliferative and differentiative manifestations of cellular immune potential. Prog Allergy. 1967;10:1–36. [PubMed] [Google Scholar]
- Möller G. 19S antibody production against soluble lipopolysaccharide antigens by individual lymphoid cells in vitro. Nature. 1965 Sep 11;207(5002):1166–1168. doi: 10.1038/2071166a0. [DOI] [PubMed] [Google Scholar]
- NEVEU T., BRANELLEC A., BIOZZI G. PROPRI'ET'ES ADJUVANTES DE CORYNEBACTERIUM PARVUM SUR LA PRODUCTION D'ANTICORPS ET SUR L'INDUCTION DE L'HYPERSENSIBILIT'E RETARD'EE ENVERS LES PROT'EINES CONJUGU'EES. Ann Inst Pasteur (Paris) 1964 May;106:771–777. [PubMed] [Google Scholar]
- NOSSAL G. J., MAKELA O. Autoradiographic studies on the immune response.I. The kinetics of plasma cell proliferation. J Exp Med. 1962 Jan 1;115:209–230. doi: 10.1084/jem.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOTA N. R., LIACOPOULOS-BRIOT M., STIFFEL C., BIOZZI G. L'IMMUNO-CYTO-ADH'ERENCE: UNE M'ETHODE SIMPLE ET QUANTITATIVE POUR L'ETUDE IN VITRO DES CELLULES PRODUCTRICES D'ANTICORPS. C R Hebd Seances Acad Sci. 1964 Aug 3;259:1277–1280. [PubMed] [Google Scholar]
- OSIPOVA P. V., KARASIK O. A. SPETSIFICHESKOE PRILIPANIE 'ERITROTSITOV K POVERKHNOSTI LIMFATICHESKIKH KLETOK IN VITRO. Biull Eksp Biol Med. 1964 Oct;58:100–103. [PubMed] [Google Scholar]
- Playfair J. H., Papermaster B. W., Cole L. J. Focal antibody production by transferred spleen cells in irradiated mice. Science. 1965 Aug 27;149(3687):998–1000. doi: 10.1126/science.149.3687.998. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- SCHOOLEY J. C. Autoradiographic observations of plasma cell formation. J Immunol. 1961 Mar;86:331–337. [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. II. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN ANTIGEN DOSE REQUIREMENT FOR SUSTAINED SYNTHESIS, ANAMNESIS, AND SENSITIVITY TO X-IRRADIATION. J Exp Med. 1964 Jan 1;119:21–39. doi: 10.1084/jem.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- UHR J. W. THE HETEROGENEITY OF THE IMMUNE RESPONSE. Science. 1964 Jul 31;145(3631):457–464. doi: 10.1126/science.145.3631.457. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Finkelstein M. S. The kinetics of antibody formation. Prog Allergy. 1967;10:37–83. [PubMed] [Google Scholar]
- Vazquez J. J., Makinodan T. Cytokinetic events following antigenic stimulation. Fed Proc. 1966 Nov-Dec;25(6):1727–1733. [PubMed] [Google Scholar]
- Wigzell H. Antibody synthesis at the cellular level: some studies on natural anti-sheep red cell antibodies in the mouse. J Immunol. 1966 Nov;97(5):608–611. [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]
- ZAALBERG O. B. A SIMPLE METHOD FOR DETECTING SINGLE ANTIBODY-FORMING CELLS. Nature. 1964 Jun 20;202:1231–1231. doi: 10.1038/2021231a0. [DOI] [PubMed] [Google Scholar]
- Zaalberg O. B., van der Meul V. A., van Twisk J. M. Antibody production by single spleen cells: a comparative study of the cluster and agar-plaque formation. Nature. 1966 Apr 30;210(5035):544–545. doi: 10.1038/210544a0. [DOI] [PubMed] [Google Scholar]


