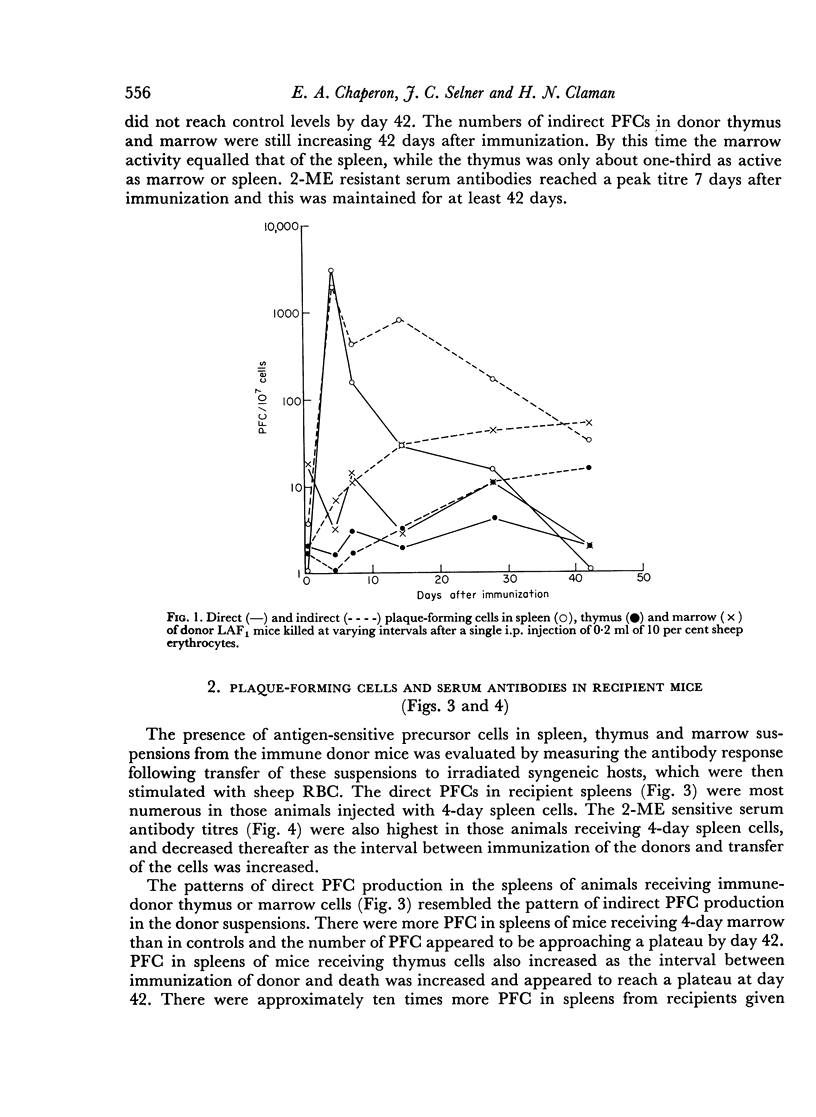
Abstract
The appearance of 19S and 7S antibody-forming cells (producers) in mice following a single i.p. injection of sheep erythrocytes, and their recirculation between spleen, thymus and marrow, were studied by the direct and indirect Jerne plaque techniques. The appearance and distribution of antigen-sensitive precursor cells (memory cells) in the same tissues were determined by transferring aliquots of thymus, marrow and spleen cells to irradiated recipients, restimulating with antigen, and counting the plaque-forming cells (PFCs) in the recipient spleens. 2-ME sensitive and 2-ME resistant serum antibodies in donors and recipients were also titrated.
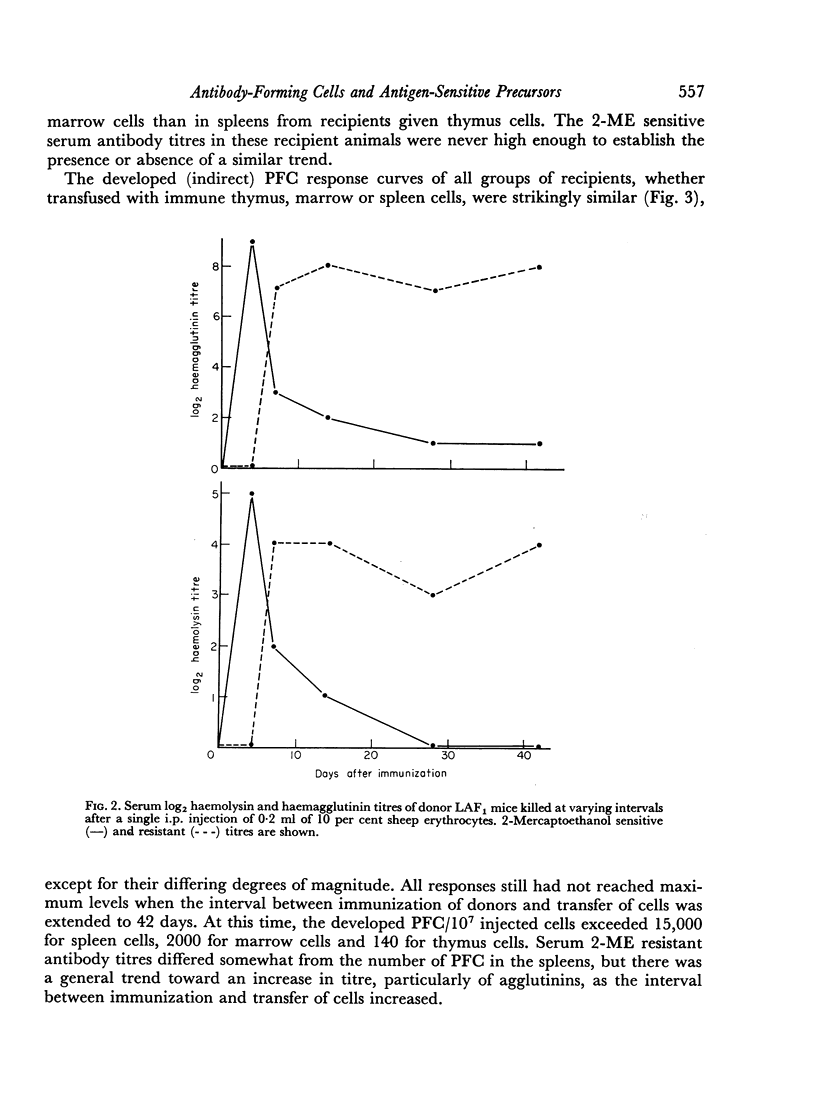
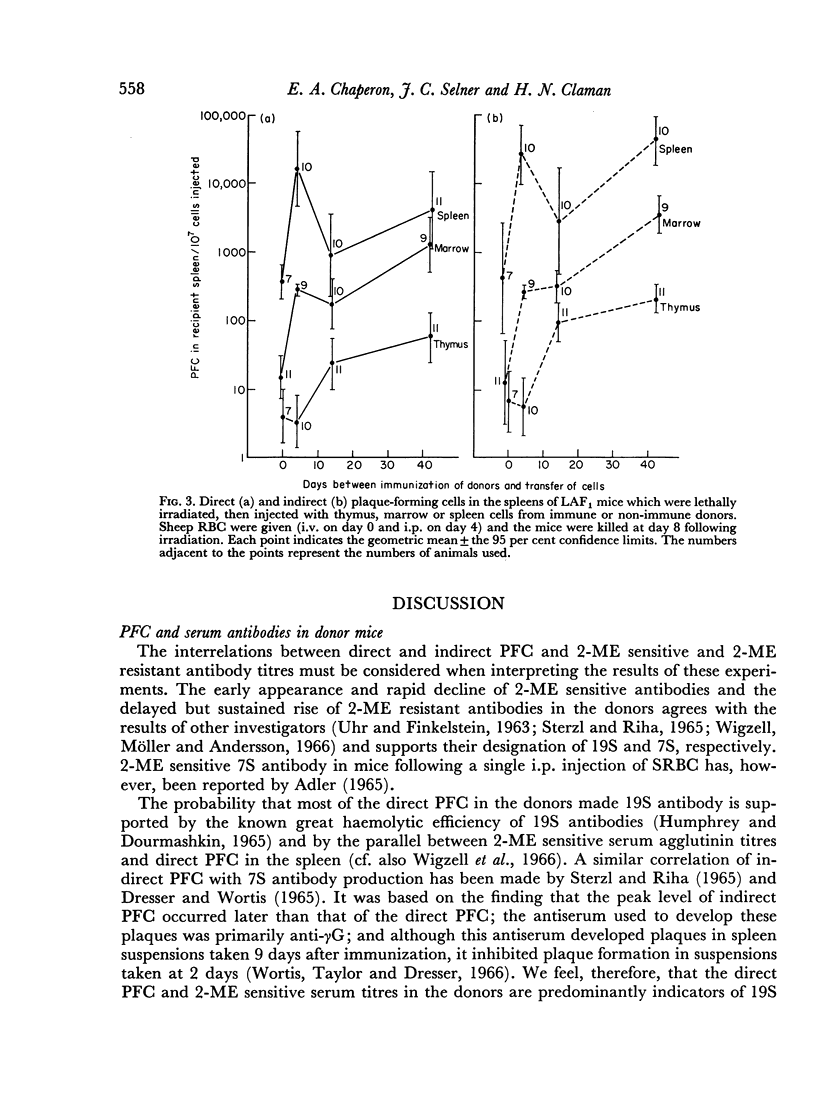
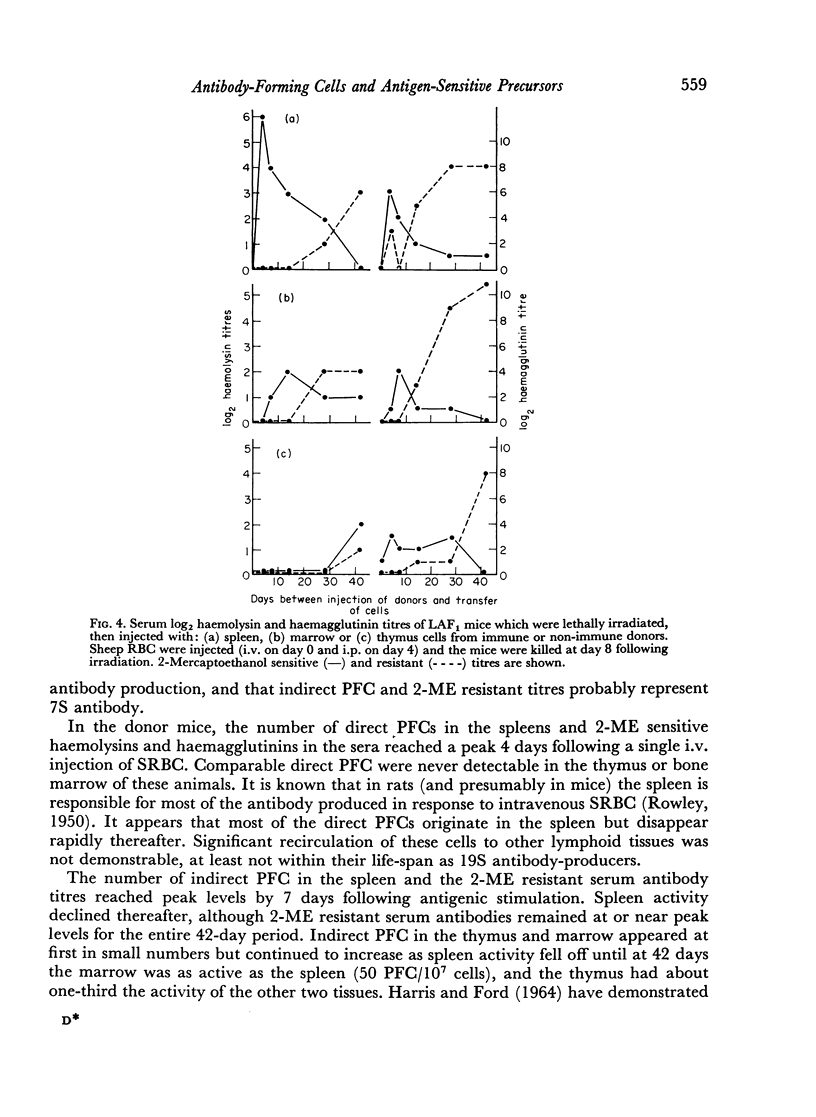
19S antibody production in mice reached a peak 4 days after antigen and declined rapidly thereafter. Direct PFCs were found in the spleen, but not in thymus or marrow. These cells probably do not recirculate, at least not within their life-span as 19S antibody producers. Production of 7S antibody reached a peak 7 days after immunization. Indirect PFCs were most numerous in the spleen at this time and persisted in the body for several weeks. These cells probably recirculate since increasing numbers were found in the thymus and marrow with increasing intervals after immunization.
The appearance and distribution of antigen-sensitive precursor cells showed a pattern similar to that of the 7S producers, suggesting that they recirculate in a similar manner.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER F. L. STUDIES ON MOUSE ANTIBODIES. I. THE RESPONSE TO SHEEP RED CELLS. J Immunol. 1965 Jul;95:26–38. [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Immunocompetence of transferred thymus-marrow cell combinations. J Immunol. 1966 Dec;97(6):828–832. [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN H. DISTRIBUTION OF ANTIBODY PLAQUE FORMING CELLS IN VARIOUS TISSUES OF SEVERAL STRAINS OF MICE INJECTED WITH SHEEP ERYTHROCYTES. Proc Soc Exp Biol Med. 1964 Nov;117:526–530. doi: 10.3181/00379727-117-29628. [DOI] [PubMed] [Google Scholar]
- GENGOZIAN N., MAKINODAN T., SHEKARCHI I. C. Transplantation of antibody-forming cells in lethally irradiated mice. J Immunol. 1961 Feb;86:113–122. [PubMed] [Google Scholar]
- Galton M., Reed P. B. Entry of lymph node cells into the normal thymus. Transplantation. 1966 Mar;4(2):168–177. doi: 10.1097/00007890-196603000-00006. [DOI] [PubMed] [Google Scholar]
- HARRIS J. E., FORD C. E. CELLULAR TRAFFIC OF THE THYMUS: EXPERIMENTS WITH CHROMOSOME MARKERS. EVIDENCE THAT THE THYMUS PLAYS AN INSTRUCTIONAL PART. Nature. 1964 Feb 29;201:884–885. doi: 10.1038/201884a0. [DOI] [PubMed] [Google Scholar]
- HOLLINGSWORTH J. W. Compatibility factors influencing the acceptance of rat bone marrow graft by the irradiated mouse. Yale J Biol Med. 1958 Dec;31(3):157–163. [PMC free article] [PubMed] [Google Scholar]
- LANDY M., SANDERSON R. P., BERNSTEIN M. T., LERNER E. M., 2nd INVOLVEMENT OF THYMUS IN IMMUNE RESPONSE OF RABBITS TO SOMATIC POLYSACCHARIDES OF GRAM-NEGATIVE BACTERIA. Science. 1965 Mar 26;147(3665):1591–1592. doi: 10.1126/science.147.3665.1591. [DOI] [PubMed] [Google Scholar]
- MARSHALL A. H., WHITE R. G. The immunological reactivity of the thymus. Br J Exp Pathol. 1961 Aug;42:379–385. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- STONER R. D., BOND V. P. ANTIBODY FORMATION BY TRANSPLANTED BONE MARROW, SPLEEN, LYMPH NODES AND THYMUS CELLS IN IRRADIATED RECIPIENTS. J Immunol. 1963 Aug;91:185–196. [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S. Antibody formation. IV. Formation of rapidly and slowly sedimenting antibodies and immunological memory to bacteriophage phi-X 174. J Exp Med. 1963 Mar 1;117:457–477. doi: 10.1084/jem.117.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E., Melletz E. W., Breuninger-Peck E. Facilitation of immune hemolysis by an interaction between red cell-sensitizing antibody and gamma-globulin allotype antibody. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1310–1317. doi: 10.1073/pnas.54.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Möller G., Andersson B. Studies at the cellular level of the 19S immune response. Acta Pathol Microbiol Scand. 1966;66(4):530–540. doi: 10.1111/apm.1966.66.4.530. [DOI] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]


